 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The sense of agency (SoA) refers to the feeling of being in control of one’s actions and the subsequent consequence of these actions. Emotional context seems to alter the strength of sense of agency. The present study explored the influence of acute psychosocial stress on the SoA by means of the Trier Social Stress Test (TSST). Self-assessment manikin (SAM) and objective physiological indicators (e.g. heart rate, electrodermal activity, and salivary cortisol levels) were utilized to evaluate the effect of the TSST. We also employed the temporal binding effect as an implicit assessment of the participant’s SoA. The results indicated that the stress level of the experimental group after TSST was significantly higher than the control group, whilst the temporal binding scores of the experimental group decreased after TSST manipulation. In short, acute psychosocial stress with intense emotional arousal weakened the sense of agency.
1. Introduction
The sense of agency (SoA) is the feeling of control over our voluntary actions and through them, of events in the outside world (Haggard, Citation2017). As a crucial component of self-awareness, the sense of agency is a cornerstone of psychological health. SoA primarily relies on the comparison between the predicted consequences of our actions and the actual sensory feedback (Moore & Obhi, Citation2012). The match of predicted and actual outcomes lead to SoA arises, whereas if they are incongruent, the outcome will likely be attributed to an external cause. For instance, when you push the button to go up by the lift, if the door opens in time, you will feel an enhanced sense of agency over the elevator door.
It has been shown that the emotional modulation of SoA may regulate social behavior (Christensen et al., Citation2019; Cui et al., Citation2018; Yoshie & Haggard, Citation2013). Yoshie and Haggard (Citation2013) provided direct evidence for the modulation of SoA by affective significance of action outcomes. In two experiments, they showed that temporal binding effect, an implicit measure of SoA, was attenuated for negative compared to positive or neutral outcomes. The temporal binding effect refers to the phenomenon in which the perceived time points of a voluntary action and its consequence are attracted to each other (Haggard et al., Citation2002; Moore & Haggard, Citation2010). The SoA was also decreased for fear-relevant outcomes relative to neutral ones (Cui et al., Citation2018).
Though negative outcomes weakened sense of agency, few studies investigated the influence of emotion state on sense of agency. A study found that arousal states with negative emotional valence, such as fear and anger, decreased sense of agency (Christensen et al., Citation2019). A recent study has shown inconsistent results using virtual reality (VR) technology to explore stress state on embodied SoA (Stern et al., Citation2020). Fourteen participants performed virtual hand tasks into a “neutral” block or a “stress” block. They found that mild psychosocial stress did not affect SoA in a virtual reality context. Therefore, the influence of acute psychosocial stress on SoA deserved further study.
The Trier Social Stress Test (TSST) is an ecologically valid stressor that involves social evaluation and unpredictability, forcing participants to speak in front of an expressionless audience and to complete an unexpected mental arithmetic test (Kirschbaum et al., Citation1993). Stress-induced in TSST could be more persuasively verified by physical indicators. Heart rate is thought to be a standard physiological indicator of the effect of TSST (Allen et al., Citation2014). Furthermore, numerous studies have shown that TSST leads to hypothalamus–pituitary–adrenal (HPA) axis activation, and cortisol is the final product of HPA axis activation (Gaab et al., Citation2003; Kirschbaum et al., Citation1999). Research suggested that electrical skin levels are also sensitive to a wide range of stressors (Posada-Quintero et al., Citation2017).
As mentioned above, there is still a lack of systematic research on the influence of stress state on the sense of agency. We employed temporal binding effect as an implicit measure of the sense of agency. This effect was first studied using the Libet Clock paradigm (Haggard et al., Citation2002), and the interval estimation paradigm was derived afterward (Humphreys & Buehner, Citation2009; Zhao et al., Citation2013). In the present study, participants were randomly divided into an experimental and a control group. The participant’s stress state in experimental group were induced through the TSST paradigm, while the control group did not. We hypothesized that TSST would cause a significant increase in participants’ stress levels, as well as a substantial decrease in the temporal binding effect.
2. Methods
2.1. Participants
A total of 59 participants (41 females, 22.05 ± 2.43 years) were recruited. Twenty-five were excluded (see 2.7 Data analysis for details), leaving 34 eligible participants, 17 subjects per group (10 females in the experimental group, 21.20 ± 2.66 years; 13 females in the control group, 23.15 ± 2.67 years). All participants were right-handed and healthy university students aged 18–28 years old who had normal vision or corrected-to-normal vision. The incorporated criteria also included: no surgery in the last six months, no smoking, no alcohol abuse, regular life and rest, and none of the women used oral contraceptives (Allen et al., Citation2017).
Sample sizes were determined using G-Power 3.1.9.4 (Faul et al., Citation2009). A mixed-design analysis of variance with a within-subjects factor of the stage (pretest and posttest) and a between-subjects factor of the group (experimental group and control group) was conducted for temporal binding scores. We required 34 participants to detect a medium effect size (Cohen’s f = 0.25) at 80% power, setting α = 0.05, Number of groups = 2, Number of measurements = 2, Correlation among repeated measures = 0.50, and non-spherical correction ε = 1. The stopping rule targeted 34 eligible participants whose data could be further analyzed (Gu et al., Citation2021).
2.2. General procedure
Before the experiment, participants needed to complete the time estimation test online at home. Firstly, they learned different length of the sound (100 ms, 500 ms, and 900 ms) by listening and then carrying on the test. Those who did not pass the test cannot participate in the experiment. All participants gave written informed consent and were paid for their participation. The experiment was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Institute of Psychology, Chinese Academy of Sciences.
