Abstract
The COVID-19 pandemic severely affected the lives of families and the well-being of both parents and their children. Various factors, including prenatal stress, dysregulated stress response systems, and genetics may have influenced how the stress caused by the pandemic impacted the well-being of different family members. The present work investigated if emotional well-being during the COVID-19 pandemic could be predicted by developmental stress-related and genetic factors. Emotional well-being of 7–10 year-old children (n = 263) and mothers (n = 241) (participants in a longitudinal German birth cohort (POSEIDON)) was assessed during the COVID-19 pandemic using the CRISIS questionnaire at two time periods (July 2020–October 2020; November 2020–February 2021). Associations of the children’s and mothers’ well-being with maternal perceived stress, of the children’s well-being with their salivary and morning urine cortisol at 45 months, and polygenic risk scores (PRSs) for depression, schizophrenia, loneliness were investigated. Lower emotional well-being was observed in both children and mothers during compared to before the pandemic, with the children’s but not the mothers’ emotional well-being improving over the course of the pandemic. A positive association between the child and maternal emotional well-being was found. Prenatally assessed maternal perceived stress was associated with a lower well-being in children, but not in mothers. Cortisol measures and PRSs were not significantly associated with the children’s emotional well-being. The present study confirms that emotional well-being of children and mothers are linked, and were negatively affected by the COVID-19 pandemic, with differences in development over time.
1. Introduction
Stress is a major risk factor for somatic and mental health problems (Agorastos & Chrousos, Citation2022). The COVID-19 pandemic was a major stressor which affected the lives and well-being of people worldwide, but findings on mental health during the COVID-19 pandemic are heterogeneous, demonstrating both increased as well as decreased mental distress over time (Bussières et al., Citation2021; Robinson et al., Citation2022).
A wide range of factors could influence how children’s mental health developed under the influence of the life changes associated with the COVID-19 pandemic. These include parental well-being and stress perception, biological regulators of the stress response such as the functioning of the hypothalamic-pituitary-adrenal (HPA) axis, and genetic risk factors.
Children’s psychosocial well-being is closely related to that of their parents (Cobham et al., Citation2016); parents who reported higher levels of depression and anxiety or stress during the pandemic also reported higher stress levels (Russell et al., Citation2020) and behavioral and emotional problems in their children (Cusinato et al., Citation2020; Spinelli et al., Citation2020).
How mental health was affected by the pandemic could have also been determined by biological factors linked to stress regulation. The HPA axis is a central component of the biological stress response. Early-life stress, which includes prenatal stress, is linked to HPA axis dysregulation (O’Connor et al., Citation2021) and its effects can last into adulthood (van den Bergh et al., Citation2020). Early-life stress has been associated with impairments in development and health of children, and there is evidence that early programming of HPA axis functioning contributes to the effects on later life outcomes (van den Bergh et al., Citation2020). In line with this, associations between HPA axis regulation and pandemic-related stressors have been shown in both children and adults (Ahrens et al., Citation2022; Haucke et al., Citation2022; Jopling et al., Citation2021; Perry et al., Citation2022).
Genetic factors also play an important role in stress regulation (Kudielka et al., Citation2009) and stress related mental disorders (Levinson, Citation2006). Genome-wide association studies (GWAS) identify common genetic variants associated with psychiatric disorders or particular traits. Based on these results, polygenic risk scores (PRSs) can be calculated in independent target samples, reflecting the polygenic risk burden of each individual (Wray et al., Citation2014). Recent studies have demonstrated associations between PRS for depression and mental health in adults (McIntosh et al., Citation2019) adolescents and children (Kwong et al., Citation2021), including during the COVID-19 pandemic (Ahrens et al., Citation2022).
The present study investigated emotional well-being trajectories during the COVID-19 pandemic and their association with maternal perceived stress, early childhood HPA axis activity and genetic risk factors in mothers and children assessed within the frame of a longitudinal birth cohort study. We hypothesized that:
(H1) The COVID-19 pandemic had negative short-term and long-term effects on the emotional well-being of children and mothers;
(H2) The mother’s emotional well-being was positively associated with the child’s emotional well-being;
(H3) Higher maternal perceived stress during pregnancy and at child’s age of 45 months was associated with a stronger decrease in the child’s and mother’s emotional well-being during the pandemic;
(H4) The child’s HPA axis regulation at 45 months of age was associated with the change in the child’s emotional well-being during the pandemic;
(H5) Higher PRSs for depression, schizophrenia and loneliness were associated with a stronger decrease in the child’s emotional well-being during the pandemic.
