Abstract
Mary Dallman has left a legacy in neuroendocrinology, not only as the scientist who elaborated on new concepts such as rapid corticosteroid feedback pathways, but also as a role model, particularly for women who followed in her footsteps. In this contribution, I compare (i) the remarkable journey she made toward her position as the first female faculty member ever at the physiology department at USCF with that of generations after her; (ii) the contribution of our labs on rapid corticosteroid actions; and, (iii) finally, our experiences with unexpected findings for which one should always keep an open mind, a standpoint that was fervently advocated by Mary Dallman.
Introduction
On the occasion of receiving the 2010 Distinguished Research Award of the Society for the Study of Ingestive Behavior, Mary Dallman wrote an illuminating retrospective and perspective on her life and the field of neuroendocrinology, the field in which she excelled. In the paper she describes how she had already embarked on a hefty overview of her life, taking no less than eight single-spaced pages to cover the first 20 years of her scientific life, when two of her children advised her to rewrite it and focus on three important things: how it all began; her scientific discoveries; and her thoughts on a few academia-related issues she deemed of interest. “The big picture,” her children told her.
In preparation for my tribute to Mary Dallman, I reread this article and was struck by the enormous -and adventurous- journey her scientific life must have been, spanning roughly 50 years; and the landslide in scientific culture she witnessed, not in the least in the attitude toward women.
I felt I could not honor her in a more befitting way than follow this wonderful article from her hand and address the same three items, comparing our journeys. My path has been so much smoother than hers, exactly because it had been paved by women like Mary Dallman, who forced open the world of (neuro)endocrinology and physiology for those who had not been easily admitted until then. Following in her footsteps -also in the organization of this article- is a tribute to this remarkable woman.
How come a scientist, as a female in a males’ world?
In her article, Mary Dallman discloses how she first expressed her wish to become a scientist at the age of 14 when asked what she aspired to be, later, mostly because she felt she could not compete with her elder sibs who excelled in music and art. When in college she became fascinated by steroid molecules, and, urged on by supporters, went to the Columbia College of Physicians & Surgeons in 1956, the year I was born. She quit after a year. Not because she liked the subject too little, but, rather, because she liked it too much and felt surrounded by less interested male students and only (besides herself) three female students who were “dressed in drab” and “seemed to want to fade into the woodwork and make themselves invisible…” (Dallman, 2011).
Typical for those not having easy access to the academic system, she took refuge in a job as a technician. But genuine drive is not to be thwarted. So, a few years later she had her second try at entering graduate school, this time at the Rockefeller University. When interviewed by the President (amazing; I don’t think the current President would take the time to interview prospective graduate students!), in a new dress bought for the occasion, she was asked:
“what is that ring on your finger?”, and when [she] said that [she] was engaged to be married, he said “I knew it was a waste of time to interview a woman”—and that was the end of the interview.
I vividly remember asking Mary in 1998 how she had pulled it off, raising a family as well as being a successful scientist. And how she answered that for many years she would first attend to the family and only when the children were in bed, she would revert her attention to science again, working many hours at night.
Much of the anecdotes related in her article about the difficulty of women entering the academic system were situated in the sixties and seventies of the last century. Things have, fortunately, changed for the better. Nowadays, roughly 50% of all PhD students in biomedical sciences in Europe as well as the USA are female (Schaller, Citation2022; SheFigures, Citation2021). Being turned down for a graduate program as female applicant because you are engaged to be married and being told that it is a waste of time to even interview you is unthinkable, at least in Western society: no diversity and inclusivity committee of any university would tolerate it. So, we have come a long way.