The experimental procedure is shown in . Participants arrived at the laboratory about 13:00 or 15:00. They began a 20-min break during which they completed the Trait Anxiety Scale, SoA scale, and SAM scale (pretest). The heart rate and electrodermal activity pads of the physiological synthesizers were connected. Heart rate, electrodermal activity (HR base, EDA base), and the first tube of saliva cortisol (SC base) were then collected.
Figure 1. Schematic illustration of the procedure. Saliva cortisol (SC) was collected five times at baseline, before the TSST, and after the TSST 0 min/15 min/30 min. Note: Heart rate (HR) and electrodermal activity (EDA) were recorded eight times for 5 min at baseline, before the TSST, throughout the preparation/speech/arithmetic, and at 0min/15 min/30 min after the TSST manipulation. Two interval estimation tasks were performed before and after the TSST, respectively. H&E: Heart rate (HR) and electrodermal activity (EDA), HRbase(baseline), HRbef (before TSST), HRpre(preparation), HRspe(speech), HRAir(Arithmetic), HR0min(TSST0min), HR15min(TSST15min), HR30min(TSST30min), SC: Salivary cortisol; TSST: Trier Social Stress Test.
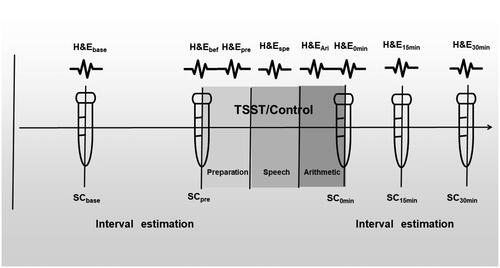
Next, the interval estimation task was conducted by E-Prime 2.0 for approximately 30 min. At the end of the task, heart rate, electrodermal activity (HR bef, EDA bef), and the second tube of saliva cortisol (SC pre) were collected.
Subsequently, the TSST was conducted which consisted of preparation, interview, and mental arithmetic for a total of 15 min. Heart rate and electrodermal activity (HR pre, HR spe, HR ari, and EDA pre, EDA spe, EDA ari) were recorded. After the TSST, heart rate, skin electrodes (HR0min, EDA0min), and the third tube of saliva cortisol (SC0min) were collected. Then, participants filled out the SAM scale (posttest).
A second interval estimation task was conducted lasting approximately 30 min. During a 5-min break within the temporal estimation task, heart rate, electrodermal activity (HR15min, EDA15min), and the fourth tube of saliva cortisol (SC15min) were collected. At the end of the interval estimation, heart rate, electrodermal activity (HR30min, EDA30min), and the fifth tube of saliva cortisol (SC30min) were collected.
2.3. Scales
2.3.1. Trait Anxiety Inventory and SoA scales
The State-Trait Anxiety Inventory (STAI) has been widely used for the assessment of anxiety in various populations. A Chinese version of the STAI's Trait Anxiety Inventory (T-AI) was used in this study. The trait anxiety subscales are all scored on a scale of 1 to 4, the higher the score, the more pronounced the individual’s anxiety trait.
The Sense of Agency scale (SoAs) contained both the positive sense of agency (SoPA) and the negative sense of agency (SoNA). It had a total of 11 items, 5 for SoPA and 6 for SoNA (Tapal et al., Citation2017).
2.3.2. SAM scale
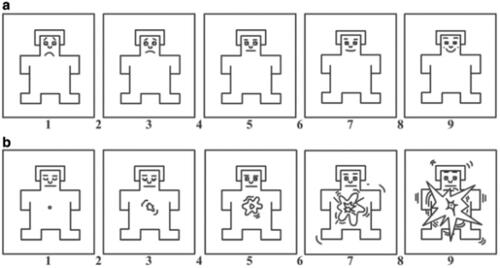
The Self-Assessment Scale (SAM) is shown in . The SAM is a nonverbal assessment technique that directly measures the valence of pleasure and arousal, associated with a person’s emotional response to various stimuli.
Figure 2. Illustration the paper version of SAM. Note: (a,b) Adapted version of the Self-Assessment Model for potency and arousal ratings. Valence and arousal were rated on a 9-point Likert scale, (a) for emotional pleasantness, where 1 represents the sadness and 9 represents the happiness, and (b) for emotional arousal, where 1 represents the calmness and 9 represents excitement.
2.4. Acute stress and control conditions
2.4.1. TSST manipulation
The Trier Social Stress Test (TSST) is a widely used method inducing acute stress responses in a laboratory setting, which consists of a 5-min preparation, a 5-min presentation, and a 5-min mental arithmetic task. In the interview presentation, the participant was guided to apply for a job position and to prepare for the following interview. Taking notes with pen and paper was allowed, but the paper would be taken away during the presentation. In the presentation phase, the participant was led to an individual interview room with three interviewers who might ask questions at any time and would be informed that there was a video camera recording. In the mental arithmetic phase, the participant was asked to subtract 13 from 1022 consecutively and to report each result of the mental arithmetic loudly. If the result was incorrect, the participant had to start again from 1022 until the end of the session. All three interviewers pretended to be very calm and reserved.
2.4.2. Control treatment
The control condition was consistent with the physical and psychological demands of the stress task, but not stressful by omitting the social and self-relevant components. Participant was only required to read a neutral text loudly to the camera, followed by 5 min of written arithmetic at the end.
2.5. Interval estimation task
The present study employed a modified version of interval estimation paradigm (Zhao et al., Citation2013). The interval estimation task was conducted by E-Prime 2.0 for approximately 30 min. The task was completed in two phases. Before the formal experiment, participants were asked to pass an online test at home in advance. Those who failed to passed the test were not allowed to enter the formal experiment. The formal experimental phase was completed in the laboratory.