2. Materials and methods
2.1. Sample
This study was part of the longitudinal birth cohort study “POSEIDON” (Pre-, Peri-, and Postnatal Stress: Epigenetic Impact on Depression) examining prenatal stress, health and child development. At three obstetric clinics in the Rhine-Neckar Region of Germany, 410 pregnant women about 4–8 weeks prior to delivery were recruited for the first study wave (T1) from October 2010 to March 2013. Five waves have been conducted so far: during the third trimester of pregnancy (T1), within a few days after childbirth (T2), six months postpartum (T3), 45 months postpartum (T4) and during the COVID-19 pandemic, when children were 7–10 years old (T5). At T4, dropouts were replaced by 101 children and their parents. Details on the recruiting process, inclusion criteria, and sample characteristics have been described previously (Send, Bardtke, Gilles, Wolf, Sütterlin, Kirschbaum, et al., Citation2019; Send et al., Citation2017).
The present work used data from study waves T1, T4, and T5. In Germany, strict restrictions were enacted in March 2020 at the beginning of the pandemic. Restrictions were slowly eased starting mid-April and reinstated in November 2020. The current study wave T5 was conducted using two repeated assessments: Baseline T5a took place between July and October 2020, while follow-up T5b took place between November 2020 and February 2021. Therefore, T5a corresponds to a phase with rather low COVID case numbers, and already partially eased restrictions after the peak of the first wave of the pandemic, and T5b to a phase characterized by stricter measures implemented to counteract the second wave of the pandemic. A total of 482 children and their parents who were part of the POSEIDON cohort in at least one study wave were invited to participate again. Of these 482 children and parents, 264 participated. The final sample comprised 263 children at T5a and 169 at T5b, and 241 mothers at T5a and 160 at T5b. Some mothers only answered questionnaires about their children, giving rise to a mismatch between the number of included children and mothers. For each analysis, only subjects with complete information of the respective measures were included. An overview of numbers of participants and samples included in the analysis is displayed in .
Figure 1. Overview of the included POSEIDON study waves: the flow diagram indicates the number of subjects participating in the POSEIDON study at the T1, T4, T5a and T5b time points, along with the number of samples that were included in the statistical analysis.
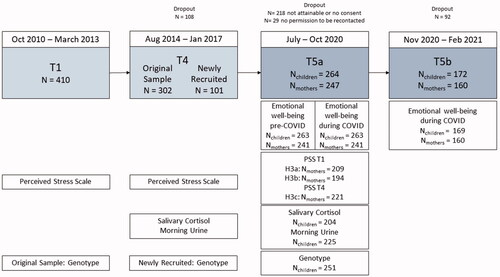
The Ethics Committee of the Medical Faculty Mannheim of the University of Heidelberg approved the study. Before participation, parents of all families provided written informed consent.
2.2. Procedure
Parents were contacted in July–August 2020 by telephone and asked whether they would be interested in participating in a short online survey. Parents who were not reachable by phone were approached by e-mail or through postal mail. If the family agreed to participate, they were provided with detailed information about the study and asked for their written informed consent. Parents who agreed to participate in the survey received an invitation by email, along with the link to the online survey and provided written consent. Participants who were either not reachable by e-mail or who preferred a paper-pencil questionnaire, were sent questionnaires by postal service. A follow-up survey was sent out between November and December 2020 to all subjects who had participated in the first online survey (T5a) and agreed to be recontacted.
Each family received three questionnaires. The primary caregiver was asked to complete the parent caregiver version of the CRISIS questionnaire (for measures, see 2.3.1) about the child. In addition, both parents were invited to answer the adult self-report version of the CRISIS. The online survey was created with REDCap, a web application for building and managing online surveys (Harris et al., Citation2019). Study data were collected and managed using REDCap electronic data capture tools.
2.3. Measures
2.3.1. Emotional well-being T5
Emotional well-being was assessed with the “Adult self-report” and the “Parent caregiver report” versions of the emotional well-being questionnaire of the “CRISIS—The Coronavirus Health Impact Survey” (V0.2) (Merikangas et al., Citation2020). These questionnaires are licensed and available at crisissurvey.org. The emotional well-being scale consists of 10 items on a 5-point Likert scale, adapted from the circumplex model of affect (Larsen & Diener, Citation1992; Posner et al., Citation2005). Construct validity of the CRISIS questionnaire has been shown in other samples (Nikolaidis et al., Citation2021). In the first part of this study wave (T5a), children’s and parents’ emotional state three months prior to the pandemic was assessed retrospectively (referred to as T5 pre) as well as the current emotional state during the pandemic. At the second time point (T5b), the current emotional state during the pandemic was assessed. As only a relatively small proportion of fathers answered the questionnaires, the present analyses include the data from mothers and children only.