But even today, there are many hurdles to take for bright female scientists, especially in the STEM (science, technology, engineering, and mathematics) field. Bostwick and Weinberg (Citation2018) showed, based on all individuals who first enrolled in a doctoral program of any public four-year university in Ohio between 2005 and 2015 in the STEM programs, that in these particular cohorts, two-thirds of PhD cohorts were less than 50% female. Importantly, women with no female peers—very similar to the situation experienced by Mary Dallman—were 12 percentage points less likely to complete their degrees within six years than men in the same cohort. An increase of one standard deviation in the share of female students in a cohort increased the probability of on-time graduation for women by nearly five percentage points. Environment matters.
And graduating is just a first career step. In her paper, Mary Dallman describes the difficulties it took her to become appointed as faculty member in the Department of Physiology, the first female ever. Nowadays, rising through the ranks is still difficult. The numbers illustrate this: In 2019, in Europe, women represented less than 25% of the heads of institutions in the higher education sector (SheFigures, Citation2021). This number is very similar to the percentage of women (24%) among the most highly paid employees at the 130 major research universities of the USA (Women’s Power Gap Study Series, Citation2022). There are multiple explanations for the slow rise through the ranks, including the fact that women are consistently less credited for what they do (Ross et al., Citation2022). And if you don’t get the credits, you may seem less productive—even at the earliest stages of one’s career (Schaller, Citation2022)—and it becomes harder to acquire grants. A recent survey among NIH grant recipients shows that the gap in earning power is not getting any smaller, women being systematically less successful in securing multiple grants than men (Nguyen et al., Citation2023).
But there are also more optimistic signs. Implicit negative bias toward women in the hiring process of a lab manager was convincingly demonstrated in 2012 (Moss-Racusin et al., Citation2012): yet, more recent data support that women, when it comes to filling tenure-track positions in the STEM fields, appear to be favored over men (Williams & Ceci, Citation2015). Similarly, older surveys show a very low representation of women amongst the membership of academies of sciences (Gibney, Citation2016), whereas a very recent study demonstrated that in the last three years’ appointments at the NAS and AAS, women made up 40% of the new membership, which is an overrepresentation compared to the percentage of female candidates (Card et al., Citation2023). All in all, the world for female scientists has considerably improved since the early days of Mary Dallman, not in the least through the perseverance of her and the likes. But it still is not a level playing field. The existence of positive examples, of mentoring and of sponsoring remains indispensable (Joëls & Mason, Citation2014). For me, Mary Dallman has been just such a positive example.
The lab: rapid feedback
One of the neuroendocrine phenomena described by Mary Dallman is that of rapid feedback. In a classical experiment (see ), she demonstrated that a stressor (for instance histamine injection) within 2 min after infusion of corticosterone to a rat did not result in the expected rise in corticosterone level as seen when exposing the organism to a stressor at 10 or 45 min after the onset of corticosterone infusion. This (Dallman & Yates, Citation1969), and later work by Morton Jones’ laboratory (reviewed in Jones & Gillham, Citation1988), was interpreted as evidence for the existence of a rapid inhibitory action by corticosterone during the rising phase of the infusion; a phenomenon sensitive to the rate of infusion. The rapid inhibitory action occurred complementary to the well-documented gene-mediated inhibitory actions by corticosterone seen 120 min after infusion with the steroid was started.
Figure 1. Rapid actions of corticosteroids in the brain. (A) Rapid feedback inhibition of the stress axis—activated by histamine administration (arrows)—was observed at 2 (but not 10 or 45; stars) min after the onset of corticosterone being infused into anesthetized female rats at a constant rate for 2 h. Delayed inhibition was observed when the rats received a stressor 120 min after corticosterone infusion started. Based on Dallman (Citation2005). (B) The mechanism of rapid inhibitory corticosteroid actions was further explored with electrophysiological methods, recording miniature excitatory postsynaptic currents (mEPSCs; typical recording in inset), each of which reflects the postsynaptic response to a spontaneously released presynaptic glutamate-containing vesicle. Changes in mEPSC frequency usually indicate an altered (presynaptic) release probability of the vesicles, whereas changes in mEPSC amplitude rather point to changes in the expression of postsynaptic receptor subunits. Panels B–D based on Tasker and Joëls (Citation2015). (C) Schematic representation how corticosterone may bind to a membrane-bound version of the glucocorticoid receptor (mbGR) on CRH-producing neurons in the PVN, and then through retrogradely transported endocannabinoids (eCB) reduce the release of glutamate-containing vesicles from presynaptic neurons. The recordings on the right show that the frequency (but not amplitude) of mEPSCs is reduced by the GR-agonist dexamethasone, compared to the control situation prior to dexamethasone. (D) In CA1 pyramidal neurons of the hippocampus, corticosteroids act via mineralocorticoid receptors (mMR) presumably located at (or in the vicinity of) the presynaptic plasma membrane. As shown on the right, this results in an increased frequency—but not amplitude—of mEPSCs during administration of (100 nM of) corticosterone.