Practice phase: Participants were trained to be familiar with different temporal intervals (100 ms, 500 ms, or 900 ms). They were told that 1 second equals 1000 ms. Participants first saw a prompt for the length of time, such as 100 ms. A fixation cross then appeared on a gray blank screen followed by a tone. The prompted time is the temporal interval between the disappearance of the fixation cross and the appearance of the subsequent tone. Each temporal interval was trained 5 times. Participants could practice again if they were not familiar with the task.
Test phase: The test phase was followed by the practice phase. A time interval was randomly selected and presented for the test phase, including 100 ms, 500 ms, and 900 ms. First, a fixation cross appeared on a gray blank screen followed by a tone. Participants needed to estimate the temporal interval between the fixation cross and tone. A total of three trials were presented and the participant passed if all answers were correct.
Experimental phase: The formal experimental phase contained voluntary and involuntary conditions. To avoid sequential effects, the order of the voluntary and involuntary conditions was counterbalanced. Each condition contained a total of 95 trials, of which 5 were practice trials (not analyzed).
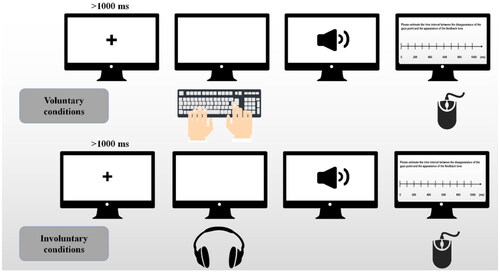
In the voluntary condition, a fixation cross appeared first and the participant pressed "Z" on the keyboard for left or "M" for right. The fixation cross disappeared followed by a "left" or "right" feedback tone. Then a fixed time interval (250 ms, 550 ms, or 850 ms) appeared. The interval between the disappearance of the fixation cross and the appearance of the feedback tone were then required to be reported for participants by clicking on a 0–1000 ms timescale.
In the involuntary condition, a fixation cross appeared and the participant heard a key beep. The fixation cross then disappeared simultaneously. A "left" or "right" feedback tone appeared after a fixed interval (250 ms, 550 ms, or 850 ms). The time interval between the disappearance of the fixation cross and the appearance of the feedback tone were required to be reported for participants by clicking on a 0–1000 ms timescale, See .
Figure 3. Diagram of the interval estimation process. Note: A fixation cross appears at the center of screen. The participant presses "Z" for left or "M" for right on the keyboard (voluntary condition) or hears a sound of key beep (involuntary condition). The fixation cross disappears followed by a fixed time interval with a feedback tone "left" or "right." The participant was asked to report the time interval by clicking on a 0–1000 ms timeline.
2.6. Physiological index collection
2.6.1. Salivary cortisol collection
A total of 5 saliva samples were collected from each participant using a biosharp (Beijing, China) BS-20-M 2 ml centrifuge collection tube. Samples were frozen in a −22 °C refrigerator within 2 h of collection until further analysis. Samples were thawed and centrifuged at 4000 rpm for 20 min before analysis by enzyme-linked immunoassay (ELISA) with biotin-labeled SC in a competitive assay (ELISA-Kit, WuHan Chundu Biotechnology Co., Ltd, China). The sensitivity of cortisol was 0.1 nmol/L. The linear regression of samples with the expected concentration correlation coefficient R-value was above 0.99. The intra-batch coefficient of variation and inter-batch coefficient of variation was less than 15%.
2.6.2. Heart rate and electrodermal activity acquisition
Heart rate and electrodermal activity were collected from participants using a BIOPAC MP160 polysomnographic recorder (USA) with a PPGED-R amplifier. The collected electrodermal activity and heart rate were recorded and analyzed by AcqKnowledge 4.0. The participant’s index finger, middle finger, and ring finger were disinfected with alcohol cotton balls and left to dry. Two electrode pads were then installed on the abdominal part of the first knuckle of the participant’s index and middle fingers. The positive and negative electrodes of electrodermal activity were connected to the two electrode pads. The heart rate recording electrode was connected to the ring finger. Participants were instructed to turn off electronic devices such as cell phones to prevent potential interference with the device signal.
2.7. Data analysis
Questionnaire analysis: Pearson correlation was conducted among trait anxiety scores, SoPA, and SoNA. SAM was analyzed with emotional valence expressed as a2a1 and emotional arousal as b2b1, where a2a1 = (a post − a pre), b2b1 = (b post − b pre), and independent samples t-test was conducted for a2a1 and b2b1.
Analysis of physiological indicators: To examine the validity of stress inducement, two-way mixed analyses of variances (ANOVAs) were conducted for heart rate (HR), skin conductance level (SCL), nonspecific skin conductance responses (NS.SCRs), and salivary cortisol (SC) with groups (experimental group and control group) as a between-subjects measure and time as a repeated measure. HR was averaged across each collecting period using the AcqKnowledge 4.0 software and was defined as the number of beats per minute (BPM). In order to get more accurate data, we set the noise rejection to 5% of peak, and the peak interval window to 40–180 BPM. There were two principal measures of relatively long-term tonic EDA states: skin conductance level (SCL) and nonspecific skin conductance responses (NS.SCRs). SCL and NS.SCRs have been widely used as indices of sympathetic nervous system arousal (Boucsein et al., Citation2012). Firstly, the acquired raw signal is down-sampled, and the low-pass filter is fixed at 1 Hz. Secondly, we set EDA preference below, baseline estimation window width 5 seconds, SCR threshold level 0.01 µs, reject SCRs under 10% of max. For SCL, in order to avoid the possibility of negative values and individual differences in the values of the raw skin conductance data, we use a discrete normalization method to transform the raw skin conductance values, x* = (x − xmin)/(xmax − xmin) (HAN Ying et al., Citation2018). The SCL was averaged across each measuring period. The NS.SCRs was the sum of the number of SCRs recorded in a 5-min period (Posada-Quintero et al., Citation2017). To examine whether the stress-induced physiological indicators would be associated with the subjective SAM reports, we conducted Pearson’s correlations between the change rate of the physiological indicators (△HR, △SCL, △NS.SCRs and △SC) and the emotional arousal (b2b1).
