Abstract
This study was designed to investigate the potential effects of chronic stress on periodontal bone remodeling and its mechanism during orthodontic tooth movement in rats. Forty-eight male SD rats aged 8 weeks were randomly divided into control group and chronic stress group. Chronic unpredictable mild stress model (CUMS) was established in the stress group, which was validated by behavioral experiment as well as cortisol (CORT) and adrenocorticotropic hormone (ATCH) levels. Then, the two groups were further divided into three distinct groups, namely group with no orthodontic force, group with 30 g orthodontic force and group with 50 g orthodontic force respectively to construct orthodontic tooth movement model. The rats were sacrificed after 14 days and maxilla on the loading side was obtained to measure tooth movement distance. It was found that compared with the control group, the chronic stress group displayed increased parathyroid hormone (PTH), amino terminal peptide of type I procollagen (PINP) and c-terminal peptide of type I collagen branch (CTX) levels as measured by enzyme-linked immunosorbent assay (ELISA). Hematoxylin and Eosin (HE) and TRAP staining showed fewer osteoblasts and more number of osteoclasts. The results of western blot showed no significant change in expression of Adenylate cyclase (AC) but increased phospholipase C (PLC) levels were noted. In addition, increased NF-κB expression was observed by immunohistochemistry. Overall, chronic stress can affect bone remodeling during orthodontic tooth movement by increasing the content of PTH in the blood and increasing PLC and NF-κB.
1. Introduction
Chronic stress refers to a series of a number of nonspecific reactions induced by the body (human or animal) under different stimuli (single or combined) such as changes in the living environment, social factors and alterations in the psychological state (Braceland, Citation1976). Stress is an inevitable part of life. Chronic stress caused by conditions such as adversity, depression, anxiety or loneliness can significantly endanger human health (Papaghiuc et al., Citation2005). In daily life, people will inevitably be often exposed to a state of psychological stress. Mild stress after a short time of stimulation or correct psychological counseling will not have a significant impact on the body, but high intensity and long-term stress can cause substantial damage to the body (Qin et al., Citation2014). Worldwide, the incidence of depression caused by chronic stress is reported to be approximately 19% (Yang et al., Citation2017).
During orthodontic tooth movement, the balance of periodontal bone osteogenesis and osteoclast is disrupted, and this process is regulated by several osteoblasts and osteoclast related factors, including Parathyroid hormone, PTH and nuclear factor κB (NF -κB) are key mediators involved in the cellular regulation of bone resorption (Pérez-Sayáns et al., Citation2010; Theoleyre et al., Citation2004). A number of studies have found that NF -κB expression was markedly increased during orthodontic tooth movement, thus indicating that it plays an important role in Periodontium remodeling (Xu et al., Citation2016). However, there is no report describing the changes of PTH expression in orthodontic teeth in stress state.
Chronic stress can cause a series of endocrine as well as metabolic changes in the body, and cause substantial organic changes or damage (Kooij, Citation2020; Wirtz & von Känel, Citation2017). This study employed animal model of chronic stress in rats to mimic the stressful state during orthodontic treatment.
Thereafter, by using different molecular and biochemical experiments, validation of chronic stress on the influence of tooth movement in the process of bone rebuilding function was carried out to elucidate the role of relevant psychological factors in orthodontic periodontal bone reconstruction mechanism. Overall,it is the first time to explore the effect of chronic stress on periodontal bone remodeling during orthodontic tooth movement, which can provide a new reference for the management of orthodontists.
2. Material and methods
2.1. Laboratory instrument reagent
2.1.1. Reagents
The various kits, antibodies and reagents used in the study have been listed below:
Type I collagen cross-linked carboxyl terminal peptide (CTX I) detection kit (Wuhan Ursun, China), corticosterone (CORT) detection kit (Wuhan Ursun, China), type I procollagen amino terminal peptide (Procollagen I) N-Terminal Propeptide, PINP) detection kit (Wuhan Ursun, China), PTH detection kit (Wuhan Ursun, China), and Adrenocorticotropic hormone (ACTH) detection reagent Box (Wuhan Ulsen, China). NF-ĸB p65 Ab (Affinity, USA), Xylene (Sinopharm), Absolute Alcohol (Sinopharm), Glycerin (Sinopharm), HE dyeing kit (MDL, China), anti-tartaric acid Acid phosphatase staining solution (Solarbio, China), protein extraction solution (MDL, China), protease inhibitors (MDL, China), BCA protein concentration determination kit (MDL, China), SDS-PAGE precast gel kit (MDL, China), medium protein molecular weight marker (Thermo, China), Anti-actin (MDL, China).