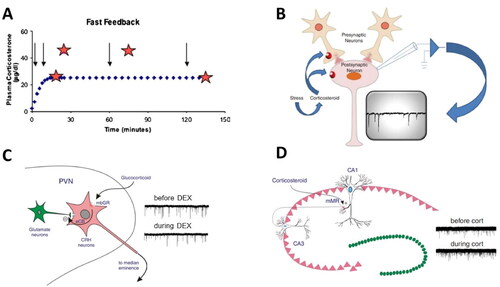
Rapid corticosteroid effects had already been described earlier, in relation to diverse functions, like mating behavior, aggression and feeding behavior in various vertebrate species (reviewed in Dallman, Citation2005). However, for many years, the rapid effects received less attention than the “classical” gene-mediated signaling, primarily due to the complete lack of understanding of a potential underlying mechanism.
This understanding was for the first time provided by the work of Jeffrey Tasker and colleagues in 2003 (Di et al., Citation2003), who demonstrated that corticosterone in vitro, presumably via glucocorticoid receptors, rapidly inhibits the release of glutamate from neurons projecting onto CRF-producing cells in the paraventricular nucleus of the hypothalamus (PVN), as inferred from a decreased frequency of miniature excitatory postsynaptic currents (mEPSCs; see ). This rapid signaling pathway involves retrograde signaling via endocannabinoids.
In her review of rapid glucocorticoid effects, Mary Dallman (Citation2005) raised the following questions: “What steroid receptor(s) is (are) involved? What is (are) the second messenger(s)? Is there a single membrane receptor and a single intracellular second messenger, or, are there many?”
Subsequent experiments by us and others revealed that the rapid and slow actions described by Dallman and Jones after stress actually form part of a complex mosaic of rapid and slow corticosteroid actions in the brain, which in part—but not exclusively—are determined by the local expression levels of mineralocorticoid and glucocorticoid receptors (MRs and GRs respectively) (for review see Joëls et al., Citation2012). For instance, in 2005, we demonstrated that rapid corticosteroid actions not only occur in the PVN but also in extrahypothalamic parts of the brain, more specifically, a non-genomic enhancement of mEPSC frequency in the CA1 area of the hippocampus. Contrary to the PVN, these corticosteroid actions were mediated by the MR rather than GR (), and do not involve retrogradely transported endocannabinoids but a presynaptic ERK1/2 signaling pathway (Karst et al., Citation2005; Olijslagers et al., Citation2008). In addition to the rapid presynaptic actions, there are also rapid postsynaptic effects on CA1 neurons by corticosterone, involving G-protein signaling (Olijslagers et al., Citation2008).
Dentate granule cells (see review Joëls et al., Citation2012) and principal neurons in the basolateral amygdala (Karst et al., Citation2010; ) also exhibit a rapid non-genomic, MR-dependent increase in excitability, whereas pyramidal neurons in the infralimbic cortex seem to respond more like neurons in the PVN (Karst et al., unpublished observation). This confirms that, as already assumed by Mary Dallman (Citation2005), the mechanisms underlying rapid corticosteroid signaling need to be unraveled on a case-by-case basis, for each brain region; very similar to the regional differences earlier observed with regard to the slow gene-mediated actions (Joëls, Citation2006).