Data exclusion: Prior to the main analysis, data from trials in which the time estimates exceeded three standard deviations from the mean of each condition were excluded (0.6% of trials). In addition, data from participants in which there was no linear upward trend in the mean of the interval estimates of 250 ms, 550 ms, and 850 ms for each condition were also excluded (Barlas, Citation2019). As a result, seven participants were excluded from the stress group and nine from the control group. At last, for the index of the SAM response to stress, HR increase (△HR) was calculated by subtracting the baseline from the maximum value among the other measurements (which was HR during the speech period of the TSST task)(Wu et al., Citation2017). △HR of the two groups was analyzed separately. In the stress group, five participants were excluded because their △HR was less than 0, which we considered as abnormal heart rates, representing participants whose heart rate did not increase but decreased during the TSST. In the control group, three participants were excluded using the boxplot of SPSS.26 (see Appendix A in supplementary materials). One participant was not included in analyses due to the data of salivary cortisol that was physiologically implausible (2 standard deviations above the response mean; Buchanan et al., Citation2014). Paired t-tests were conducted on the time estimates of the experimental and control groups to verify the differences in time estimates between voluntary and involuntary conditions. The temporal binding scores were calculated by subtracting the mean reported time interval in voluntary condition from involuntary condition (Barlas, Citation2019; Poonian & Cunnington, Citation2013).
A mixed-design ANOVA was conducted on the temporal binding scores of the two groups. The within-subjects factor was the stage (pretest and posttest) and the between-subjects factor was the group (experimental group and control group). The effect sizes and interaction were also checked, followed by a simple effects analysis if significant interaction occurred. All reported p-values were two-tailed, and the alpha value was set at 0.05.
Greenhouse-Geisser corrections were used when sphericity assumptions were violated. Post hoc comparisons were conducted using Bonferroni adjustments to correct for multiple testing. All the reported p-values were two-tailed, and the alpha was set at 0.05. In cases of (borderline) significance, ANOVAs were supplemented with partial eta squared in between-subject designs and generalized eta squared in mixed designs as measures of the effect size.
3. Results
3.1. Questionnaire results
3.1.1. T-AI and SoA scale scores
Trait anxiety scores and SoA scores were analyzed by independent samples t-test, and there was no significant difference between the two groups, ps > 0.05. Trait anxiety scores (59.53 ± 8.99) were significantly negatively correlated with SoPA (26.98 ± 5.27), r = −0.66, p < 0.01, while trait anxiety scores were significantly positively correlated with SoNA (7.77 ± 3.40), r = 0.53, p < 0.01.
3.1.2. SAM scale scores
Emotional valence was expressed as a2a1 and emotional arousal as b2b1. Independent sample t-tests were conducted for a2a1 and b2b1, with the group as the grouping variables. For emotional valence, the independent t-test showed no significant difference in the two groups (experimental group: −0.12 ± 1.45; control group: 0.65 ± 1.46), t (32) = −1.53, p = 0.14, Cohen’s d = −0.76, 95%CI [-1.78, 0.25]. For emotional arousal the independent t-test showed significant differences in the two groups (experimental group:1.82 ± 1.67; control group: −0.24 ± 1.60), t (32) = 3.67, p < 0.01, Cohen’s d = 2.06, 95%CI [0.91, 3.20].
3.2. Results of physiological indicators
3.2.1. Results of heart rate
For heart rate measures (see ), ANOVA revealed a significant interaction between time and group, F(2.77, 88.79) = 16.88, p < 0.01, generalized = 0.35, a significant main effect of time, F(2.77, 88.79) = 29.72, p < 0.01, generalized
= 0.48, and group, F(1, 32) = 13.22, p < 0.01, generalized
= 0.29. Follow-up simple effects analysis highlighted a significant increase in HR occurring after TSST in the experimental group, with HRspe, HRAri, and HR0min all significantly higher than the control group, p < 0.05, See .
Figure 4. Results of physiological indicators across both groups and times. Note: A represents the results of HR (BPM), B represents the results of SCL, C represents the results of NS.SCRs, and D represents the results of SC (nmol/L). where *represents p < 0.05, **represents p < 0.01. Error bars represent SEM.
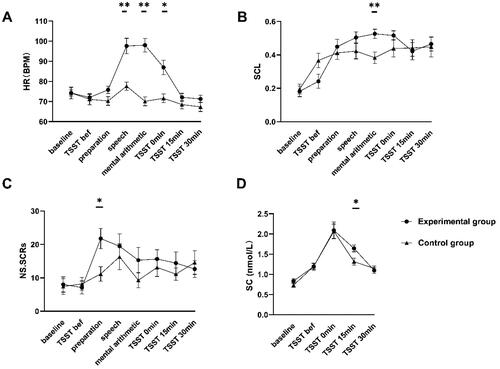
Table 1. Means and standard deviations for HR(BPM) in two groups and the simple effects of group.