2.1.2. Instruments
The various instruments used for the experiments in this study have been listed below:
Water bathtub (Leica, Germany), upright fluorescence microscope (Leica, Germany), microplate reader (BIORAD, USA), orthodontic wire (West Lake Bar, China), wide dynamic cash register zoom camera (Kopor, China), Refrigerator (Meiling, China), Electrophoresis tank (Beijing Longfang, China), Biological tissue spreader (Wuhan Junjie, China), Electronic balance (Hangzhou Wante, China), Centrifuge (Hengnuo Instruments, China), Micropipette (Gilson, France), electrothermal constant temperature water bath (Shanghai Yiheng Constant Temperature Equipment Factory, China), decoloring shaker (Haimen Qilin Bell Instrument Manufacturing Company), electrophoresis instrument (Beijing Baijing), cryogenic centrifuge (Sigma, Germany), SDS-PAGE electrophoresis system (BIO-Rad, USA), gel imaging system (UVP, USA), and ChemiDoc MP Chemiluminescence Imaging System (Bio-rad, USA)
2.2.3. Animal origin and ethics consideration
Forty-eight healthy male Sprague DawLey (SD) rats aged 8 weeks, weighing 250 ± 10 g, were used as experimental animals. All rats were purchased from Spefu (Beijing) Biotechnology Co., LTD. All experimental and animal care procedures were approved by the Animal Ethics Committee of Jiamusi University and performed in accordance with institutional guidelines.
2.2. Methods
2.2.1. Grouping and modeling of animals
48 SD rats were randomly divided into the control group and chronic stress group, with 24 rats in each group. CUMS modeling was performed in chronic stress group and the stimulation conditions included (1) fasting for 24 h; (2) No water for 24 h; (3) The cage was tilted for 24 h, that is, one side of the cage was placed at an Angle of 45° and tilted for 24 h; (4) The plantar subjected to shock for 5 min; The electric shock box was used at 1 mA and 30 V for 10 random shocks within 5 min, each lasting for 10 s; (5) The day and night were reversed for 24 h. The rats were given light at night and placed in a dark room during the day; (6) Exposure to the wet cushion material 24 h; (7) Horizontal shock for 5 min. The cage was placed on the shaking device at 160 Hz for 5 min. Modeling method: One stimulus was randomly administered each day. However, In consecutive weeks, the method of daily exposurewas not repeated, and the same stimulation could not be used in consecutive two days, and rats in each group received the same stimulation on the same day. Modeling lasted for 4 weeks. The control group was fed normally.
2.2.2. Behavior test in rats
2.2.2.1. Weight measurement
The weight of each rat was weighed at the first, second, third and fourth weeks after the start of CUMS modeling and was recorded at the end of schedule, and the weight change curve was drawn according to the results.
2.2.2.2. Sugar water preference experiment
After 4 weeks of stimulation, a 48-h sucrose adaptation training was conducted. Two identical drinking water bottles were placed in each cage, and sucrose solution (2%) was placed in these two bottles. After 24 h, one bottle was replaced with pure water and kept for 24 h. After the training, the animals were deprived of water for 24 h. Then, the two identical drinking water bottles were placed in each cage, one containing pure water and the other containing sucrose solution (2%), and rats were allowed to drink them freely for 12 h. Thereafter, amount of water remaining in the bottle was measured and the consumption of sugar water (mL) was calculated.
2.2.2.3. Tail suspension experiment
After 4 weeks of stimulation, the rats in each group were made to hang their heads downwards by fixing their tails and the immobility time (s) of the animals within 5 minutes was recorded by stopwatchs.
2.2.2.4. Forced swimming experiment
After 4 weeks of stimulation, the animals were placed in a bucket for 5 minutes to measure the swimming behavior and immobility behavior of the rats, as well as the immobility time (s) was recorded.
2.2.3. Measurement of the stress indicators by ELISA
At the end of the fourth week of modeling, orbital venous blood was collected from rats and the stress indexes of CORT as well as ACTH were detected by ELISA. The difference of stress indexes between the normal control group and chronic stress group was compared and the level of PTH was detected.
2.2.4. Application of orthodontic force to teeth
At the end of the fourth week of stress, based on the previous grouping of CUMS model, the control group and the stress group were randomly divided into three subgroups according to the different applied orthodontic force values. These included group with no orthodontic force, the group with 30 g orthodontic force and the group with 50 g orthodontic force. When the orthodontic tooth movement model was established, the stress stimulus continued to exist.
