Abstract
Prolonged or severe stress has been found to inhibit the hypothalamic-pituitary-gonadal axis (HPG) and its testosterone release. In contrast, acute stress, including competition, social evaluation, or physical challenges, shows more inconsistent response patterns. This study examined changes in cortisol and testosterone across different types and durations of stress in the same individuals. We further explored the influence of baseline levels on hormonal stress responses. Sixty-seven male officer cadets in the Swiss Armed Forces (mean age 20.46 years ± 1.33) were assessed during two different acute stressors—the Trier Social Stress Test for Groups (TSST-G) and a brief military field exercise—and in the long-term during the 15-week officer training school. Several saliva samples were collected before and after the acute stressors for cortisol and testosterone. Morning testosterone was assessed four times during officer training school. There were significant increases in cortisol and testosterone during the TSST-G and the field exercise. Baseline levels of testosterone were negatively associated with acute cortisol response during the field exercise but not during the TSST-G. Morning saliva testosterone decreased during the first 12 weeks of officer training school and increased again in week 15, with no differences to baseline levels. The findings suggest that group stress tests such as the TSST-G or field exercises in groups may be particularly challenging for young men. The results also point to an adaptive role of testosterone during acute challenges during prolonged stress.
1. Introduction
Leadership training for future military officers can be described as a period with stressful events. During this period, cadets are pushed to their physical and mental limits to develop their leadership skills and achieve training requirements. They are constantly exposed to dominance hierarchies and competition with highly motivated comrades (Bernton et al., Citation1995; Kreuz et al., Citation1972; Taylor et al., Citation2017). However, exposure to different performance requirements and stressors during officer training could lead to adaptive psychological and biological coping mechanisms.
Stress induces mental and physical adaptations to the given demands. These responses include a range of physiological systems, including the endocrine system and in particular cortisol and testosterone. Cortisol, a steroid hormone end product of the hypothalamic-pituitary-adrenocortical (HPA) axis, provides energy by mobilizing glucose and helps the individual cope with social threats or challenges (Dickerson et al., Citation2009; McEwen, Citation2019). Cortisol also inhibits immune, digestive, and reproductive functions in the short term (Sapolsky et al., Citation2000). In cases of extreme or prolonged stress, however, cortisol can have a negative effect on mental and physical health (Agorastos & Chrousos, Citation2022; Cohen et al., Citation2012; Dziurkowska & Wesolowski, Citation2021; McEwen, Citation2017). It also has a suppressive effect on the hypothalamic-pituitary-gonadal (HPG) axis and its end product testosterone (Aakvaag et al., Citation1978; Lieberman et al., Citation2016; Rivier & Rivest, Citation1991; Szivak et al., Citation2018). In particular, during short- and long-term military field exercises with physical stress, a significant increase in cortisol levels and a simultaneous decrease in testosterone levels have been found (Bernton et al., Citation1995; Friedl et al. Citation2000; Gomez-Merino et al., Citation2003; Kreuz et al., Citation1972; Lieberman et al., Citation2016; Morgan et al., Citation2000; Nindl et al., Citation2007; Szivak et al., Citation2018; Tait et al., Citation2022). Testosterone, another steroid hormone released predominantly by the gonads after activation of the HPG axis, is involved in multiple physiological functions and reproduction, and is associated with a number of status-relevant behaviors such as aggression, competitiveness, and dominance (e.g. Archer, Citation2006; Chichinadze & Chichinadze, Citation2008; Mazur & Booth, Citation1998; Vermeer et al., Citation2020). Similar to cortisol, concentrations are highest in the morning, fall continuously throughout the day, and reach their nadir in the evening (Dabbs, Citation1990). Chronic stress from several weeks of military training involving physical exertion and loss of energy can disrupt this circadian rhythm and lead to very low morning testosterone levels. However, after training stress and during recovery phase, testosterone levels rise again (Bernton et al., Citation1995). Therefore, testosterone production has been shown to be negatively affected by periods of food restrictions, sleep deprivation, psychological stress or strenuous physical exercise (Friedl et al., Citation2000; Morgan et al., Citation2000; Nindl et al., Citation2006, Citation2007; Szivak et al., Citation2018).
In contrast to the consistent findings on cortisol responses to extreme or chronic stress, the results on testosterone responses are equivocal. Several studies found an increase in testosterone in response to competitive stressors, performance tasks, status-threatening tasks, or physical challenges (Bedgood et al., Citation2014; Casto & Edwards, Citation2016; D’Andrea et al., Citation2020; Lennartsson et al., Citation2012; Turan et al., Citation2022, Citation2015). Most peak testosterone levels after physical and psychosocial stressors were reached within a period of 30 min (D’Andrea et al., Citation2020; Lennartsson et al., Citation2012). However, diverging results, i.e. testosterone decline or unchanged concentrations, have also been found for physical or social-evaluative stressors (Minas et al., Citation2022; Pletzer et al., Citation2021; Schoofs & Wolf, Citation2011). Possible explanations for these different results could have to do with the contextual demands of the stressor and the nature of the stress itself. For instance, some studies using social evaluation additionally manipulated dominance or competition (Bedgood et al., Citation2014; Deuter et al., Citation2016). These transient testosterone changes are caused by threats to status in a social or competitive context. In studies with physical stressors, differences depended on the type, duration, and intensity of the activity. Moderate or high-intensity physical activity during short time intervals (D’Andrea et al., Citation2020) or extreme challenges (e.g. skydiving; White et al., Citation2019) were associated with an increase in testosterone, whereas excessive physical training negatively affected testosterone (Minas et al., Citation2022).
Evidence further suggests that baseline hormone levels are associated with hormonal stress response. For instance, in some studies with competitive or social-evaluative stressors, pre-stress cortisol and testosterone levels were negatively related to testosterone (Bedgood et al., Citation2014; Maestripieri et al., Citation2010; Mehta & Josephs, Citation2006) and cortisol (Mehta et al., Citation2008; Stephens et al., Citation2016) responses, respectively. More specifically, individuals with lower baseline cortisol showed increased testosterone reactivity during competitive tasks and social evaluation (Bedgood et al., Citation2014; Maestripieri et al., Citation2010; Mehta & Josephs, Citation2006). And individuals with higher baseline testosterone showed lower cortisol reactivity during ultimately successful competition (Mehta et al., Citation2008) and during social evaluation (Stephens et al., Citation2016). Thus an inhibitory effect, like the effect cortisol has on the HPG axis and on testosterone secretion (Aakvaag et al., Citation1978; Rivier & Rivest, Citation1991), can also be demonstrated for testosterone on the HPA axis and on its cortisol secretion (Rubinow et al., Citation2005; Toufexis et al., Citation2014). In contrast, there is also evidence that indicated no relationship between baseline levels of cortisol and testosterone and the hormonal stress response during public speaking (Pletzer et al., Citation2021). Accordingly, the underlying mechanisms have not yet been conclusively explored.