3.2.2. Results of skin conductance levels (SCL)
For SCL measures (see ), ANOVA revealed a significant interaction between time and group, F(4.18, 133.77) = 2.91, p = 0.02, generalized = 0.08, a significant main effect of time, F(4.18, 133.77) = 18.22, p < 0.01, generalized
= 0.36, and group, F(1, 32) = 0.39, p = 0.54, generalized
= 0.01. Follow-up simple effects analysis highlighted a significant increase in SCL during the mental arithmetic of TSST in the experimental group, with SCLAri, significantly higher than the control group, p = 0.003. Significantly differences were found in two groups before the TSST phase, with the control group being higher than the experimental group, p = 0.04, See .
Table 2. Means and standard deviations for SCL in two groups and the simple effects of group.
For NS.SCRs measures (see ), ANOVA revealed a significant main effect of time, F(3.88, 124.24) = 4.23, p < 0.01, generalized = 0.12, the main effect of group was insignificant, F(1, 32) = 1.70, p = 0.20, generalized
= 0.05, in absence of significant interaction effect (p = 0.29), See .
Table 3. Means and standard deviations for NS.SCRs in two groups and the simple effects of group.
3.2.3. Results of salivary cortisol (SC)
For SC measures (see ), ANOVA revealed a significant main effect of time, F(2.18, 69.75) = 37.43, p < 0.01, generalized = 0.54; the main effect of group was insignificant, F(1, 32) = 1.83, p = 0.19, generalized
= 0.05, in absence of significant interaction effect (p = 0.48), see .
Table 4. Means and standard deviations for SC in two groups and the simple effects of group.
Finally, to examine whether the stress-induced physiological indicators would be associated with the subjective SAM reports, we conducted Pearson’s correlations between the change rate of the physiological indicators (△HR, △SCL, △NS.SCRs and △SC) and the emotional arousal(b2b1) for all participants excluding abnormal physiological data (N = 50). Pearson correlations between △HR and emotional arousal(b2b1) were significant, r = 0.32, p = 0.03. In contrast, there was no significant correlation between the subjective SAM reports and the other physiological measures (SCL, NS.SCRs and SC), ps > 0.1.
3.3. Results of interval estimation
The mean time intervals reported for the different conditions in the two groups are shown in . The paired t-test for the reporting interval showed that the voluntary condition reported interval (M = 374.24 ms, SD = 80.34 ms) was significantly shorter than the involuntary condition (M = 398.44 ms, SD = 78.45 ms), t(33) = −2.69, p = 0.01, Cohen’s d = −0.46, 95% CI [−42.48, −5.91], indicating that the interval between behavior and subsequent outcomes in the voluntary condition was subjectively compressed to a greater extent than that in the involuntary condition and that there was a temporal binding effect in the voluntary condition (see ).
Figure 5. Paired t-test for reported time between voluntary and involuntary conditions. It shows that the reported interval for the voluntary condition is significantly shorter than that for the involuntary condition, p < 0.05.
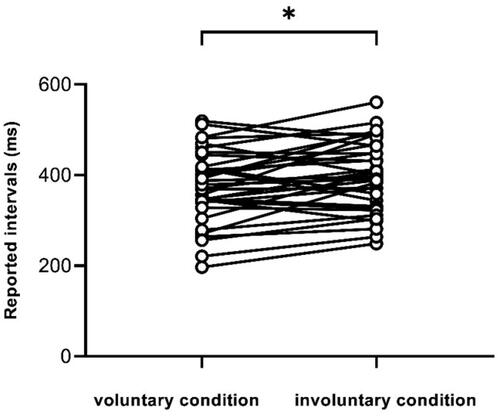
Table 5. The reported intervals for the two groups with different conditions.
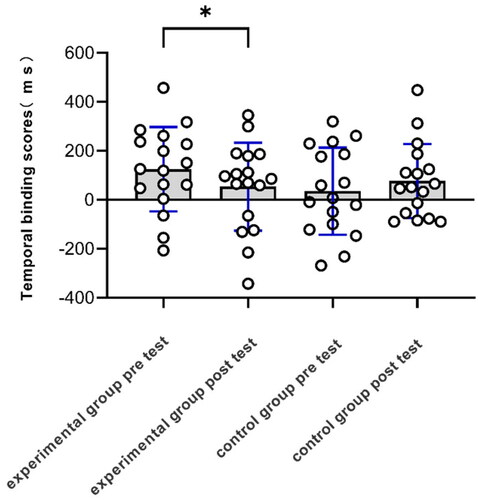
A mixed-design ANOVA with between-subjects factor “group” (experimental and control groups) and within-subjects factor “stage” (pretest and posttest) was conducted for the temporal binding scores. To excluded the effect of heart rate variation on the temporal binding scores, we used HR increase (△HR) as a covariate. The ANOVA showed no significant main effects for stage, group, and △HR, ps > 0.05. The interaction effect between stage and group was significant, F (1,1) = 4.98, p = 0.03, = 0.14. No significant interactions were observed for HR increase and stage, p > 0.1. The three-way mixed ANOVA (between-factor “group,” within-factors “stage” and “time” (250 ms, 550 ms, and 850 ms)) showed significant main effects for time, p < 0.01. No significant interactions were observed for time and group, stage and time and group (see Appendix B in supplementary materials).