The rats were first anesthetized with 3% phenobarbital solution at 35 mg/kg, and the were fixed in supine position on the animal operating table after the effect of anesthesia. The rat’s right upper first molars and middle incisors were ground into a retention groove with a depth of about 1 mm by using dental high speed turbine, and the other end of the tension spring was ligated as well as fixed to the retention groove of the incisors with stainless steel wire. The 30 g and 50 g forceNi-Ti tension springs were ligated between the right incisor and the first molar with a dynamometer. Thereafter, the ligature wires were glued around the incisor ligature wires with glass ions to prevent the ligature wires from falling off. The orthodontic model was checked daily and maintained carefully. The whole afterloading process lasted for 14 days.
2.2.5. Measurement of the tooth movement distance
At the end of the experiment, rats were sacrificed by exposure to excessive carbon dioxide, maxillary bone tissue was extarcted, and the distance between the right maxillary first molar and the second molar was measured by Vernier caliper. The average value was calculated by the same person after the three measurements.
2.2.6. Elisa assay
The blood was collected from orbit of rats, and the serum was processed according to ELISA kit instructions to detect the contents of CORT, ACTH, PTH, PINP and CTX in the serum of rats.
2.2.7. Sectioning of the tissues for HE and TRAP staining
The bone tissue specimens were fixed with formaldehyde and dehydrated with EDTA. After embedding and fixation with wax blocks, they were sectioned continuously with a thickness of 4 um along the long axis of the tooth body. During sectioning, they were sectioned vertically along the long axis of the first molar after completing the slices for HE staining and the TRAP. HE staining sections were used to observe the morphological changes of the periodontal bone in 1/3 of the proximal and distal lateral root of the right maxillary first molar. The situation of osteoclasts in the proximal and distal root region of the right maxillary first molars was observed in TRAP staining sections.
2.2.8. Western blot analysis
The total protein from the maxillary tissue in the right tooth moving region was obtained. The electrophoresis apparatus was adjusted to 80 V for the sample to pass through the concentrated gel and the separated gel (the voltage was about 8 V/cm). After the gel electrophoresis, the protein bands separated from the gel were transferred to PVDF membrane by transfer electrophoresis, and the membrane was transferred to 65 V for 2 h. The transfer membrane was thereafter removed, placed in the sealing solution, and incubated at the room temperature for 1 h. The membrane were incubated with the diluted primary antibody respectively at 4 °C for overnight. After that the membrane was washed, the secondary antibody was added, and the membrane was again washed with 1 × TBST to remove the free secondary antibody. The rafter, the two liquids in the ELC kit were mixed, the mixture was evenly spread on the PVDF membrane surface, and the reaction was performed for 4 minutes at the room temperature. Finally, the solution was discarded and the membrane was visualized with chemiluminescence imaging system.
2.2.9. Immunohistochemistry
The slices were subjected to conventional dewaxing, heating in microwave and then placed in citrate buffer solution with pH = 6. After being removed, primary antibody was added and incubated at 37 °C for 1–2 h. After washing, the secondary antibody was added and incubated at 37 °C for 30 min. The slices were then observed under a light microscope. The mean optical density (MOD) was calculated by image-Pro Plus 6.0 software to represent the immunity of the various corresponding antigens.
2.2.10. Statistical analysis
SPSS 21.0 software was used for the statistical analysis. One-way anOVA was employed to observe the normal distribution as well as the homogeneity of variance of data and to analyze the mean of the repeated samples. T-test was used to analyze multiple comparative analysis between groups. p < 0.05 was considered as statistically significant.
3. Results
3.1. Cums model was successfully established
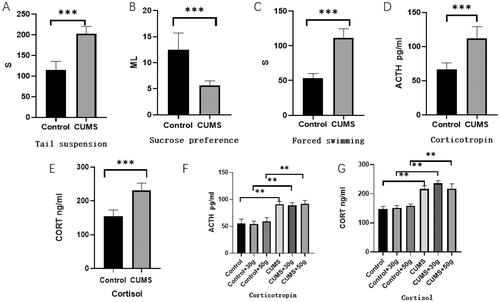
It was fund that Compared with the control group, the sugar water preference rate of the chronic stress group was significantly decreased (p < 0.01), whereas the immobility time of rats in suspended tail experiment and forced swimming experiment was significantly prolonged (p < 0.01). In addition, the weight gain was also significantly reduced (p < 0.01). These findings suggested that the model rats displayed significant chronic stress symptoms. Moreover, further experiments showed that the concentrations of CORT and ACTH in serum of CUMS rats were significantly increased (p < 0.01), which proved that the rat model of chronic unpredictability stress was successfully constructed, as shown in .