In summary, research indicates that stress-induced cortisol and testosterone responses depend on the context, type, and duration of the stressor, and on pre-stress hormone levels. However, to our knowledge, only few studies are available on endocrine changes across different types and durations of stress in the same individuals. Therefore, we aimed to examine endocrine changes in young male officer candidates during various short- and long-term stressors. First, we were interested in whether a stressor involving social threat and peer evaluation elicits a hormonal response in young male officer candidates. We assume that the group setting during the Trier Social Stress Test (TSST-G) (von Dawans et al., Citation2011) exposes officer candidates to status threats and challenges, and we therefore expect an increase in cortisol and testosterone. Second, we examined cortisol and testosterone during a brief military field exercise (e.g. Lieberman et al., Citation2016). We hypothesize that the high psychological and physical demands of the stressor will lead to an increase in cortisol and a decrease in testosterone. Third, we examined changes in morning testosterone during long-term training stress and expected a decline in testosterone over the course of training followed by recovery at the end of training. Finally, we explored the influence of baseline cortisol and testosterone levels on acute hormonal responses in both laboratory and field stress conditions.
2. Methods
2.1. Participants and procedure
This study was part of a longitudinal research project on resilience in aspiring military officers (Zueger et al., Citation2022). The study protocol was reviewed and approved by the ethics committee of Northwest and Central Switzerland (Ethikkommission Nordwest- und Zentralschweiz EKNZ; ID no. 2017-00841). Study participants were cadets at the Swiss Armed Forces infantry officer training school (Inf OS 1-10/17) in 2017. Cadets attend officer training school (OS) for 15 weeks to achieve the rank of lieutenant. The training is the same for all cadets and is highly structured in terms of content and time (e.g. regulated sleep, wake-up, and meal times, scheduled theory lessons, tactical training, field exercises). The study participants represented a sample of healthy young men and women, as only mentally and physically healthy candidates are admitted to officer training school (i.e. only about 10% of all soldiers are selected for OS). Detailed written and oral information on the study procedures were given, and written informed consent was obtained from voluntary participants (n = 99). Exclusion criteria were defined as female sex, due to hormonal cycles influencing physiological stress response (Kajantie & Phillips, Citation2006) and small sample size (n = 5). Due to military scheduling, 21 participants were not able to take part in all study conditions and were therefore excluded from analysis. During officer training, six participants dropped out of the study (military dropouts n = 3, study withdrawal n = 3). The final sample consisted of 67 men aged 20.46 years (± 1.33; see ). During the first week of officer training, study participants completed sociodemographic questionnaires. The military school command recorded anthropometric measurements (weight, height). To examine short- and long-term endocrine changes, we collected saliva samples during weeks 2, 9, 12, and 15 of OS (see ).
Figure 1. Study procedure including data and hormone sampling during officer training, TSST-G and field exercise.
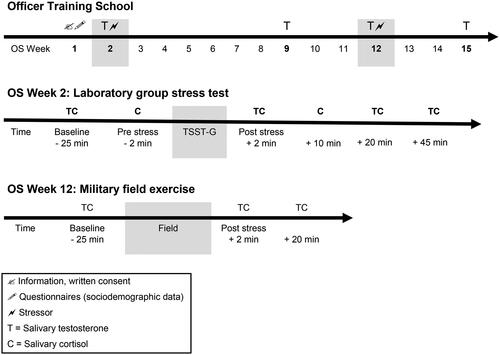
Table 1. Study characteristics.
2.2. Stress exposures
2.2.1. Laboratory group stress test
The Trier Social Stress Test for groups (TSST-G) (von Dawans et al., Citation2011) was conducted in a laboratory setting in week 2 of OS. The TSST-G is a group version of the TSST (Kirschbaum et al., Citation1993). The TSST-G protocol for a group of up to 6 participants is a standardized motivated performance task that combines high levels of social-evaluative threat with uncontrollability. The standardized laboratory stressor consists of a brief preparation period followed by an examination phase when participants are required to speak freely about their suitability for employment in a mock job interview and to perform mental arithmetic fluently in front of a fake expert panel (one man and one woman). The stress exposure was embedded in the officer training schedule between 1:30 and 6:00 p.m. to control diurnal fluctuations in hormonal secretion. Participants gathered in groups of six in a classroom. Meanwhile, first saliva samples were collected and questions about their recent activities and behaviors (e.g. sports, sleep, sexual behavior) were asked. Subsequently, each group was guided to the testing room, where participants were asked to sit down at a numbered supplemental table (1-6). Participants were separated from each other by dividing walls. An introduction to the subsequent task (mock job interview) was given orally. Participants were told that they were being filmed, and an expert panel was evaluating their non-verbal behavior during the testing session. After a brief preparation period, a second sample of saliva was collected. The fake expert panel then entered the room and started the camera to make them believe they were being videotaped. The male panel member required each participant, in random order (from 1 to 6), to perform a speaking task for about 2 min (a total of 12 min). After finishing these interviews, participants were asked to perform a subtraction task as fast and accurately as possible within 80 s (a total of 8 min). Immediately after the testing, the expert panel left the room after switching off the camera. Participants were asked to collect their saliva before they were guided back to the classroom for recovery and more data collection.
2.2.2. Military field exercise
In addition to the laboratory-induced stress condition, cadets were also examined during a brief field exercise on the second day of OS week 12–13, called the “perseverance week”: During perseverance week, cadets are mentally (e.g. limited privacy, performance pressure) and physically (e.g. nutritional restrictions, sleep deprivation) pushed to their limits. The first task of the exercise was to infiltrate an open area without being caught by a fake enemy. Next, cadets had to crawl through a tight sewer (1.50 m of height, 1.5 km of length), where there were insects and knee-high water. They completed the exercise fully equipped (e.g. rifle, loaded rucksack of ≈20 kg) and in small groups and were instructed not to leave the sewer until their group was complete. This field exercise was conducted in the late evening hours (starting at 8:15 p.m.) in late autumn in low temperatures but dry weather.