2.3.2. Maternal perceived stress T1 & T4
At T1, prenatal perceived stress of the mothers was assessed with the Perceived Stress Scale (PSS) (Cohen et al., Citation1983). The self-report questionnaire consists of 14 items measuring the experienced level of stress during the last month (of pregnancy). Higher values regarding the sum score of all items indicate higher perceived stress. At T4 the perceived stress of the mothers was assessed again using the PSS. For families who were newly recruited at T4, no PSS data from T1 was available (see ). During the pandemic, the PSS was not assessed.
2.3.3. HPA axis measures T4
The nocturnal activity of the HPA axis was assessed through the cortisol concentration in the morning urine of the children at T4. Details are described elsewhere (Send, Bardtke, Gilles, Wolf, Sütterlin, Wudy, et al., Citation2019).
Stress reactivity was operationalized by salivary cortisol concentration before, 10, 30 and 40 minutes after finishing the stress test at T4. Briefly, the children and the researcher played a game, where the children had to attach colored magnets to a matching animal. A red stop light indicated that the time was up. The researcher switched the light remotely before the children could finish. Therefore, the children experienced failure and received negative feedback repeatedly. Full details are described elsewhere (Send, Bardtke, Gilles, Wolf, Sütterlin, Kirschbaum, et al., Citation2019).
While the urine cortisol measure represents an integrative measure of basal nocturnal HPA axis activity, the salivary measures were used to calculate an area under the curve (AUCi; see below), a measure of the reactivity of the HPA axis to an acute stressor.
2.3.4. Polygenic risk scores
For children of the original cohort, DNA was extracted from cord blood. For children newly recruited at T4, DNA was extracted from saliva using the ORAgene sampling kit (DNA Genotek, Ottawa, Ontario, Canada). The Illumina Psych Array, V1.0 for original cohort and V1.3 for newly recruited children (Illumina, Inc., San Diego, CA, USA), was used for genome-wide genotyping. Quality control and filtering was performed using PLINK 1.9 (Chang et al., Citation2015), according to recommendations published in Turner et al. (Citation2011). We removed participants with a mismatch between phenotypic and genotypic sex, > 0.02 missingness, or a heterozygosity rate > |0.2|. We removed SNPs with a minor allele frequency (MAF) of < 0.01, missing data > 0.02, or deviating from Hardy-Weinberg equilibrium (HWE) with a p-value < 10−6.
A SNP set filtered for high quality SNPs (MAF > 0.20, missingness = 0, HWE p > .02) and LD pruning (pairwise r2 < 0.1 within a 200 SNP window) was used to filter for relatedness and population structure and cryptically related (π̂ > 0.20) subjects were excluded at random. Principal components were generated to control for population stratification and to remove outliers (> 6 SD on one of the first 20 principal components). After quality control, genotypic data was available for 251 subjects, who also had T5 data.
PRSice 2.1.6 (Choi & O’Reilly, Citation2019) was used to calculate PRS, including GWAS SNPs with an info score > 0.9, and using a p-value threshold of nominal significance (p < .05). Besides PRSs for depression (PRS-Depression), we calculated PRS for schizophrenia (PRS-Schizophrenia) and loneliness (PRS-Loneliness), which have previously been linked to depressive symptoms and well-being (Day et al., Citation2018; Okbay et al., Citation2016). The PRS-Depression was based on GWAS summary statistics from Howard et al. (Citation2019), excluding German samples, the PRS-Schizophrenia, which was based on Ripke (Ripke et al., Citation2014) and the PRS-Loneliness was based on Day (Day et al., Citation2018).
2.4. Statistical analysis
All statistical analyses were performed in the R statistical environment version 3.5.1. For emotional well-being, inverted items were recoded and mean scores were calculated for the three time points, with higher value corresponding to better emotional well-being (range 1–5). Cronbach’s alpha was calculated for the CRISIS emotional well-being scale for children and mother at each assessed time-point (range 0.82–0.88). For perceived stress, a total sum score of the PSS was calculated (Cohen et al., Citation1983). If only one item was missing, it was imputed with the mean of the other 13 items. Internal reliability was determined by calculating Cronbach’s alpha at T1 (0.88) and T4 (0.85).