Figure 1. CUMS rat model was successfully established. Tail suspension (A), Sucrose preference (B), and Forced swimming (C) in rats with or without exposure to CUMS. The sugar and water preference rate of rats in the stress group decreased significantly (p < 0.01), and the immobility time in the tail suspension experiment as well as the forced swimming experiment increased significantly. The concentration of ACTH (D,F) and CORT (E,G) in the serum of CUMS model rats increased significantly before and after the orthodontic application *p < 0.05, **p < 0.01, ***p < 0.001, compared with the control group.
3.2. Effects of chronic stress on periodontal bone remodeling in orthodontic mobile area
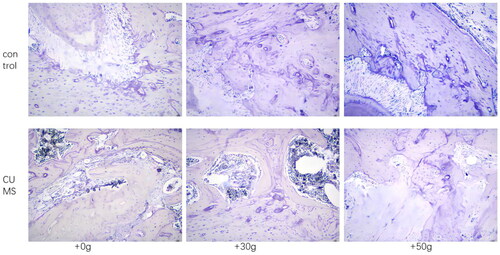
After 14 days of orthodontic tooth movement, CORT and ACTH concentrations were significantly increased in the chronic stress group compared with control group (p < 0.01), which proved that rats in the chronic stress group remained in the chronic stress state during orthodontic after loading, as shown in . However, compared with the control group, the tooth movement distance of the chronic stress group was increased (p < 0.01), CTX concentration in serum increased, PINP concentration decreased. The results indicated that osteoclastic activity was markedly increased but osteogenic activity decreased in chronic stress group, and the balance of bone metabolism was disrupted as shown in . In addition, by observing the HE staining section of the tooth movement area, it was found that compared with the control group, the density of bone trabecular bone in the orthodontic movement area of the rats in the chronic stress group was significantly reduced and the number of bone absorption lacunae was significantly increased, as shown in . Moreover, in TRAP stained sections, it was found that compared with the control group, the number of osteoclasts in jaw bone in the tooth movement area of rats in the chronic stress group was significantly increased as shown in . These results suggested that chronic stress could effectively inhibit the formation of new bone by osteoblasts and promote the absorption of bone tissue by affecting osteoclasts during orthodontic tooth movement.
Figure 2. Orthodontic tooth movement can alter the bone metabolism balance changes. (A) The tooth movement distance of rats in the stress group was markedly increased. The concentration of CTX (C) in the serum was increased but the concentration of PINP (B) was decreased. These results suggest that the osteoclast activity of rats in the stress group increased, but the osteogenic activity decreased. The balance of bone metabolism was disrupted. *p < 0.05, **p < 0.01, ***p < 0.001, compared with the control group.
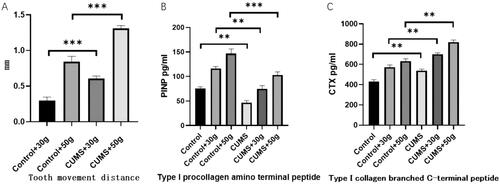
Figure 3. HE staining showed that the alveolar bone fibers in the tooth movement area in the CUMS group were sparse and osteoclast lacuna was substantially increased.
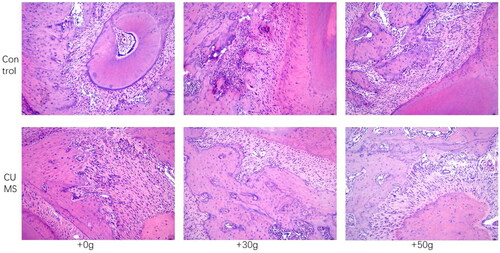
Figure 4. TRAP staining revealed increased staining of osteoclasts in the alveolar bone in the tooth movement area in the CUMS group.