A pairwise t-test showed that the temporal binding scores of the pretest (M = 124.98 ms, SD = 172.21 ms) were significantly greater than the posttest (M = 53.54 ms, SD = 179.66 ms) in the stress group, t(16) = 2.85 p = 0.01, Cohen’s d = 0.69, 95% CI [18.28, 124.60], whereas pretest temporal binding scores in the control group (M = 35.04 ms, SD = 178.14 ms) was not significantly different from posttest (M = 76.78 ms, SD = 151.43 ms), t(16) = 1.19, p = 0.25, Cohen’s d = 0.28, 95% CI [-116.30, 32.28] (see ). An independent t-test showed no significant difference for the temporal binding scores of the pretest (M = 124.98 ms, SD = 41.77 ms) in the stress group and the temporal binding scores of the pretest (M = 53.54 ms, SD = 178.14 ms) in the control group, t(32) = −1.50, p = 0.14, Cohen’s d = 0.51, 95% CI [-32.46, 212.35].
Figure 6. Temporal binding scores for experimental and control groups. Temporal binding scores of the pretest was significantly greater than posttest in the experimental group, while there was no difference in the control group.
4. Discussion
The present study examined the effect of acute psychosocial stress on the sense of agency using a persuasive examination manipulation of stress. Generally, the results revealed that stressed participants had significantly lower temporal binding scores, whereas non-stressed participants kept relatively steady temporal binding scores, indicating that acute psychosocial stress leads to a decrease in the sense of agency.
4.1. The validity of the TSST manipulation
No difference in trait anxiety and sense of agency was found between experimental and control groups by examining the results of the T-AI Questionnaire and SoAs questionnaire. While we integrated the data from the two groups, it was found that trait anxiety scores were significantly negatively correlated with SoPA scores and positively correlated with SoNA scores, suggesting a potential association between anxiety and the sense of agency. The relationship between trait anxiety and SoPA/SoNA could be further investigated by larger sample sizes.
In order to draw a persuasive conclusion, massive objective and subjective indicators were carefully examined to make sure the validity of the evoking of stress. Using the SAM scale, we tested the positive change in emotional arousal before and after TSST in the experimental group while negative in the control group, indicating that the TSST manipulation succeeded in arising participants’ emotional reactions.
As supplements to self-reported results, physiological indicators were also analyzed, including heart rate, skin electricity, and salivary cortisol. A total of eight time periods of heart rate were collected, each for 5 min. Independent t-tests revealed a significant difference between the heart rates of the two groups during the two phases of TSST and the 5 min after the end of TSST. The difference can be found in all three time periods, which is consistent with the results of previous studies (Lin et al., Citation2020).
The same independent t-test for skin conductance level (SCL) was performed for both groups, and the results demonstrated a significant difference between the two groups during the final mental arithmetic phase of TSST. The SCL was measured by the amount of sweat secreted from fingertips contributing to the change of conductance. There was a gradual accumulation of sweat secretion from the fingertips, and the SCL of the experimental group started to rise at the beginning of TSST and reached a peak at the end of the cardio phase (Posada-Quintero et al., Citation2017). The results of the NS.SCRs of the two groups showed significant differences in the preparation phase of TSST. Participants in the experimental group had significantly higher skin conductance responses in the preparation phase, indicating that the autonomic nervous system was activated at the beginning of the TSST, where the volatility was greatest.
Salivary cortisol was also collected on five occasions, and the results showed a significant difference between the two groups 15 min after the end of TSST. Salivary cortisol is a product of the activation of the HPA axis in humans undergoing acute stress. Therefore, its concentration rises slowly, usually reaching its peak secretion 10–30 min after the end of TSST (Liu et al., Citation2017). We detected salivary cortisol concentration in the experimental group 15 min after the end of TSST, indicating that the HPA axis was activated in participants under acute psychosocial stress.
Combining the above subjective scores on the SAM scale and objective data on a range of physiological indicators, we conclude that our TSST setting is reasonable and well-validated for the acute psychosocial stress state of the experimental group.
4.2. Examination of the temporal binding effect
As for the implicit measure of the temporal binding effect, the pairwise t-test revealed that the reported interval was significantly shorter under voluntary conditions than involuntary conditions, identifying that the interval between action and subsequent outcome in voluntary conditions was subjectively compressed.
A mixed-design ANOVA was conducted on the temporal binding scores of the two groups, revealing a significant interaction between pre- and post-measures and groups. Simple effect analysis found a significant difference between the pretest and posttest in the experimental group, but not in the control group. Considering that the temporal binding scores after TSST significantly decreased, it can be inferred that acute psychosocial stress has a negative effect on the sense of agency.
Currently, there are two main approaches in empirical research on how affective information moderates SoA. One is to manipulate the affective validity of action effects by presenting positive or negative action outcomes (Christensen et al., Citation2016; Wen et al., Citation2015), and the other is to directly manipulate participants’ emotional states (Caspar et al., Citation2016; Christensen et al., Citation2019; Saarikallio et al., Citation2019). Our study falls into the latter category, and the findings are consistent with the results of experiments on sexual arousal (Render & Jansen, Citation2021), fear and anger (Christensen et al., Citation2019) where a diminished sense of agency was detected by experimental manipulation that placed participants in specific emotional states. Our results are different from another study that reported that stress did not impair SoA (Stern et al., Citation2020). There are three main reasons for this difference between the two studies. First, we adopt a group design instead of inducing stress in different blocks to exclude the interference from each other. Second, we adopted the Pretest-posttest designs that we can directly explore the influence of stress on sense of agency. Third, we are in a normal situation rather VR context, the induced stress in our study may be relatively large. Additionally, SoA contains two forms called embodied SoA and non-embodied SoA respectively. The former focuses on the immediate connection between the intention to perform the action and the action itself, and the latter focuses on the link between the action performed by the agent and the intended outcome. Based on the experimental manipulation, it is obvious that our study applies the TSST paradigm to the effect of non-embodied SoA, conversely, the Stern team focus on examining embodied SoA in VR context. Further studies are need to clarify these issues.