2.3. Hormone collection and assays
Cadets were requested to provide 2 mL saliva using tubes (SaliCaps, IBL International GmbH, Hamburg, Germany). During TSST-G, six saliva samples were assessed for cortisol ≈25 and ≈2 min before and immediately after (≈2 min), and 10, 20, and 45 min after stress exposure. For testosterone, four saliva samples were assessed ≈25 min before and immediately after (≈2 min), and 20 and 45 min after the TSST-G (see ). Cadets were advised not to eat, brush teeth, or smoke for a minimum of 1 h before the TSST-G. During the field exercise, three saliva samples for cortisol and testosterone were collected before (≈25 min), shortly after, and 20 min after the exercise (see ). Due to the restrictive rules during the perseverance week, cadets were not allowed to smoke or drink coffee or alcohol. Furthermore, food intake was restricted to an energy bar only, and cadets were instructed not to eat for at least 2 h before the field exercise. To obtain morning testosterone, four saliva samples were collected in the morning at 7:15 a.m. in OS weeks 2, 9, 12, and 15 (see ). Cadets were advised not to eat, brush teeth, exercise, or smoke before the sampling. After collection, all samples were kept frozen and sent to the biochemical laboratory of the Institute of Psychology at the University of Zurich. Upon receipt, the samples were stored at −20 °C until analysis. Free salivary cortisol and testosterone were analyzed using luminescence immunoassay (LIA, IBL International GmbH, Germany). The limit of detection was 0.10 nmol/L for cortisol and 1.09 pg/mL for testosterone. The inter- and intra-assay coefficients of variation (CV) for cortisol and testosterone were < 10% and < 5%, respectively.
2.4. Statistical analysis
Data analysis was performed using the statistical program R version 4.1. for Macintosh. Mixed effects models were applied to account for non-independency in repeated measures. Analyses were conducted using the lme4 package and associated packages. Mixed effect models were used to assess alterations in testosterone and cortisol over time in both acute stress settings (TSST-G and military field exercise) and over the course of the OS (15 weeks of officer training). We modeled a random intercept model (i.e. the intercept was allowed to vary across cadets). For each model, theoretically relevant variables (e.g. age, body mass index, start and duration of stressor) were included in the analyses as covariates and standardized for ease of interpretation. As hormones were not expected to linearly develop with time, time points were included as categorical predictors. Furthermore, time points were modeled as categorial predictors because growth, non-linear and polynomial time trend modeling yielded poor model fit, and also to better account for unequal time intervals between each measurement. Pairwise comparisons of consecutive time points were conducted to assess between time points. P-values were adjusted using the multivariate t method estimating the multivariate t distribution implemented in the package emmeans. To test hypotheses, we further included baseline hormone levels (of cortisol or testosterone) to investigate the interaction with hormone response (of testosterone or cortisol) over time. To compare model fit (with and without interaction) chi-square likelihood ratio tests were applied. To investigate differences between responder groups, we applied independent Welch’s t-test for continuous variables. Except for testosterone data during TSST-G, all cortisol and testosterone data were log-transformed to better approximate a normal distribution of residuals. Outliers (> ± 3 standard deviation from the mean) were found for testosterone (TSST-G: n = 1, Field: n = 3) and for cortisol (TSST-G: n = 7, Field: n = 1), and were winsorized prior to all analyses. Two-tailed tests with a significance level of p < .05 (alpha = .05) were used to test all hypotheses. Violations of model assumptions were checked and additionally, the robustness of the results was tested with robust mixed effect models using the robustlmm package.
3. Results
3.1. Sample characteristics and preliminary analyses
shows descriptive data on sample and stressor characteristics (see Supplementary Material, Table 1 for all raw values of cortisol and testosterone). Of the 67 participating cadets, some were not able to provide all saliva samples due to sick leave or some restrictions on stressor duration and time provided by the military command and were therefore missing (9.23% of 737 testosterone samples and 3.65% of 603 cortisol samples). These missing samples were treated using listwise deletion.
In TSST-G, 34 of 67 cadets were classified as cortisol responders (defined as an increase from baseline to a peak of more than 15.5%) (Miller et al., Citation2013) with an average increase cortisol of 3.66 nmol/L (SD 2.63 nmol/L), and 33 cadets were classified as non-responders with no change or decrease of 0.28 nmol/L (SD 1.67 nmol/L). Of the 34 cortisol responders, 94% reached their cortisol peak within 20 min following the TSST-G. During the field exercise, 59 cadets responded with an average increase of cortisol of 20.95 nmol/L (SD 17.874 nmol/L) and three cadets showed a decrease of − 2.98 nmol/L (SD 2.997 nmol/L). Cortisol peaks were reached immediately after the field exercise from 21 cadets and within 20 min of the finish from 38 cadets. There were no significant differences between cortisol responders compared to cortisol non-responders during both stressors (TSST-G, military field exercise) in terms of age, BMI, duration of stressor, or baseline cortisol levels (ps > .05). Regarding group size, the average number of group members was n = 6 for TSST-G and n = 7 for the field exercise.
From all cadets, peak levels of testosterone during the TSST-G and field exercise were reached at different time points. During the TSST-G, testosterone levels peaked immediately after stress in 40 cadets, with an average increase of 17.329 pg/mL (SD 13.116 pg/mL), 20 min afterwards in 10 cadets with an average increase of 15.951 pg/mL (SD 12.521 pg/mL), and 45 min after the TSST-G in 9 cadets, with an average increase of 25.989 pg/mL (SD 16.062 pg/mL) from baseline. Eight cadets showed an average decrease of −9.936 pg/mL (SD 4.957 pg/mL) testosterone following the TSST-G. During the field exercise, peak levels of testosterone were immediately reached after the exercise in 48 cadets, with an average increase of 38.031 pg/mL (SD 37.607 pg/mL) and 20 min afterwards in 5 cadets, with an average increase of 58.804 pg/mL (SD 44.732 pg/mL) from baseline testosterone. Nine cadets showed an average decrease of −19.451 pg/mL (SD 17.184 pg/mL) in testosterone. There were no significant differences between cadets with increasing testosterone levels and cadets with decreasing testosterone levels during both stressors in terms of age, BMI, duration of stressor, and morning or baseline testosterone levels (ps > .05).