3.3. The influence mechanism of chronic stress on periodontal bone remodeling
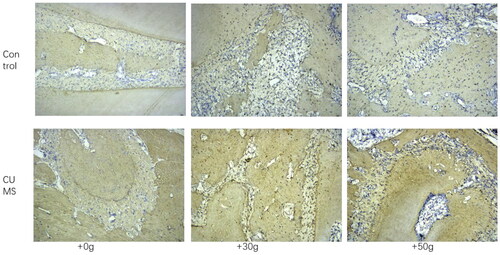
It was noted that compared with the control group, serum PTH concentration in the chronic stress group was significantly increased as observed by ELISA (). Western blot results indicated that AC content in jaw tissue was not significantly affected, but phospholipase C(PLC) content was significantly increased (). Furthermore, immunohistochemical results of bone tissues revealed that the expression of NF-κB in mandible in orthodontic movement area of rats in chronic stress group was substantially increased (). These results suggested that the chronic stress can promote the secretion of the PTH in rats, and these hormones can potentially influence the periodontal bone remodeling of rats by promoting NF-κB expression.
Figure 5. Before exposure (A) and after exposure (B) to orthodontic stress, the PTH content in the CUMS group was found to be significantly increased. *p < 0.05, **p < 0.01, ***p < 0.001, compared with the control group.
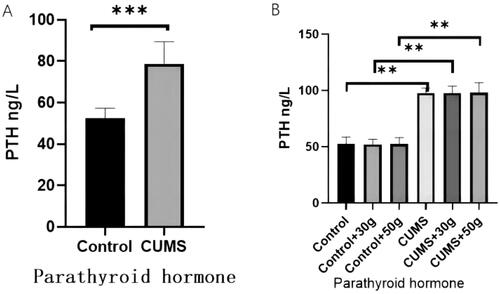
Figure 6. The AC content in the jaw bone tissue of the CUMS group did not change significantly, but the PLC content was significantly increased.
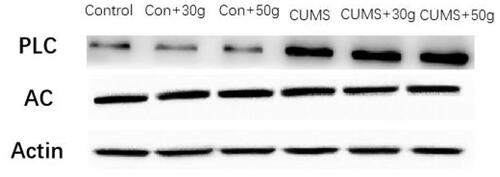
Figure 7. The expression of NF-ĸB in the jaw bone of orthodontic tooth movement area was substntially increased in CUMS group.
4. Discussion
Chronic stress refers to the continuous emotional changes and psychological pressure caused by the failure of an independent individual to adjust to and stabilize homeostasis in the face of long-term specific and continuous environmental changes. Accumulating evidences over the course of several years have confirmed that chronic stress can serve as an important cause or promoting factor of various major human diseases (Sharif et al., Citation2018; Wirtz & von Känel, Citation2017). Recently, bone remodeling in chronic stress has become a hot research topic. For instance, Yang et al. proved in a mouse model that GABAergic neuron (GABA)in the hypothalamus could mediate chronic stress-induced bone loss in the neural circuit, thereby indicating that GABA can potentially act on a specific set of somatostatin neurons and trigger bone loss without the stressors. In addition, Yang et al. also reported that the sympathetic nervous system and glutamate neurons in the bundle nucleus could effectively regulate bone loss caused by stress, and identified the central nervous mechanism of chronic stress affecting bone metabolism (Yang et al., Citation2020). Foertsch et al. found that in comparison with the normal control rats the length of tibia and femur in chronic stress rats was significantly decreased, the thickness of growth plates was increased, and the mineral deposition of growth plates was markedly reduced, thus indicating that chronic stress can interfere with endochondral ossification during the growth of long bones. In addition, the trabecular thickness and bone mineral density in the bones of chronic stress rats was significantly increased, and the expression of tyrosine hydroxylase in the bone marrow cells of the growth plates was also enhanced, thereby suggesting that the local adrenergic signal transduction might be involved in the effects of chronic stress on skeletal phenotype (Foertsch et al., Citation2017). Raymond W. Lam et al. concluded in past experiments that rodents in CUMS model can cause bone loss, fluoxetine significantly increased the cross-sectional area, trabecular bone area, structural strength and osteoblasts/bone area. This could be a potential treatment for bone loss caused by depression (Lam et al., Citation2022). Moreover, Yirmiya et al. found that chronic stress-induced bone loss was accompanied by a significant reduction in the number of osteoblasts leading to limited bone formation. Chronic stress-induced bone loss was associated with significantly elevated levels of bone norepinephrine and could be blocked by the β adrenergic antagonist propranolol, thus suggesting that the sympathetic nervous system can mediate the skeletal effects of stress-induced depression (Yirmiya et al., Citation2006).