Morning urinary cortisol concentrations were corrected for urinary creatinine concentration by dividing cortisol by creatinine concentration. As the urinary cortisol concentrations corrected for creatinine and the salivary cortisol concentrations at the four time points were skewed to the right, log10-transformations were applied, and successfully reduced skewness. The area under the curve with respect to increase (AUCi) was calculated (Pruessner et al., Citation2003) in relation to baseline cortisol concentration. AUCi reflects the course of cortisol concentration in response to stress induction. Outliers three standard deviations above or below the mean were winsorized to the closest value within three standard deviations.
To investigate emotional well-being over time, linear mixed models were calculated and means and differences between the time points were estimated using estimated marginal means (H1). As a sensitivity analysis, we repeated the models adding the time interval between T5a and T5b as a covariate. Pearson correlations were performed to test associations between the child’s and the mother’s emotional well-being (H2). Linear multiple regression analysis was used to investigate the relationship between perceived stress as independent variable and the change in the emotional well-being of the child (H3a) and the mother (H3bc) as dependent variable. For maternal perceived stress, the child’s sex (H3a) and age (H3abc) were added as covariates because of possible influences of these variables on emotional well-being.
To investigate the relationship between the HPA axis measures and the change in the child’s well-being, the child’s AUCi and the child’s urinary cortisol level were tested as independent variables and the change in the emotional well-being as dependent variable (H4) in linear regression models. For the AUCi, time of measurement was added as a covariate. For urinary cortisol, the time of urine sample and whether the children wore a diaper were included as covariates.
Linear regression models were calculated to explore an association between the PRS-Depression, PRS-Schizophrenia, PRS-Loneliness as independent variables and the change in the emotional well-being during the pandemic as the dependent variable, correcting for the first five genetic principal components, age and sex (H5).
The significance level was set to p < .05. Subjects were excluded pairwise from the respective analyses when needed.
A post-hoc power analysis was calculated for the primary hypothesis, changes in emotional well-being of children over the three assessed time points, using GPower 3.1.9.7 (Faul et al., Citation2009). With a total sample size of n = 169, an observed effect of f = 0.24, a mean correlation of repeated measures of r ∼ 0.6 and three measurements, the achieved power for this analysis was > 0.99.
3. Results
3.1. Descriptive statistics
An overview of variables is provided in . From a total of N = 264 children and N = 247 mothers, n = 1 child and n = 6 mothers were excluded from the analyses in T5a due to missing values. From a total of N = 172 children and N = 160 mothers, n = 3 children were excluded from the analyses in T5b due to missing values. Mean age of the children at T5a was M = 7.75 (SD = 0.71) years and at T5b M = 8.02 (SD = 0.76) years. Mean age of the mothers at T5a was M = 40.73 (SD = 4.49) years and at T5b M = 40.94 (SD = 4.62) years. Data on morning urine cortisol was available for n = 226 children and data on salivary cortisol (AUCi) for n = 205 children. Regarding maternal perceived stress, data from n = 210 mothers for T1 and n = 241 for T4 was included in the sample.
Table 1. Descriptive statistics.
3.2. H1: Emotional well-being in course of the pandemic
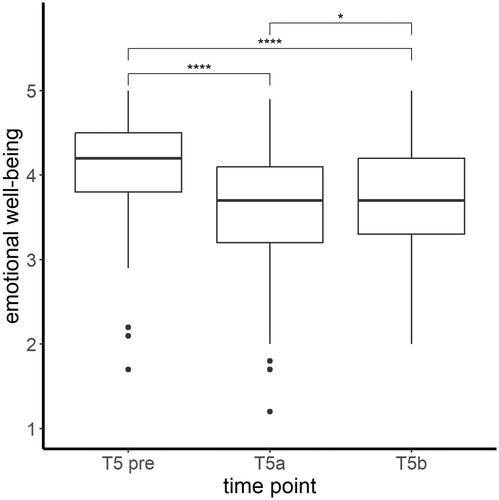
The children’s emotional well-being differed significantly before and over the course of the pandemic (time: Χ2(2, N = 169) = 152.79, p < .001), as depicted in . A worse emotional well-being during the pandemic (T5a) compared to before the pandemic (T5 pre) was observed (t(336) = 11.82, p < .001). In the course of the pandemic (T5b) the emotional well-being of the children improved compared to the beginning of the pandemic (T5a) (t(336) = −2.77, p = .006), but was still worse compared to before the pandemic (t(336) = 9.05, p < .001).