3.2. Testosterone changes during officer training school
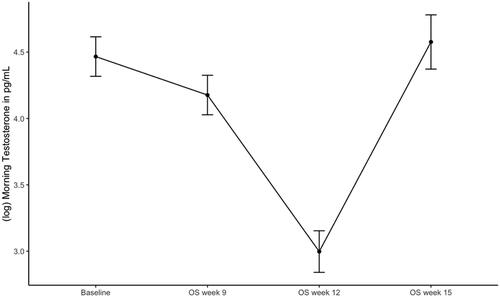
Contrast analysis of the mixed effects model revealed that after controlling for age and BMI, there was a significant decrease in log testosterone from OS week 2 to 9, ß = −0.290, SE = 0.095, t(159) = −3.064, 95% CI [−0.515, −0.064], p < .01 and a significant decrease in log testosterone from OS week 9 to 12, ß = −1.180, SE = 0.098, t(162) = −12.066, 95% CI [−1.413, −0.946], p < .001 (). Log testosterone significantly increased from OS week 12 to the end of OS in week 15, ß = 1.580, SE = 0.121, t(177) = 13.073, 95% CI [1.291, 1.867], p < .001. Robust analyses confirmed these findings (see Supplementary Material, Table 2 for results of standard and robust analyses).
3.3. Endocrine response to the TSST-G
3.3.1. Cortisol
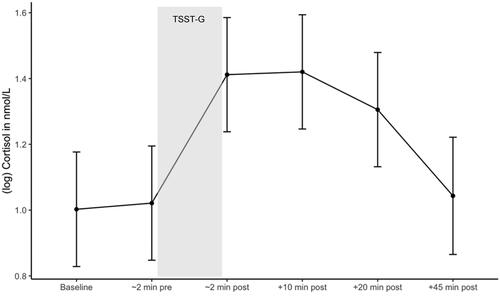
Contrast analysis of the mixed effects model revealed that after controlling for age, BMI, and stressor start, there was a significant increase in log cortisol from baseline (immediately before TSST-G start) to immediately after the TSST-G, ß = 0.390, SE = 0.069, t(320) = 5.667, 95% CI [0.214, 0.567], p < .001 and a significant decrease in log cortisol during recovery from 20 min to 45 min following the TSST-G, ß = −0.263, SE = 0.072, t(321) = −3.657, 95% CI [−0.448, −0.079], p < .01 (). Robust analyses confirmed these findings (see Supplementary Material, Table 3 for results of standard and robust analyses).
Figure 3. Cortisol response during the TSST-G. Estimated marginal means of log cortisol during the TSST-G. Error bars are confidence intervals of estimated marginal means.
We further examined whether baseline testosterone was associated with cortisol response during the TSST-G. Including the interaction term did not improve the model fit significantly (χ2(5) = 8.000, p > .05). Therefore, baseline testosterone did not appear to have an impact on cortisol response during the TSST-G over time. There was also no significant main effect of baseline testosterone on cortisol response, ß = 0.156, SE = 0.089, t(62.275) = 1.753, 95% CI [−0.014, 0.325], p = .085.
3.3.2. Testosterone
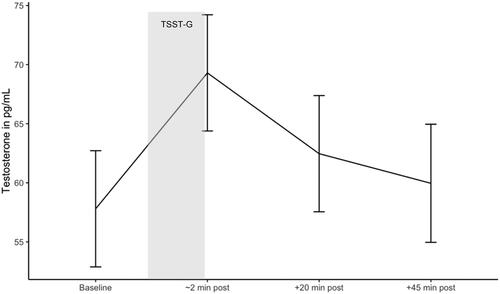
Contrast analysis of the mixed effects model revealed that after controlling for age, BMI, and stressor start, there was a significant increase in testosterone from baseline (before TSST-G start) to immediately after the TSST-G, ß = 11.502, SE = 1.826, t(192.132) = 6.299, 95% CI [7.150, 15.850], p < .001, and a significant decrease in testosterone during recovery from immediately after the TSST-G to 20 min following the TSST-G, ß = −6.840, SE = 1.830, t(192) = −3.745, 95% CI [−11.190, −2.490], p < .01 (). Robust analyses confirmed these findings (see Supplementary Material, Table 4 for results of standard and robust analyses).
Figure 4. Testosterone response during the TSST-G. Estimated marginal means of testosterone during the TSST-G. Error bars are confidence intervals of estimated marginal means.
We further examined whether baseline cortisol was associated with testosterone response during the TSST-G. Including the interaction term did not improve the model fit significantly (χ2(3) = 3.773, p > .05). Therefore, baseline cortisol did not appear to have an impact on testosterone response during the TSST-G over time. There was a significant main effect of baseline cortisol on testosterone response, ß = 9.369, SE = 2.682, t(61.896) = 3.493, 95% CI [4.238, 14.500], p = .001.
We further explored if morning testosterone was associated with testosterone response during the TSST-G. We tested if the model with the interaction time * morning testosterone and the model without this interaction term differed. Results revealed a non-significant difference between the two models. Thus, the model with the interaction term was not significantly different (χ2(3) = 5.312, p >.05).
3.4. Endocrine response to the military field exercise
3.4.1. Cortisol
Results of the contrast analysis of the mixed effects model revealed that after controlling for age, BMI, stressor start, and duration, there was a significant increase in cortisol from before to immediately after the field exercise, ß = 1.526, SE = 0.136, t(122) = 11.244, 95% CI [1.222, 1.830], p < .001 (). Robust analyses confirmed these findings.
Figure 5. Cortisol response during the military field exercise. Estimated marginal means of log cortisol during the field exercise. Error bars are confidence intervals of estimated marginal means.