Chronic stress primarily leads to activation of the classic neuroendocrine system [the hypothalamus—pituitary—adrenal (HPA) axis] and the sympathetic nervous system (SNS), thus affecting the prefrontal cortex and hippocampus of function and dysfunction. HPA axis and SNS activation process of a variety of stress hormones constitutes the endocrine regulation network of the chronic stress (McEwen, Citation2017). As the downstream hormone regulated by thyroid hormone, a number of studies have found that thyroid hormone secretion can significantly increase under the chronic stress, and thyroid hormone level and T-cell lymphoid tissue hyperplasia response of decrease in mice (Frick et al., Citation2009). In our experiment, it was found that the level of parathyroid hormone of the same family also changed in chronic stress state. Moreover, compared with the control group, ELISA results showed that the serum concentration of PTH in chronic stress group was significantly increased. Parathyroid hormone is a polypeptide molecule synthesized and secreted by the main cells of the parathyroid gland, which participate in the regulation of calcium and phosphorus homeostasis in the body and can affect bone reconstruction (Luo et al., Citation2018). In recent years, domestic and foreign scholars have been investigated the mechanisms of action of PTH on vertebra and limb bone remodeling (Milstrey et al., Citation2017). A number of clinical and basic studies have reported that intermittent administration of PTH during orthognathic surgery and distraction osteogenesis can accelerate the bone defect repair and promote fracture healing as well as new bone formation (Neer et al., Citation2001; Tang et al., Citation2014). PTH binds to the specific receptors on the target cell membranes and can activate two major signaling pathways, cyclic adenosine phosphate (cAMP)/protein kinase A (PKA) or PLC/protein kinase C (PKC), to play A biological role (Choi et al., Citation2018). Moreover, another study conducted on oral and maxillofacial remodeling found that PTH could exhibit dual effect, namely, intermittent low-dose PTH promoted the formation of the cranial and maxillofacial new bone and can aid to markedly improve the biomechanical properties of new bone. On the contrary, exposure of osteoblasts to continuous and high-dose application of PTH resulted in secretion of a large amount of nuclear factor -κB receptor-activating factor (RANKL), inhibited the expression of osteoprotectin (OPG), increased osteoclast activity, and finally enhanced the rate of bone resorption greater than bone formation (Choi et al., Citation2018). In this study, it was found that when the rats in the stress state were subjected to orthodontic, PLC in the jaw bone tissue increased significantly. In addition, immunohistochemical results of bone tissue indicated that in the chronic stress group rats, NF-ĸB expression was increased in jaw, which in turn can enhance the activity of osteoclasts.
Terzioqlu et al. used rat stress model to establish that chronic stress can increase the plasma PTH level by upregulating calcium-sensitive receptors on the main cells of the parathyroid gland (Terzioglu-Usak et al., Citation2018). The action of parathyroid hormone in the body has been reported to be bidirectional (Poole & Reeve, Citation2005). Intermittent exposure to higher-than-average parathyroid hormone can promote osteogenesis, whereas the continuous exposure to high parathyroid hormone modulates the expression of RANKL in osteoblasts can inhibit both OPG expression and bone formation (Locklin et al., Citation2003). Thus, persistent chronic stress may promote bone loss through increased parathyroid hormone concentrations. We found that compared with the control group, the serum PINP content in the stress group decreased but CTX content increased, thus suggesting that the osteoclastic activity increased and osteogenic activity decreased in the stress group, and the balance of bone metabolism was disrupted. In the tissue sections, it was observed that the density of bone trabecular and the number of osteoclasts and osteoclast lacunae increased in the stress group, which suggested that osteoclasts were activated and the density of jaw bone decreased under the stress state. These changes were found to be gradually significant with the increase of orthodontic force, and the tooth movement distance of the stress group was significantly greater than that of the control group under the same orthodontic force, thereby indicating that the stress can effectively promote the absorption of jaw bone and increase the sensitivity of alveolar bone to orthodontic force. Further mechanism study found that thyroid hormone concentration in the stress group was significantly increased, and there was a significant correlation noted with the change of periodontal bone mass, thus suggesting that stress state under the regulation of parathyroid hormone concentration might act as the key factor causing the decrease of periodontal bone mass in rats. PLC and NF-κB are key proteins involved in the parathyroid pathway. The content of PLC and NF-κB in the jaw was found to be significantly higher in the stress group than in the control group. These findings confirmed that chronic stress on the bone remodeling during orthodontic tooth movement was primarily mediated through the action of parathyroid hormone on the PLC/PKC signaling pathway, thus regulating the effect of NF-κB on bone metabolism. The major aim of this study was to explore the chronic stress state in the process of orthodontic tooth movement and to establish the correlation of periodontal bone reconstruction for clinical patients in chronic stress state to provide more humanistic care and realistic situation of treatment. Here, we have only studied the influence of chronic stress on bone metabolism in orthodontic process. In the future research, this research group will further study the mechanism. It is hoped to provide help for clinicians in the treatment of orthodontic patients under chronic stress.