Figure 2. Metaplasticity of corticosterone responses in the basolateral amygdala. (A) Principal neurons in the basolateral amygdala (BLA) respond within minutes to corticosterone with an increase in the mEPSC frequency. A similar increase is also seen when corticosterone is applied for a second time, within 60 min after the first administration. However, with longer delays between the first and second administration, this increase in mEPSC frequency alters into a decrease. Each dot represents the percentual change in mEPSC frequency observed in a particular BLA neuron tested at the delay (after the first administration) indicated on the x-axis. The shift from increased to decreased mEPSC frequency with a delay of >1 h was dubbed “metaplasticity.” (B) Interestingly, some degree of metaplasticity also occurs when corticosterone is administered after the β-agonist isoproterenol, although the effect was less clearly seen and seemed to occur already after a delay of ∼30 min. (C) The metaplasticity observed in relation to isoproterenol and corticosterone may be of relevance for the naturally occurring waves of stress mediators. We tested three conditions, i.e. (i) a situation with only a wave of (a low dose of) isoproterenol (left (i.e. first) wave), reflecting a situation of arousal (top); (ii) a situation with subsequent waves of moderately high doses of isoproterenol and corticosterone (right (i.e. second) wave) respectively, such as may occur during moderate stress (middle); and (iii) a situation with subsequent waves of high doses of isoproterenol and corticosterone, such as may occur during severe stress (bottom). Whereas in the former two situations, BLA neuronal activity is only temporarily high, the latter situation caused a prolonged increase in excitability. All panels are based on Karst and Joëls (Citation2016).
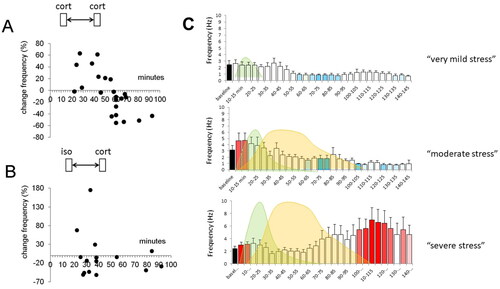
The nature of the receptor involved in rapid corticosteroid signaling remains elusive. Rapid non-genomic actions in the hippocampus and amygdala do require the expression of the “classical” MR-encoding gene (Karst et al., Citation2005, Citation2010). The receptor molecule mediating rapid effects appears to be located close to the plasma membrane rather than in the nucleus, given the effectiveness of corticosterone conjugated to bovine serum albumin—which cannot pass the plasma membrane—to evoke rapid actions. However, to date all attempts to visualize an MR close to the plasma membrane have failed (Karst et al., Citation2022). It cannot be entirely excluded that a hitherto unknown membrane-variant of the MR, GR or both, is involved in the rapid signaling pathways, as has been previously found for the estrogen receptor GPER1 (Alexander et al., Citation2017).
In 2005, Mary Dallman wrote “the overall notion … is that rapid effects are ancient functions of hormonal steroids and sterols that serve to accentuate behaviors that are evolutionarily important for fitness of the individual under stress.” This notion has been completely confirmed in subsequent studies by us and others, involving an extensive series of behavioral experiments in rodents and humans, often in combination with genetic or pharmacological interventions to specifically activate MR or GR (). The current view is that rapid corticosteroid actions, mostly via MRs, in concert with actions exerted by other stress mediators—belonging to the class of monoamines or neuropeptides—, serve to quickly focus attention on relevant information, promoting the selection of simple strategies that are geared to preserve the stressed individual and its close ones. This is essential for immediate survival. However, it comes at a price: these rapid actions would render the organism rather inflexible and self-centered in its solutions to adapt to an ever-changing environment. It is therefore essential that the rapid actions are complemented by well-documented slow, gene-mediated corticosteroid actions, generally involving intracellularly located GRs. These slow actions (i) facilitate remembering salient information for future use, in a context-dependent—and more flexible—manner; (ii) support taking rational decisions; and iii) promote making altruistic choices.