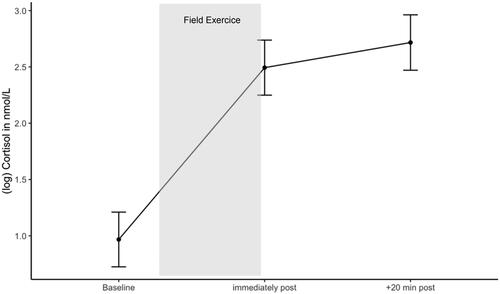
We further explored whether baseline testosterone was associated with cortisol response during the field exercise. We tested if the model with the interaction time * baseline testosterone and the model without this interaction term differed. Results revealed a significant difference between the two models. Including the interaction term improved the model fit significantly (χ2(2) = 6.838, p = .033). Thus, results of the mixed effect model revealed that higher baseline testosterone levels showed a smaller cortisol increase from before the field exercise to immediately after, ß = −0.316, SE = 0.134, t(119.809) = −2.355, 95% CI [−0.576, −0.055], p = .020, and subsequently a smaller cortisol increase 20 min after the field exercise, ß = −0.289, SE = 0.135, t(120.066) = −2.146, 95% CI [−0.550, −0.027], p = .034. Cadets with lower baseline testosterone showed a greater cortisol response during the field exercise. Robust analyses confirmed these findings (see Supplementary Material, Table 5 for results of standard and robust analyses).
3.4.2. Testosterone
Results of the contrast analysis of the mixed effects model revealed that after controlling for age, BMI, duration and start of stress, testosterone significantly increased during the field exercise, from before to immediately afterwards, ß = 0.809, SE = 0.168, t(114) = 4.820, 95% CI [0.433, 1.190], p < .001, and subsequently decreased within 20 min of the end of the exercise, ß = −1.603, SE = 0.176, t(117) = −9.100, 95% CI [−1.998, −1.210], p < .001 ().
Figure 6. Testosterone response during the military field exercise. Estimated marginal means of log testosterone during the field exercise. Error bars are confidence intervals of estimated marginal means.
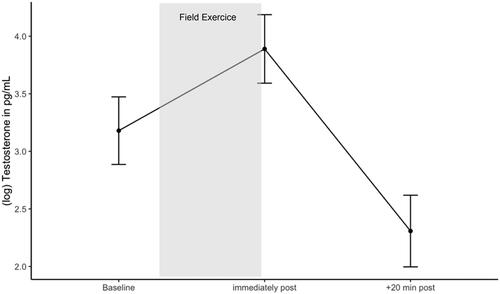
We further examined if baseline cortisol was associated with testosterone response during the field exercise. Including the interaction term did not improve the model fit significantly (χ2(2) = 0.787, p > .05). Therefore, baseline cortisol did not appear to have an impact on testosterone response during the field exercise over time. There was no significant main effect of baseline cortisol on testosterone response, ß = 0.216, SE = 0.111, t(57.751) = 1.948, 95% CI [0.006, 0.425], p = .056 (see Supplementary Material, Table 6 for results of standard and robust analyses).
We further explored if morning testosterone was associated with testosterone response during the field exercise. We tested if the model with the interaction time * morning testosterone and the model without this interaction term differed. Results revealed a non-significant difference between the two models. Thus, the model with the interaction term was not significantly different (χ2(2) = 1.980, p > .05).
4. Discussion
The aim of this study was to examine changes in cortisol and testosterone in young male military officer cadets during different types of stressors. We found that cadets showed increases in cortisol and testosterone during two acute stressor types, the TSST-G and a brief military field exercise. An unexpected finding was the significant increase in testosterone during the strenuous field exercise, with a sharp drop during recovery. Baseline cortisol and testosterone levels were not related to the hormonal response following the TSST-G, but baseline testosterone levels were negatively associated with cortisol response during the field exercise. Over the course of officer training school, morning testosterone decreased during the first 12 weeks and was lowest during what is called the perseverance week (a high-stress week). At the end of OS, morning testosterone levels returned to baseline levels. Various acute stressors in the military environment resulted in a transient co-activation of both the HPA and HPG axes and their hormone release, whereas intense and prolonged stress resulted in inhibition of the HPG axis and its testosterone release. These findings contribute novel information on testosterone changes during the group version of the TSST, TSST-G, and a short military stressor. In the following, we discuss possible factors that affect the hormonal response to the different type of stressors.
In addition to the well-known cortisol increase following social-evaluative stress in groups (von Dawans et al., Citation2011), testosterone also increased in most cadets. These results are consistent with some studies using the TSST version for individuals, where dominance and competition was manipulated (Bedgood et al., Citation2014; Deuter et al., Citation2016; Lennartsson et al., Citation2012; Turan et al., Citation2015). In line with the hypothesis, testosterone may be triggered when status is challenged in a social and competitive environment. As testosterone is linked to various status-relevant behaviors, such as social dominance (Chichinadze & Chichinadze, Citation2008), competitiveness (Archer, Citation2006), or status-related motivation and improving one’s social status (Vermeer et al., Citation2020), this group setting might therefore have promoted an increase of testosterone to cope with contextual demands. For individuals who are highly motivated and aspire to a leadership position, a group stress test with comrades may be particularly challenging or competitive due to status-related characteristics in the situation (e.g. maintain social status).
An intriguing finding of the current study is that in the hormonal response during the military field exercise, there is a co-activation of both HPA and HPG-axes. Despite the high physical and psychological demands of the strenuous field exercise, as reflected by an increase in cortisol, our results show that this brief stressor triggered testosterone release. More specifically, 48 cadets’ testosterone levels increased significantly and strongly from pre- to post-exercise, whereas nine cadets showed the expected decrease in testosterone during the exercise. Although cortisol changes were comparable to other military field studies (Lieberman et al., Citation2016; Morgan et al., Citation2000; Szivak et al., Citation2018); the testosterone responses from pre to post differed but were similar to those found in studies with physical exercises of moderate or high intensity (for a review, see D’Andrea et al., Citation2020). It should be noted that the field exercise in this study may be less threatening than survival training exercises are (Lieberman et al., Citation2016; Morgan et al., Citation2000) and therefore differ in acute testosterone response. Further, collection time points of the endocrine measures also differed from ours, insofar as we were able to collect saliva immediately before and after the exercise instead of hours later. The associated physiological changes and metabolic mobilization with the hormonal increases may have aided the cadets’ performance during this exercise. In addition, contextual characteristics such as competition and maintaining social status may also have played a considerable role, because the cadets had to meet performance targets and were required to complete the exercise with their comrades in small groups. It is possible that cadets were pushing each other to perform at their best and to further maintain their social status within their group and class. However, these arguments are speculative, because the performance of each cadet or group was not measured and perceptions of contextual conditions (e.g. competitiveness) were not assessed.