5. Conclusion
Chronic stress can affect bone remodeling during orthodontic tooth movement by increasing the content of PTH in the blood and increasing PLC and NF-κB.
Acknowledgments
We thank Heilongjiang Provincial Department of Education for supporting this project.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
Notes on contributors
Li Xiaotong
Li Xiaotong holds a master's degree in Stomatology from Jiamusi University. Her current research focuses on the relationship between chronic stress and orthodontic tooth movement.
Yu Jiazhi
Yu Jiazhi studied in Jiamusi University for a master's degree in Stomatology. At present, he mainly studies the relationship between chronic stress and orthodontic tooth movement and the role of MiRNA in orthodontic tooth movement.
Li Xiaoguang
Li Xiaoguang is an associate professor of stomatology at Jiamusi University. Her research interests include the relationship between chronic stress and orthodontic tooth movement as well as invisible orthodontic research.
Zhao Gang
Zhao Gang is a professor of stomatology at Jiamusi University. His research interests include the relationship between chronic stress and orthodontic tooth movement, drug therapy for oral squamous cell carcinoma, etc.
References
- Braceland, F. J. (1976). The stress of life. Psychiatric Annals, 6(12), 65–66. https://doi.org/10.3928/0048-5713-19761201-13
- Choi, H., Magyar, C. E., Nervina, J. M., & Tetradis, S. (2018). Different duration of parathyroid hormone exposure distinctively regulates primary response genes Nurr1 and RANKL in osteoblasts. PLoS One, 13(12), e0208514. https://doi.org/10.1371/journal.pone.0208514
- Foertsch, S., Haffner-Luntzer, M., Kroner, J., Gross, F., Kaiser, K., Erber, M., Reber, S. O., & Ignatius, A. (2017). Chronic psychosocial stress disturbs long-bone growth in adolescent mice. Disease Models & Mechanisms, 10(12), 1399–1409. https://doi.org/10.1242/dmm.030916
- Frick, L. R., Rapanelli, M., Bussmann, U. A., Klecha, A. J., Arcos, M. L. B., Genaro, A. M., & Cremaschi, G. A. (2009). Involvement of thyroid hormones in the alterations of T-cell immunity and tumor progression induced by chronic stress. Biological Psychiatry, 65(11), 935–942. https://doi.org/10.1016/j.biopsych.2008.12.013
- Kooij, M. (2020). The impact of chronic stress on energy metabolism. Molecular and Cellular Neuroscience, 107, 103525. https://doi.org/10.1016/j.mcn.2020.103525
- Lam, R. W., Wong, H.-K., Kumarsing, R. A., Chua, A. N., Ho, R. C., McIntyre, R. S., & Ho, C. S. (2022). Fluoxetine improves bone microarchitecture and mechanical properties in rodents undergoing chronic mild stress - an animal model of depression. Translational Psychiatry, 12(1), 339. https://doi.org/10.1038/s41398-022-02083-w
- Locklin, R. M., Khosla, S., Turner, R. T., & Riggs, B. L. (2003). Mediators of the biphasic responses of bone to intermittent and continuously administered parathyroid hormone. Journal of Cellular Biochemistry, 89(1), 180–190. https://doi.org/10.1002/jcb.10490
- Luo, Y., Li, S.-Y., Tian, F.-M., Song, H.-P., Zhang, Y.-Z., & Zhang, L. (2018). Effects of human parathyroid hormone 1-34 on bone loss and lumbar intervertebral disc degeneration in ovariectomized rats. International Orthopaedics, 42(5), 1183–1190. https://doi.org/10.1007/s00264-018-3821-2
- McEwen, B. S. (2017). Neurobiological and systemic effects of chronic stress. Chronic Stress, 1, 247054701769232. https://doi.org/10.1177/2470547017692328
- Milstrey, A., Wieskoetter, B., Hinze, D., Grueneweller, N., Stange, R., Pap, T., Raschke, M., & Garcia, P. (2017). Dose-dependent effect of parathyroid hormone on fracture healing and bone formation in mice. The Journal of Surgical Research, 220, 327–335. https://doi.org/10.1016/j.jss.2017.07.019
- Neer, R. M., Arnaud, C. D., Zanchetta, J. R., Prince, R., Gaich, G. A., Reginster, J. Y., Hodsman, A. B., Eriksen, E. F., Ish-Shalom, S., Genant, H. K., Wang, O., & Mitlak, B. H. (2001). Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. The New England Journal of Medicine, 344(19), 1434–1441. https://doi.org/10.1056/NEJM200105103441904
- Papaghiuc, C., Constantin, B., Mihalache, C., Oprea, V., & Carja, E. (2005). Effects of psychological stress on health. Revista medico-chirurgicala a Societatii de Medici si Naturalisti din Iasi, 109(4), 705–708.