Figure 3. Rapid and delayed effects of stress hormones on cognitive processing. Summary of behavioral effects described in rodents (r) and humans (h) observed shortly after elevation of stress hormones (top left) and with a delay of at least 1 h (top right). The behavioral paradigms that were employed (left) are arranged from (top to bottom) those involving emotional circuits (including the amygdala); via rather routine-based circuits (e.g. involving the striatum); more flexible context-dependent paradigms (involving e.g. the hippocampus); to those related to executive functions like decision-making (involving e.g. the prefrontal cortex). Overall, the studies indicate that directly after stress emotional and routine-based circuits are enhanced in activity, while higher cognitive functions are suppressed. By contract, starting 1 h after stress, the opposite effect is seen. The rapid effects (top-left arrow) most likely involve noradrenergic activation, as well as the effects of neuropeptides like CRH and corticosteroids acting via MR. The delayed effects (arrow on lower right) can be mostly explained by gene-mediated actions via GR.
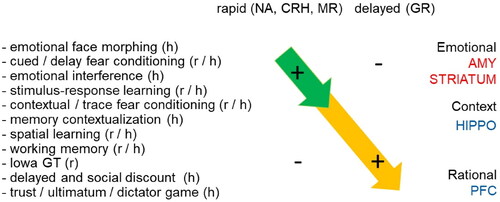
Both the rapid and delayed effects, in balance, are important for the fitness of the individual, e.g. with regard to its resilience to develop psychopathology (De Kloet & Joëls, Citation2023). Due to genetic predisposition, some individuals may display a slight disbalance between the rapid and delayed actions of stress mediators; a disbalance which could be exacerbated by subsequent exposure to life events, especially when occurring in the vulnerable developmental period. Such a disbalance may change the way in which individuals process information and render them susceptible to the precipitation of psychiatric disorders, especially in the face of challenging life conditions. Eventually this may also affect the fitness of the species, from an evolutionary point of view (Korte et al., Citation2005).
Final note: the academic world
In her reminiscences (Dallman, Citation2011), Mary Dallman ended by stating that young and eager academics these days feel crippled
through requiring assurances that every study has a certain bottom line, and through making the working life of the investigator dependent on producing positive results for what is hypothesized. It is very important that in the coming years, a new functional model is found for these institutions that acknowledges that outcomes can be predicted, but that the predictions may not be correct.
I couldn’t agree more. The pressure in the system is building, especially on young people, to produce (positive) results. That has the potential risk that one is only open to the expected result, whereas unexpected outcomes can be illuminating, when one has a ready mind. Let me give two examples from our own work, to illustrate how unexpected results can give rise to entirely new insights.
The first example dates back from the early nineties, when I had just embarked on investigating corticosteroid effects in the brain (Joëls et al., Citation1991). It so happened that, one day, I had tested corticosteroid actions on brain slices and at the end of the day wanted to use the same slices to run some control experiments on serotonin (5-HT) which a reviewer had requested, as part of an entirely different experiment. To my surprise, 5-HT was not giving the clear hyperpolarization of CA1 pyramidal neurons we expected -due to activation of K+ channels-; but, rather, a half-baked small hyperpolarization. What was wrong? At first, I thought the 5-HT had gone off, because it is sensitive to oxidation. So, I made a fresh stock; only to find the same small hyperpolarization. And then it slowly dawned on me that perhaps that earlier corticosteroid exposure of the slices had something to do with it. That asked for a new, dedicated set of experiments testing exactly that: Does brief corticosterone exposure several hours before application of 5-HT affect the subsequent 5-HT1A receptor mediated responses? And this turned out to be the case, in a dose-dependent manner. Thus, moderately low doses of corticosterone (predominantly activating MR) were associated with small responses, while both in the absence of corticosterone and with simultaneous activation of MR and GR by high doses of corticosterone large 5-HT1A receptor mediated responses were observed. It turned out to be one of the clearest examples of a U-shaped dose dependency for corticosteroids in the CA1 area (Joëls, Citation2006).