Consistent with the hypothesis and in line with other military studies (Lieberman et al., Citation2016; Morgan et al., Citation2000), testosterone decreased dramatically and cortisol increased concurrently during the brief recovery phase in the field exercise. The low testosterone levels before and at the end of the field exercise can be attributed to the diurnal decline in the evening hours besides the high physical demands (e.g. restricted food and sleep and high workload) during the field training. These acute endocrine changes point to a concomitant suppression of the HPA axis on the HPG axis during this overall highly stressful training session.
Unsurprisingly, morning testosterone levels fell with increasing stress load during OS. These testosterone alterations were comparable to those documented in individuals undergoing stressors such as long-term military training courses including high-intensity and extreme physical demands, reduced energy intake, inadequate sleep, and psychological stress (Bernton et al., Citation1995; Friedl et al., Citation2000; Kreuz et al., Citation1972; Nindl et al., Citation2007; Tait et al., Citation2022). The increase in morning testosterone in the last OS week indicates recovery and is associated with the low physical and mental demands in this final training week.
In contrast to previous research that focused specifically on the influence of baseline hormones on stress response, the present analysis is exploratory. Whereas baseline hormone levels and hormonal stress response appear to be independent during TSST-G, baseline testosterone levels were associated with cortisol responses during the stress of the field exercise. For instance, cadets with higher testosterone levels showed smaller increases in cortisol during the exercise. Although we did not expect this finding, our results are consistent with other studies (Mehta et al., Citation2008; Rubinow et al., Citation2005; Stephens et al., Citation2016) showing that pre-stress testosterone reduces the stress response and inhibits cortisol reactivity under certain conditions (e.g. competition). Further, our results show that the stressor type (TSST-G versus military field exercise) and context (laboratory versus field) may be associated with the impact of baseline hormones on hormonal stress response.
This study further extends and contributes to the existing knowledge by documenting that despite prolonged high stress and an accompanying decline in morning testosterone, some individuals are still able to adapt to acute challenges. Specifically, the increases in cortisol and testosterone levels in the two acute stress conditions suggest that both the HPA and HPG axes were activated. Thus, the two hormones were in lockstep during these two challenges in groups. However, the magnitude of hormonal changes was greater during the field exercise than in the laboratory, most likely due to the duration and physical intensity of the stressor. As a result, cortisol and testosterone levels are highly dependent on the type, intensity and duration of the stressor or exercise.
There are some limitations to this study. First, our sample of military cadets consisted of young men only. However, this could also be seen as a strength, as dominance and competition among these men occur in a more natural setting and do not need to be manipulated. Future studies are required to test whether women and men in a civilian or nonmilitary setting would show similar hormone changes in group stress tests. Second, there was no control condition in a group setting without stress or a single-participant stress test to examine what effect being a member of a group has on the hormonal change. Therefore, it cannot be conclusively determined whether the testosterone stress response is different between the TSST (individual version) versus the TSST-G (group version). Third, the samples collected in the mornings were planned to analyze testosterone levels only, and too little saliva was left to assess cortisol levels. To analyze HPA and HPG activation during acute and chronic stress, future research should collect more saliva samples. Fourth, we did not assess feelings of dominance or competitiveness or status-related personality traits. It would be important to test how dominant and competitive cadets are and if their personality traits may be associated with the acute hormonal response. Fifth, based on the present study design, the hormonal response of cadets to the laboratory and the field stressor could possibly have been affected by the course of the officer training school, which is characterized by physical and psychological demands, i.e. sleep deprivation, a highly structured daily routine and challenging training exercises. Finally, concerns have been raised in the literature about assessing saliva samples in field studies.
Future research might benefit from examining whether the TSST-G would also trigger a co-activation of the HPA and HPG axes in female, nonmilitary, and non-leadership samples. Additionally, assessing female officer cadets would provide insights into whether the group setting in the laboratory and the field is as effective as it is for male cadets. Moreover, the correlations between hormonal changes and performance should be investigated to derive insights for military training. The collection of feelings of dominance and competitiveness as well as status-related personality traits could also be used to identify the relationships with hormonal changes and performance. Finally, collecting additional samples for cortisol would help to elucidate individual differences in HPA and HPG changes during both acute and prolonged stress.
5. Conclusion
We found that during acute challenges in small groups, such as during the TSST-G and a brief military exercise in the field, cortisol and testosterone levels increase among young male officer candidates. Pre-stress levels of testosterone suppressed the cortisol stress response during the field exercise. These findings provide novel evidence for cortisol and testosterone responses during group stress tests in laboratory and field stressors in young healthy men. Further, although prolonged periods of high stress lead to dramatic decreases in testosterone, it seems that testosterone is adaptive, depending on the type and context of the stressor. Taken together, for an individual it may be more adaptive to produce a rise in testosterone in the right context instead of maintaining constantly elevated testosterone levels. The present findings have relevance not only for military or operational forces but also for society—for example the finding that during acute psychological and physical challenges, group settings may trigger hormonal responses to cope with the contextual demands.
Supplemental Material
Download MS Word (49.6 KB)Acknowledgements
We would like to acknowledge the most valuable help of the command of the Infantry Officer School in conducting the study and thank Dr. Firouzeh Farahmand for conducting the biochemical analyses and Ellen Russon for proofreading the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Regula Zueger
Regula Zueger is a psychologist and PhD student with a research interest in the psychobiology of stress and resilience.
Hubert Annen
Hubert Annen is the head of Military Psychology and Pedagogy Studies at the Swiss Military Academy at ETH Zurich.
Ulrike Ehlert
Ulrike Ehlert is a Professor of clinical psychology and psychotherapy at the University of Zurich.