Another issue should be mentioned. The sense of agency in our study differs from the concept of the locus of control. The locus of control was considered a personality trait. There are two types of the locus of control: internal and external. The first is characterized by the participant’s belief that the things happening to him or her are a consequence of his or her actions, and the second is because the subject belief that things occurring are due to external factors (de Dios-Duarte et al., Citation2022). The important aspect of sense of agency is the experience of how one’s action affects the external environment. Further studies are needed to testify whether there is a difference of SoA between two types of the locus of control.
5. Conclusion
The present study investigated the effect of acute psychosocial stress on the sense of agency using the Trier Social Stress Test paradigm. The finding suggests that acute psychosocial stress can lead to strong emotional arousal, while significantly attenuating the time-compression effect as well as the sense of agency. With the support of physiological data, our research points out the potential association between acute psychosocial stress and the sense of agency, inspiring further exploration of physiological impacts through the change of the two variables.
Supplemental Material
Download MS Word (19.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All raw data obtained in this experiment including questionnaires, physiological measurements and time estimates, and data used for statistical analysis in the article (excluding excluded data), are publicly available on the figshare website: https://doi.org/10.6084/m9.figshare.21758537.v2.
Additional information
Funding
Notes on contributors
Yayun Chu
Yayun Chu,Postgraduate students at the Institute of Psychology, Chinese Academy of Sciences, Beijing, China.
Guanhua Huang
Guanhua Huang,Postgraduate students at the Institute of Psychology, Chinese Academy of Sciences, Beijing, China.
Yunyun Li
Yunyun Li, PhD, Postdoctorat the Institute of Psychology, Chinese Academy of Sciences, Beijing, China.
Qin Chen
Qin Chen,Postgraduate students at the Institute of Psychology, Chinese Academy of Sciences, Beijing, China.
Jiajia Liu
Jiajia Liu, Postgraduate students at the Institute of Psychology, Chinese Academy of Sciences, Beijing, China.
Ke Zhao
Ke Zhao,PhD,Associate professor at the Institute of Psychology, Chinese Academy of Sciences, Beijing, China.
Xiaolan Fu
Xiaolan Fu, PhD,Professor at the Institute of Psychology, Chinese Academy of Sciences, Beijing, China.
References
- Allen, A. P., Kennedy, P. J., Cryan, J. F., Dinan, T. G., & Clarke, G. (2014). Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neuroscience and Biobehavioral Reviews, 38, 94–124. https://doi.org/10.1016/j.neubiorev.2013.11.005
- Allen, A. P., Kennedy, P. J., Dockray, S., Cryan, J. F., Dinan, T. G., & Clarke, G. (2017). The Trier Social Stress Test: Principles and practice. Neurobiology of Stress, 6, 113–126. https://doi.org/10.1016/j.ynstr.2016.11.001
- Barlas, Z. (2019). When robots tell you what to do: Sense of agency in human- and robot-guided actions. Consciousness and Cognition, 75, 102819–102819. https://doi.org/10.1016/j.concog.2019.102819
- Boucsein, W., Fowles, D. C., Grimnes, S., Ben-Shakhar, G., Roth, W. T., Dawson, M. E., & Filion, D. L. (2012). Publication recommendations for electrodermal measurements. Psychophysiology, 49(8), 1017–1034. https://doi.org/10.1111/j.1469-8986.2012.01384.x
- Buchanan, T. W., Laures-Gore, J. S., & Duff, M. C. (2014). Acute stress reduces speech fluency. Biological Psychology, 97, 60–66. https://doi.org/10.1016/j.biopsycho.2014.02.005
- Caspar, E. A., Christensen, J. F., Cleeremans, A., & Haggard, P. (2016). Coercion changes the sense of agency in the human brain. Current Biology, 26(5), 585–592. https://doi.org/10.1016/j.cub.2015.12.067
- Christensen, J. F., Di Costa, S., Beck, B., & Haggard, P. (2019). I just lost it! Fear and anger reduce the sense of agency: A study using intentional binding. Experimental Brain Research, 237(5), 1205–1212. https://doi.org/10.1007/s00221-018-5461-6
- Christensen, J. F., Yoshie, M., Di Costa, S., & Haggard, P. (2016). Emotional valence, sense of agency and responsibility: A study using intentional binding. Consciousness and Cognition, 43, 1–10. https://doi.org/10.1016/j.concog.2016.02.016
- Cui, Q., Zhao, K., Chen, Y.-H., Zheng, W., & Fu, X. (2018). Opposing subjective temporal experiences in response to unpredictable and predictable fear-relevant stimuli. Frontiers in Psychology, 9, 360. https://doi.org/10.3389/fpsyg.2018.00360
- de Dios-Duarte, M. J., Arias, A., Durantez-Fernández, C., Niño Martín, V., Olea, E., Barba-Pérez, M. Á., Pérez-Pérez, L., Cárdaba-García, R. M., & Barrón, A. (2022). Flare-ups in Crohn’s disease: Influence of stress and the external locus of control. International Journal of Environmental Research and Public Health, 19(20), 13131. https://doi.org/10.3390/ijerph192013131