The second example concerns the rapid corticosteroid actions in principal neurons of the basolateral amygdala (Karst et al., Citation2010; ). Here, we found that corticosterone application to slices prepared from naïve mice at the trough of the circadian rhythm causes a rapid MR-dependent increase in mEPSC frequency, similar to what had been observed in the hippocampus, though more persistently. That is, once the mEPSC frequency had been increased by corticosterone in amygdalar neurons, the mEPSC frequency remained high for hours, whereas in the hippocampus the effects were transient. When we wanted to wrap up that set of experiments on rapid-onset corticosteroid actions in the BLA with a final test of some MR- and GR-ligands, the most experienced electrophysiologist in our lab, Henk Karst, was unable to reproduce the earlier observed increase in mEPSC frequency. Had it occurred to a less experienced experimenter, we would probably have endlessly repeated the experiments, until we could reproduce the earlier results. However, coming from Henk Karst, it simply had to be true! So, we started to wonder what could have changed in the experimental conditions, compared to the earlier measurements. Many possibilities were considered: the chemicals in the buffer; the food in the animal house; the supplier of the mice etcetera. Until we realized that the only change that had taken place since the earlier experiments was in the person taking care of the animals: Recently, the former caretaker (a rather subdued person) had retired and the new guy had started with gusto, rattling the buckets and enthusiastically cleaning the cages. We wondered if the mice, inadvertently, had been stressed? To test this, we administered corticosterone to slices from entirely naïve (and unstressed) mice and compared these with responses in slices from mice that had been stressed prior to the slice preparation. And sure enough, if the mice were stressed prior to slice preparation, a decrease rather than increase in mEPSC frequency was observed. The former—contrary to the latter—was accomplished via a GR-dependent pathway involving retrograde endocannabinoid transport, similar to the PVN. In other words, in basolateral amygdalar cells, corticosterone rapidly and persistently increases excitability via an MR-dependent pathway, which changes the state of these cells such that subsequent corticosteroid exposure induces a completely different effect. This phenomenon of dependence of rapid corticosteroid responses on the recent (stress) history of the individual was dubbed “metaplasticity.” Metaplastic rapid corticosteroid signaling turned out to be relevant for the overall excitability during ultradian corticosteroid pulses, causing an increased excitability with increasing amplitude of the ultradian pulses and, conversely, decreased excitability when the amplitude of pulses declines (Den Boon et al., Citation2019). Metaplasticity is also important for the response to “normal” sequential waves of stress mediators to which brain cells are exposed after stress, since corticosterone actions in amygdalar neurons were also found to depend on prior exposure to monoamines like noradrenaline, acting via β-adrenoceptors (Karst & Joëls, Citation2016; ). The susceptibility of basolateral amygdala neurons to metaplastic corticosteroid actions gives them a special position in the mediation of stress responses, with strong and sustained responses after exposure to high sequential doses of a β-adrenoceptor agonist and corticosterone, such as may occur after a strong stressor. This could explain why very emotional information is so well retained (LaLumiere et al., Citation2017).
It is the inquisitive mind that characterizes the true scientist, and this is what Mary Dallman described so well in 2011: “…Unexpected results that change perceptions of how ‘the secret in the middle’ … may pave the way to new understanding …”
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Marian Joëls
Marian Joels is professor in Neurobiology of Environmental Factors at the University of Groningen and University Medical Center Groningen. She and her group members investigate the effects of stress on the brain, in health and disease, using a variety of techniques, ranging from in vitro electrophysiology in animal models to experimental psychology in humans. In recent years, research efforts have particularly focused on the effects of stress experienced early in life for later life cognitive processing.