References
- Aakvaag, A., Bentdal, Ø., Quigstad, K., Walstad, P., Rønningen, H., & Fonnum, F. (1978). Testosterone and testosterone binding globulin (TeBG) in young men during prolonged stress. International Journal of Andrology, 1(1–6), 1–11. https://doi.org/10.1111/j.1365-2605.1978.tb00573.x
- Agorastos, A., & Chrousos, G. P. (2022). The neuroendocrinology of stress: The stress-related continuum of chronic disease development. Molecular Psychiatry, 27(1), 502–513. https://doi.org/10.1038/s41380-021-01224-9
- Archer, J. (2006). Testosterone and human aggression: An evaluation of the challenge hypothesis. Neuroscience and Biobehavioral Reviews, 30(3), 319–345. https://doi.org/10.1016/j.neubiorev.2004.12.007
- Bedgood, D., Boggiano, M. M., & Turan, B. (2014). Testosterone and social evaluative stress: The moderating role of basal cortisol. Psychoneuroendocrinology, 47, 107–115. https://doi.org/10.1016/j.psyneuen.2014.05.007
- Bernton, E., Hoover, D., Galloway, R., & Popp, K. (1995). Adaptation to chronic stress in military trainees: Adrenal androgens, testosterone, glucocorticoids, IGF-1, and immune function. Annals of the New York Academy of Sciences, 774(1), 217–231. https://doi.org/10.1111/j.1749-6632.1995.tb17383.x-i1
- Casto, K. V., & Edwards, D. A. (2016). Testosterone, cortisol, and human competition. Hormones and Behavior, 82, 21–37. https://doi.org/10.1016/j.yhbeh.2016.04.004
- Chichinadze, K., & Chichinadze, N. (2008). Stress-induced increase of testosterone: Contributions of social status and sympathetic reactivity. Physiology & Behavior, 94(4), 595–603. https://doi.org/10.1016/j.physbeh.2008.03.020
- Cohen, S., Janicki-Deverts, D., Doyle, W. J., Miller, G. E., Frank, E., Rabin, B. S., & Turner, R. B. (2012). Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences of the United States of America, 109(16), 5995–5999. https://doi.org/10.1073/pnas.1118355109
- D’Andrea, S., Spaggiari, G., Barbonetti, A., & Santi, D. (2020). Endogenous transient doping: Physical exercise acutely increases testosterone levels—results from a meta-analysis. Journal of Endocrinological Investigation, 43(10), 1349–1371. https://doi.org/10.1007/s40618-020-01251-3
- Dabbs, J. M. (1990). Salivary testosterone measurements: Reliability across hours, days, and weeks. Physiology & Behavior, 48(1), 83–86. https://doi.org/10.1016/0031-9384(90)90265-6
- Deuter, C. E., Schächinger, H., Best, D., & Neumann, R. (2016). Effects of two dominance manipulations on the stress response: Cognitive and embodied influences. Biological Psychology, 119, 184–189. https://doi.org/10.1016/j.biopsycho.2016.06.004
- Dickerson, S. S., Gruenewald, T. L., & Kemeny, M. E. (2009). Psychobiological responses to social self threat: Functional or detrimental? Self and Identity, 8(2–3), 270–285. https://doi.org/10.1080/15298860802505186
- Dziurkowska, E., & Wesolowski, M. (2021). Cortisol as a biomarker of mental disorder severity. Journal of Clinical Medicine, 10(21), 5204. https://doi.org/10.3390/jcm10215204
- Friedl, K. E., Moore, R. J., Hoyt, R. W., Marchitelli, L. J., Martinez-Lopez, L. E., & Askew, E. W. (2000). Endocrine markers of semistarvation in healthy lean men in a multistressor environment. Journal of Applied Physiology (Bethesda, Md. : 1985), 88(5), 1820–1830. https://doi.org/10.1152/jappl.2000.88.5.1820
- Gomez-Merino, D., Chennaoui, M., Burnat, P., Drogou, C., & Guezennec, C. Y. (2003). Immune and hormonal changes following intense military training. Military Medicine, 168(12), 1034–1038. https://doi.org/10.1093/milmed/168.12.1034
- Kajantie, E., & Phillips, D. I. W. (2006). The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology, 31(2), 151–178. https://doi.org/10.1016/j.psyneuen.2005.07.002
- Kirschbaum, C., Pirke, K.-M., & Hellhammer, D. H. (1993). The ‘Trier Social Stress Test’ – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. https://doi.org/10.1159/000119004
- Kreuz, L. E., Rose, R. M., & Jennings, R. (1972). Suppression of plasma testosterone levels and psychological stress. Archives of General Psychiatry, 26(5), 479–482. https://doi.org/10.1001/archpsyc.1972.01750230089017
- Lennartsson, A. K., Kushnir, M. M., Bergquist, J., Billig, H., & Jonsdottir, I. H. (2012). Sex steroid levels temporarily increase in response to acute psychosocial stress in healthy men and women. International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology, 84(3), 246–253. https://doi.org/10.1016/j.ijpsycho.2012.03.001
- Lieberman, H. R., Farina, E. K., Caldwell, J., Williams, K. W., Thompson, L. A., Niro, P. J., Grohmann, K. A., & McClung, J. P. (2016). Cognitive function, stress hormones, heart rate and nutritional status during simulated captivity in military survival training. Physiology & Behavior, 165, 86–97. https://doi.org/10.1016/j.physbeh.2016.06.037
- Maestripieri, D., Baran, N.M., Sapienza, P., & Zingales, L. (2010). Between-and within-sex variation in hormonal responses to psychological stress in a large sample of college students. Stress (Amsterdam, Netherlands), 13(5), 413–424. https://doi.org/10.3109/10253891003681137
- Mazur, A., & Booth, A. (1998). Testosterone and dominance in men. The Behavioral and Brain Sciences, 21(3), 353–397. https://doi.org/10.1017/S0140525X98001228
- McEwen, B. S. (2017). Neurobiological and systemic effects of chronic stress. Chronic Stress, 1, 247054701769232. https://doi.org/10.1177/2470547017692328
- McEwen, B. S. (2019). What is the confusion with cortisol? Chronic Stress, 3, 247054701983364. https://doi.org/10.1177/2470547019833647
- Mehta, P. H., Jones, A. C., & Josephs, R. A. (2008). The social endocrinology of dominance: Basal testosterone predicts cortisol changes and behavior following victory and defeat. Journal of Personality and Social Psychology, 94(6), 1078–1093. https://doi.org/10.1037/0022-3514.94.6.1078