Figure 2. Mean levels and range of emotional well-being (CRISIS score) of the children at all three assessed time points. Significant differences in post hoc tests ****p < .001; *p < .05.
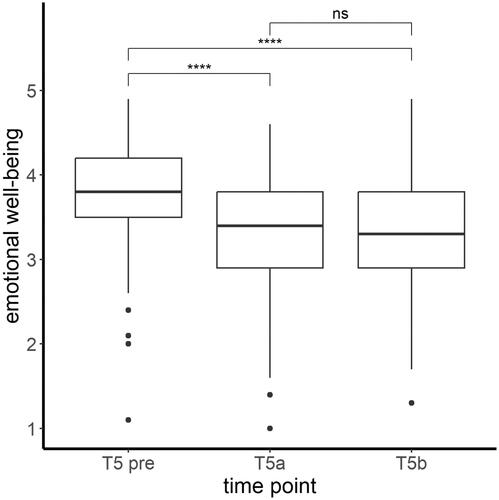
The same overall effect of emotional well-being was observed for mothers as the ANOVA showed significant differences in the mothers’ emotional well-being before and during the pandemic (time: Χ2(2, N = 158) = 135.11, p < .001), as depicted in . A lower emotional well-being was observed when comparing T5a (t(314) = 10.28, p < .001) and T5b (t(314) = 9.84, p < .001) to well-being before the pandemic (T5 pre). In the course of the pandemic (T5a to T5b), the emotional well-being of the mothers did not change significantly (t(314) = −0.44, p = .66).
Figure 3. Mean levels and range of emotional well-being (CRISIS score) of the mothers at all three assessed time points. Significant differences in post hoc tests ****p < .001; ns = not significant.
Sensitivity analyses did not indicate a significant influence of time interval between T5a and T5b on well-being of the children and mothers over the three assessed time points (all p > .5) and results did not change substantially when including it as a covariate to the analyses.
3.3. H2: Association between the mother’s and the child’s emotional well-being
The emotional well-being of the mother and child was positively associated at all time points, as shown by correlation coefficients of moderate size: T5 pre (n = 241, r = 0.43, p < .001), T5a (n = 241, r = 0.59, p < .001) and T5b (n = 156, r = 0.49, p < .001).
3.4. H3: Association between maternal perceived stress and change in the emotional well-being
H3a: The model predicting the change in the child’s emotional well-being during the pandemic with prenatal perceived stress of the mother at T1, age and sex of the child explained 6% of the variance of the change in the child’s emotional well-being (F(3, 205) = 4.58, p = .004, R2 = 0.063). Regression parameters are shown in . Prenatal perceived stress of the mother at T1 significantly predicted the change in the child’s emotional well-being (β= -0.018; p < .001), with higher PSS values at T1 being associated with a stronger decrease in child’s well-being during T5a compared to before the pandemic (T5 pre).
H3b: The model predicting the change in the mother’s emotional well-being during the pandemic with prenatal perceived stress at T1 was not significant (F(2, 191) = 1.229, p = .29, R2 = 0.013).
H3c: Furthermore, the model predicting the change in the mother’s emotional well-being during the pandemic with perceived stress at T4 was not significant (F(2, 218) = 0.89, p = .41, R2 = 0.008).
Table 2. Parameter estimation to predict change in the child’s emotional well-being.
3.5. H4: Association between HPA axis reactivity and the change in the child’s emotional well-being
The model predicting the change in the child’s emotional well-being with cortisol concentration in morning urine was not significant (F(3, 221) = 0.46; p = .71, R2 = 0.006). The model predicting the change in the child’s emotional well-being with AUCi was not significant (F(2, 201) = 0.51; p = .60, R2 = 0.005).
3.6. H5: Association between polygenic risk for MDD, schizophrenia and loneliness and the change in the child’s emotional well-being
The change in the child’s emotional well-being during the pandemic could not be predicted by PRS for depression (F(8, 242) = 0.62; p = .76, R2 = 0.02), schizophrenia (F(8, 242) = 0.64; p = .74, R2 = 0.02) or loneliness (F(8, 242) = 0.65; p = .73, R2 = 0.02).