References
- Alexander, A., Irving, A. J., & Harvey, J. (2017). Emerging roles for the novel estrogen-sensing receptor GPER1 in the CNS. Neuropharmacology, 113(Pt B), 1–7. https://doi.org/10.1016/j.neuropharm.2016.07.003
- Bostwick, V.K., & Weinberg, B.A. (2018). Nevertheless she persisted? Gender peer effects in doctoral STEM programs. Working paper 25028, National Bureau of Economic Research. http://www.nber.org/papers/w25028
- Card, D., DellaVigna, S., Funk, P., & Iriberri, N. (2023). Gender gaps at the academies. Proceedings of the National Academy of Sciences of the United States of America, 120, e221242110.
- Dallman, M. F. (2005). Fast glucocorticoid actions on brain: Back to the future. Frontiers in Neuroendocrinology, 26(3-4), 103–108. https://doi.org/10.1016/j.yfrne.2005.08.001
- Dallman, M. F. (2011). Retrospective and perspective on the occasion of receiving the SSIBs Distinguished Research Award. Physiology & Behavior, 104(4), 530–534. https://doi.org/10.1016/j.physbeh.2011.04.047
- Dallman, M. F., & Yates, F. E. (1969). Dynamic asymmetries in the corticosteroid feedback path and distribution-metabolism-binding elements of the adrenocortical system. Annals of the New York Academy of Sciences, 156(2), 696–721. https://doi.org/10.1111/j.1749-6632.1969.tb14008.x
- De Kloet, E. R., & Joëls, M. (2023). The cortisol switch between vulnerability and resilience. Molecular Psychiatry. https://doi.org/10.1038/s41380-022-01934-8
- Den Boon, F.S., de Vries, S., Baelde, M., Joels, M. & Karst, H. (2019). Circadian and Ultradian Variations in Corticosterone Level Influence Functioning of the Male Mouse Basolateral Amygdala. Endocrinology. 16(4), 791-802. https://doi.org/10.1210/en.2018-00767
- Di, S., Malcher-Lopes, R., Halmos, K. C., & Tasker, J. G. (2003). Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: A fast feedback mechanism. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(12), 4850–4857. https://doi.org/10.1523/JNEUROSCI.23-12-04850.2003
- Gibney, E. (2015). Women under-represented in world’s science academies. Nature. doi:10.1038/nature.2016.19465
- Joëls, M. (2006). Corticosteroid effects in the brain: U-shape it. Trends in Pharmacological Sciences, 27(5), 244–250. https://doi.org/10.1016/j.tips.2006.03.007
- Joëls, M., Hesen, W., & de Kloet, E. R. (1991). Mineralocorticoid hormones suppress serotonin-induced hyperpolarization of rat hippocampal CA1 neurons. The Journal of Neuroscience, 11(8), 2288–2294. https://doi.org/10.1523/JNEUROSCI.11-08-02288.1991
- Joëls, M., & Mason, C. (2014). A tale of two sexes. Neuron, 82(6), 1196–1199. https://doi.org/10.1016/j.neuron.2014.05.021
- Joëls, M., Sarabdjitsingh, R. A., & Karst, H. (2012). Unraveling the time domains of corticosteroid hormone influences on brain activity: Rapid, slow and chronic modes. Pharmacological Reviews, 64(4), 901–938. https://doi.org/10.1124/pr.112.005892
- Jones, M. T., & Gillham, B. (1988). Factors involved in the regulation of adrenocorticotropin/beta-lipotropic hormone. Physiological Reviews, 68(3), 743–818. https://doi.org/10.1152/physrev.1988.68.3.743
- Karst, H., Berger, S., Erdmann, G., Schütz, G., & Joëls, M. (2010). Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proceedings of the National Academy of Sciences of the United States of America, 107(32), 14449–14454. https://doi.org/10.1073/pnas.0914381107