- Mehta, P. H., & Josephs, R. A. (2006). Testosterone change after losing predicts the decision to compete again. Hormones and Behavior, 50(5), 684–692. https://doi.org/10.1016/j.yhbeh.2006.07.001
- Miller, R., Plessow, F., Kirschbaum, C., & Stalder, T. (2013). Classification criteria for distinguishing cortisol responders from nonresponders to psychosocial stress: Evaluation of salivary cortisol pulse detection in panel designs. Psychosomatic Medicine, 75(9), 832–840. https://doi.org/10.1097/PSY.0000000000000002
- Minas, A., Fernandes, A. C. C., Maciel Júnior, V. L., Adami, L., Intasqui, P., & Bertolla, R. P. (2022). Influence of physical activity on male fertility. Andrologia, 54(7), e14433. https://doi.org/10.1111/and.14433
- Morgan, C. A., Wang, S., Mason, J., Southwick, S. M., Fox, P., Hazlett, G., Charney, D. S., & Greenfield, G. (2000). Hormone profiles in humans experiencing military survival training. Biological Psychiatry, 47(10), 891–901. https://doi.org/10.1016/S0006-3223(99)00307-8
- Nindl, B. C., Barnes, B. R., Alemany, J. A., Frykman, P. N., Shippee, R. L., & Friedl, K. E. (2007). Physiological consequences of U.S. Army Ranger training. Medicine and Science in Sports and Exercise, 39(8), 1380–1387. https://doi.org/10.1249/MSS.0b013e318067e2f7
- Nindl, B. C., Rarick, K. R., Castellani, J. W., Tuckow, A. P., Patton, J. F., Young, A. J., & Montain, S. J. (2006). Altered secretion of growth hormone and luteinizing hormone after 84 h of sustained physical exertion superimposed on caloric and sleep restriction. Journal of Applied Physiology (Bethesda, Md. : 1985), 100(1), 120–128. https://doi.org/10.1152/japplphysiol.01415.2004
- Pletzer, B., Poppelaars, E. S., Klackl, J., & Jonas, E. (2021). The gonadal response to social stress and its relationship to cortisol. Stress (Amsterdam, Netherlands), 24(6), 866–875. https://doi.org/10.1080/10253890.2021.1891220
- Rivier, C., & Rivest, S. (1991). Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: Peripheral and central Mechanisms. Biology of Reproduction, 45(4), 523–532. https://doi.org/10.1095/biolreprod45.4.523
- Rubinow, D. R., Roca, C. A., Schmidt, P. J., Danaceau, M. A., Putnam, K., Cizza, G., Chrousos, G., & Nieman, L. (2005). Testosterone suppression of CRH-stimulated cortisol in men. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 30(10), 1906–1912. https://doi.org/10.1038/sj.npp.1300742
- Sapolsky, R. M., Romero, L. M., & Munck, A. U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 21(1), 55–89. https://doi.org/10.1210/edrv.21.1.0389
- Schoofs, D., & Wolf, O. T. (2011). Are salivary gonadal steroid concentrations influenced by acute psychosocial stress? A study using the Trier Social Stress Test (TSST). International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology, 80(1), 36–43. https://doi.org/10.1016/j.ijpsycho.2011.01.008
- Stephens, M. A. C., Mahon, P. B., McCaul, M. E., & Wand, G. S. (2016). Hypothalamic-pituitary-adrenal axis response to acute psychosocial stress: Effects of biological sex and circulating sex hormones. Psychoneuroendocrinology, 66, 47–55. https://doi.org/10.1016/j.psyneuen.2015.12.021
- Szivak, T. K., Lee, E. C., Saenz, C., Flanagan, S. D., Focht, B. C., Volek, J. S., Maresh, C. M., & Kraemer, W. J. (2018). Adrenal stress and physical performance during military survival training. Aerospace Medicine and Human Performance, 89(2), 99–107. https://doi.org/10.3357/AMHP.4831.2018
- Tait, J. L., Drain, J. R., Corrigan, S. L., Drake, J. M., & Main, L. C. (2022). Impact of military training stress on hormone response and recovery. PloS One, 17(3), e0265121. https://doi.org/10.1371/journal.pone.0265121
- Taylor, M. K., Hernández, L. M., Kviatkovsky, S. A., Schoenherr, M. R., Stone, M. S., & Sargent, P. (2017). The “yin and yang” of the adrenal and gonadal systems in elite military men. Stress (Amsterdam, Netherlands), 20(3), 258–264. https://doi.org/10.1080/10253890.2017.1333594
- Toufexis, D., Rivarola, M. A., Lara, H., & Viau, V. (2014). Stress and the reproductive axis. Journal of Neuroendocrinology, 26(9), 573–586. https://doi.org/10.1111/jne.12179
- Turan, B., Hurst-Wajszczuk, K., & Edwards, D. A. (2022). Hormone and enzyme reactivity before, during, and after a music performance: Cortisol, testosterone, and alpha-amylase. Comprehensive Psychoneuroendocrinology, 9, 100111. https://doi.org/10.1016/j.cpnec.2022.100111
- Turan, B., Tackett, J. L., Lechtreck, M. T., & Browning, W. R. (2015). Coordination of the cortisol and testosterone responses : A dual axis approach to understanding the response to social status threats. Psychoneuroendocrinology, 62, 59–68. https://doi.org/10.1016/j.psyneuen.2015.07.166
- Vermeer, A. B., Krol, I., Gausterer, C., Wagner, B., Eisenegger, C., & Lamm, C. (2020). Exogenous testosterone increases status-seeking motivation in men with unstable low social status. Psychoneuroendocrinology, 113, 104552. https://doi.org/10.1016/j.psyneuen.2019.104552
- von Dawans, B., Kirschbaum, C., & Heinrichs, M. (2011). The Trier Social Stress Test for Groups (TSST-G): A new research tool for controlled simultaneous social stress exposure in a group format. Psychoneuroendocrinology, 36(4), 514–522. https://doi.org/10.1016/j.psyneuen.2010.08.004
- White, S. F., Lee, Y., Phan, J. M., Moody, S. N., & Shirtcliff, E. A. (2019). Putting the flight in “fight-or-flight”: Testosterone reactivity to skydiving is modulated by autonomic activation. Biological Psychology, 143, 93–102. https://doi.org/10.1016/j.biopsycho.2019.02.012
- Zueger, R., Niederhauser, M., Utzinger, C., Annen, H., & Ehlert, U. (2022). Effects of resilience training on mental, emotional, and physical stress outcomes in military officer cadets. Military Psychology, 1–11. https://doi.org/10.1080/08995605.2022.2139948