- Pérez-Sayáns, M., Somoza-Martín, J. M., Barros-Angueira, F., Rey, J. M. G., & García-García, A. (2010). RANK/RANKL/OPG role in distraction osteogenesis. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 109(5), 679–686. https://doi.org/10.1016/j.tripleo.2009.10.042
- Poole, K., & Reeve, J. (2005). Parathyroid hormone - a bone anabolic and catabolic agent. Current Opinion in Pharmacology, 5(6), 612–617. https://doi.org/10.1016/j.coph.2005.07.004
- Qin, H.-Y., Cheng, C.-W., Tang, X.-D., & Bian, Z.-X. (2014). Impact of psychological stress on irritable bowel syndrome. World Journal of Gastroenterology, 20(39), 14126–14131. https://doi.org/10.3748/wjg.v20.i39.14126
- Sharif, K., Watad, A., Coplan, L., Amital, H., Shoenfeld, Y., & Afek, A. (2018). Psychological stress and type 1 diabetes mellitus: What is the link? Expert Review of Clinical Immunology, 14(12), 1081–1088. doi: https://doi.org/10.1080/1744666X.2018.153878
- Tang, Z.-L., Zhang, W-j., Wang, D.-X., Chen, J.-M., Ma, H., & Wu, D.-R. (2014). An experimental study addressing the promotion of mandibular defect repair through the intermittent subcutaneous injection of parathyroid hormone. Journal of Oral and Maxillofacial Surgery : Official Journal of the American Association of Oral and Maxillofacial Surgeons, 72(2), 419–430. https://doi.org/10.1016/j.joms.2013.07.037
- Terzioglu-Usak, S., Elibol, B., Dalli, T., Guler, C., & Aysan, E. (2018). Effect of restraint stress on plasma PTH concentration and its molecular targets expressions in Wistar rats. International Journal of Endocrinology and Metabolism, 16(4), e66979. https://doi.org/10.5812/ijem.66979
- Theoleyre, S., Wittrant, Y., Tat, S. K., Fortun, Y., Redini, F., & Heymann, D. (2004). The molecular triad OPG/RANK/RANKL: Involvement in the orchestration of pathophysiological bone remodeling. Cytokine & Growth Factor Reviews, 15(6), 457–475. https://doi.org/10.1016/j.cytogfr.2004.06.004
- Wirtz, P. H., & von Känel, R. (2017). Psychological stress, inflammation, and coronary heart disease. Current Cardiology Reports, 19(11), 111. https://doi.org/10.1007/s11886-017-0919-x
- Xu, F., Dong, Y., Huang, X., Chen, P., Guo, F., Chen, A., & Huang, S. (2016). Pioglitazone affects the OPG/RANKL/RANK system and increase osteoclastogenesis. Molecular Medicine Reports, 14(3), 2289–2296. https://doi.org/10.3892/mmr.2016.5515
- Yang, F., Liu, Y., Chen, S., Dai, Z., Yang, D., Gao, D., Shao, J., Wang, Y., Wang, T., Zhang, Z., Zhang, L., Lu, W. W., Li, Y., & Wang, L. (2020). A GABAergic neural circuit in the ventromedial hypothalamus mediates chronic stress–induced bone loss. The Journal of Clinical Investigation, 130(12), 6539–6554. https://doi.org/10.1172/JCI136105
- Yang, Y., Hu, Z., Du, X., Davies, H., Huo, X., & Fang, M. (2017). miR-16 and fluoxetine both reverse autophagic and apoptotic change in chronic unpredictable mild stress model rats. Frontiers in Neuroscience, 11, 428. https://doi.org/10.3389/fnins.2017.00428
- Yirmiya, R., Goshen, I., Bajayo, A., Kreisel, T., Feldman, S., Tam, J., Trembovler, V., Csernus, V., Shohami, E., & Bab, I. (2006). Depression induces bone loss through stimulation of the sympathetic nervous system. Proceedings of the National Academy of Sciences of the United States of America, 103(45), 16876–16881. https://doi.org/10.1073/pnas.0604234103