- Faul, F., Erdfelder, E., Buchner, A., & Lang, A.-G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. https://doi.org/10.3758/BRM.41.4.1149
- Gaab, J., Blattler, N., Menzi, T., Pabst, B., Stoyer, S., & Ehlert, U. (2003). Randomized controlled evaluation of the effects of cognitive-behavioral stress management on cortisol responses to acute stress in healthy subjects. Psychoneuroendocrinology, 28(6), 767–779. https://doi.org/10.1016/s0306-4530(02)00069-0
- Gu, J., Li, Y., Zhao, K., & Fu, X. (2021). Disappearing and appearing: Temporal binding effects are consistent across situations. Consciousness and Cognition, 93, 103166–103166. https://doi.org/10.1016/j.concog.2021.103166
- Haggard, P. (2017). Sense of agency in the human brain. Nature Reviews Neuroscience. 18(4), 196–207. https://doi.org/10.1038/nrn.2017.14
- Haggard, P., Clark, S., & Kalogeras, J. (2002). Voluntary action and conscious awareness. Nature Neuroscience, 5(4), 382–385. https://pubmed.ncbi.nlm.nih.gov/11896397 https://doi.org/10.1038/nn827
- Humphreys, G. R., & Buehner, M. J. (2009). Magnitude estimation reveals temporal binding at super-second intervals. Journal of Experimental Psychology. Human Perception and Performance, 35(5), 1542–1549. https://doi.org/10.1037/a0014492
- Kirschbaum, C., Kudielka, B. M., Gaab, J., Schommer, N. C., & Hellhammer, D. H. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine, 61(2), 154–162. https://doi.org/10.1097/00006842-199903000-00006
- Kirschbaum, C., Pirke, K. M., & Hellhammer, D. H. (1993). The Trier Social Stress Test – A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. https://doi.org/10.1159/000119004
- Lin, L., Leung, A. W. S., Wu, J., & Zhang, L. (2020). Individual differences under acute stress: Higher cortisol responders performs better on N-back task in young men. Int J Psychophysiol, 150, 20–28. https://doi.org/10.1016/j.ijpsycho.2020.01.006
- Liu, J. J. W., Ein, N., Peck, K., Huang, V., Pruessner, J. C., & Vickers, K. (2017). Sex differences in salivary cortisol reactivity to the Trier Social Stress Test (TSST): A meta-analysis. Psychoneuroendocrinology, 82, 26–37. https://doi.org/10.1016/j.psyneuen.2017.04.007
- Moore, J. W., & Haggard, P. (2010). Intentional binding and higher order agency experience. Consciousness and Cognition, 19(1), 490–491. https://doi.org/10.1016/j.concog.2009.11.007
- Moore, J. W., & Obhi, S. S. (2012). Intentional binding and the sense of agency: A review. Consciousness and Cognition, 21(1), 546–561. https://doi.org/10.1016/j.concog.2011.12.002
- Poonian, S. K., & Cunnington, R. (2013). Intentional binding in self-made and observed actions. Experimental Brain Research, 229(3), 419–427. https://doi.org/10.1007/s00221-013-3505-5
- Posada-Quintero, H. F., Florian, J. P., Orjuela-Cañón, A. D., & Chon, K. H. (2017). Electrodermal activity is sensitive to cognitive stress under water. Frontiers in Physiology, 8, 1128. https://doi.org/10.3389/fphys.2017.01128
- Render, A., & Jansen, P. (2021). Influence of arousal on intentional binding: Impaired action binding, intact outcome binding. Atten Percept Psychophys, 83(1), 103–113. https://doi.org/10.3758/s13414-020-02105-z
- Saarikallio, S. H., Randall, W. M., & Baltazar, M. (2019). Music listening for supporting adolescents’ sense of agency in daily life. Frontiers in Physiology, 10, 2911. https://doi.org/10.3389/fpsyg.2019.02911
- Stern, Y., Koren, D., Moebus, R., Panishev, G., & Salomon, R. (2020). Assessing the relationship between sense of agency, the bodily-self and stress: Four virtual-reality experiments in healthy individuals. Journal of Clinical Medicine, 9(9), 2931. https://doi.org/10.3390/jcm9092931
- Tapal, A., Oren, E., Dar, R., & Eitam, B. (2017). The Sense of Agency Scale: A measure of consciously perceived control over one’s mind, body, and the immediate environment. Frontiers in Psychology, 8, 1552. https://doi.org/10.3389/fpsyg.2017.01552
- Wen, W., Yamashita, A., & Asama, H. (2015). The influence of action-outcome delay and arousal on sense of agency and the intentional binding effect. Consciousness and Cognition, 36, 87–95. https://doi.org/10.1016/j.concog.2015.06.004
- Wu, J., Sun, X., Wang, L., Zhang, L., Fernández, G., & Yao, Z. (2017). Error consciousness predicts physiological response to an acute psychosocial stressor in men. Psychoneuroendocrinology, 83, 84–90. https://doi.org/10.1016/j.psyneuen.2017.05.029
- Ying, H., Yu-Qi, D., & Jing-Gang, B. (2018). The physiological data representation of emotion in learning analysis – Prospects for the application of galvanic skin response. Modern Educational Technology, 28(10), 12–19. (in Chinese). https://doi.org/10.3969/j.issn.1009-8097.2018.10.002
- Yoshie, M., & Haggard, P. (2013). Negative emotional outcomes attenuate sense of agency over voluntary actions. Current Biology, 23(20), 2028–2032. https://doi.org/10.1016/j.cub.2013.08.034
- Zhao, K., Chen, Y.-H., Yan, W.-J., & Fu, X. (2013). To bind or not to bind? Different temporal binding effects from voluntary pressing and releasing actions. PLoS One, 8(5), e64819. https://doi.org/10.1371/journal.pone.0064819