- Karst, H., Berger, S., Turiault, M., Tronche, F., Schütz, G., & Joëls, M. (2005). Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proceedings of the National Academy of Sciences of the United States of America, 102(52), 19204–19207. https://doi.org/10.1073/pnas.0507572102
- Karst, H., den Boon, F. S., Vervoort, N., Adrian, M., Kapitein, L. C., & Joëls, M. (2022). Non-genomic steroid signaling through mineralocorticoid receptor: Involvement of a membrane-associated receptor? Molecular and Cellular Endocrinology, 541, 111501. https://doi.org/10.1016/j.mce.2021.111501
- Karst, H., & Joëls, M. (2016). Severe stress hormone conditions cause an extended window of excitability in the mouse basolateral amygdala. Neuropharmacology, 110(Pt A), 175–180. https://doi.org/10.1016/j.neuropharm.2016.07.027
- Korte, S. M., Koolhaas, J. M., Wingfield, J. C., & McEwen, B. S. (2005). The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neuroscience and Biobehavioral Reviews, 29(1), 3–38. https://doi.org/10.1016/j.neubiorev.2004.08.009
- LaLumiere, R. T., McGaugh, J. L., & McIntyre, C. K. (2017). Emotional modulation of learning and memory: Pharmacological implications. Pharmacological Reviews, 69(3), 236–255. https://doi.org/10.1124/pr.116.013474
- Moss-Racusin, C. A., Dovidio, J. F., Brescoll, V. L., Graham, M. J., & Handelsman, J. (2012). Science faculty’s subtle gender biases favor male students. Proceedings of the National Academy of Sciences of the United States of America, 109(41), 16474–16479. https://doi.org/10.1073/pnas.1211286109
- Nguyen, M., Chaudhry, S.,I., Desai, M. M., Dzirasa, K., Cavazos, J. E., & Boatright, D. (2023). Gender, racial, and ethnic and inequities in receipt of multiple national institutes of health research project grant. JAMA Network Open, 6(2), e230855. https://doi.org/10.1001/jamanetworkopen.2023.0855
- Olijslagers, J. E., de Kloet, E. R., Elgersma, Y., van Woerden, G. M., Joëls, M., & Karst, H. (2008). Rapid changes in hippocampal CA1 pyramidal cell function via pre- as well as postsynaptic membrane mineralocorticoid receptors. The European Journal of Neuroscience, 27(10), 2542–2550. https://doi.org/10.1111/j.1460-9568.2008.06220
- Ross, M. B., Glennon, B. M., Murciano-Goroff, R., Berkes, E. G., Weinberg, B. A., & Lane, J. I. (2022). Women are credited less in science than men. Nature, 608(7921), 135–145. https://doi.org/10.1038/s41586-022-04966-w
- Schaller, M. D. (2022). The gender gap amongst doctoral students in the biomedical sciences. BioRXiv. https://doi.org/10.1101/2022.10.18.512765
- SheFigures. (2021). https://ec.europa.eu/research-and-innovation/en/knowledge-publications-tools-and-data/interactive-reports/she-figures-2021
- Tasker, J. F., & Joëls, M. (2015). The synaptic physiology of the central nervous system response to stress. In J. A. Russell & M. J. Shipston (Eds.), Neuroendocrinology of stress (1st ed., pp. 43–70, Chapter 3). John Wiley & Sons, Ltd.
- Williams, W. M., & Ceci, S. J. (2015). National hiring experiments reveal 2:1 faculty preference for women on STEM tenure track. Proceedings of the National Academy of Sciences of the United States of America, 112(17), 5360–5365. https://doi.org/10.1073/pnas.1418878112
- Women’s Power Gap Study Series. (2022). https://www.womenspowergap.org/wp-content/uploads/2021/02/WPG-Power-Gap-at-Elite-Universities-2021-Study-4.pdf