4. Discussion
The present study investigated the well-being of mothers and children during the COVID-19 pandemic, as well as its relation to prenatal perceived stress, early childhood HPA axis activity, and polygenic risk.
Consistent with our hypotheses (H1), the emotional well-being of children and mothers worsened during the pandemic compared to before the pandemic. The children’s and the mothers’ emotional well-being worsened at the beginning of the pandemic compared to pre-pandemic but the children’s emotional well-being improved during the second part of this study. Other studies have also shown that emotional well-being worsened in adults (Adams et al., Citation2021; Daly et al., Citation2022; Fancourt et al., Citation2021) and children (Bignardi et al., Citation2020; Ravens-Sieberer et al., Citation2020) at the beginning of the pandemic. There have been inconsistent findings considering emotional well-being over the course of the pandemic. Our results are in line with previous results showing improvement of emotional well-being among children (Raw et al., Citation2021) as the pandemic went by.
When interpreting the results, it is important to consider the circumstances, e.g. whether there was a lockdown and how strict restrictions were. Restrictions during the first assessment starting in July 2020, were already partly eased in Germany. During the second assessment beginning in November 2020, the second lockdown began, accompanied by more restrictions. On account of increasing coronavirus infections, a “hard lockdown” followed in December. Therefore, adults and children were affected by more restricting circumstances during the second assessment compared to the first. In addition, seasonal effects may have negatively affected emotional well-being, as the second assessment took place in winter, from November 2020 to February 2021 (Meesters & Gordijn, Citation2016). Another aspect could be that mothers already had additional difficulties with parenting (Spinelli et al., Citation2020) for a long period since the first lockdown. The combination of strict restrictions, depressive symptoms caused by seasonal effects and parenting difficulties could explain a worse emotional well-being in adults at both assessed time points during the COVID-19 pandemic.
Interestingly, children had a better emotional well-being in the second assessment compared to the first. This finding might be explained by adaptation to their environment and developing better coping mechanisms over the course of the pandemic. Also the social environment, e.g. families and schools, may have developed better strategies to support children compared to the beginning of the pandemic (Masten & Motti-Stefanidi, Citation2020).
A moderate association between the emotional well-being of the mother and the child during the pandemic (H2) was observed. This association has been shown by several studies (Russell et al., Citation2020; Spinelli et al., Citation2020) underlining that a parent’s well-being is a mediator for children’s emotional and behavioral problems (Achterberg et al., Citation2021). Especially during stressful times like the COVID-19 pandemic, parents play an important role for the mental health of their children (Russell et al., Citation2020).
A significant association between the prenatal perceived stress of the mother and the change in the emotional well-being of the child (H3a) was found. Previous findings have shown that prenatal stress has an impact on children’s development and health (van den Bergh et al., Citation2020). Our findings suggest that prenatal maternal stress has an impact on children’s well-being several years later in life, particularly under stressful circumstances such as the periods studied here. Recent studies have examined the association between prenatal stress and children’s mental health starting in the pregnancy during the pandemic (Buthmann et al., Citation2022; Duguay et al., Citation2022; Provenzi et al., Citation2023). Therefore these studies are of children who are much younger compared to our present sample; our results from 7 to 10 year old children add to the literature. In order to examine how children’s development and mental health are affected by prenatal maternal health later in life, subsequent study waves should investigate this association in the present sample at later stages.
There was no significant association between the perceived stress of the mother assessed in previous study waves and the change in the emotional well-being of the mother (H3b and c). Results from another longitudinal study suggest that increases in depression and anxiety symptoms during the COVID-19 pandemic in mothers occurred universally, regardless of previous mental health history (Racine et al., Citation2021). Depending on current life circumstances, the amount of stress experienced, and its perception and response to it can vary. For example, during pregnancy (T1) and when children are at preschool age (T4) different stressors may have impacted the mothers’ perceived stress, but could be limited to the existing circumstances at the assessed time.
There was no significant association between the change in the emotional well-being of the child and the measures of HPA axis activity at 45 months (H4). In the same longitudinal birth cohort, prenatal stress was shown to be associated with a hyporegulation of the children’s HPA axis, as indicated by lower cortisol levels after a stress test (Send, Bardtke, Gilles, Wolf, Sütterlin, Kirschbaum, et al., Citation2019) and lower cortisol and cortisone levels in the first morning urine of the then 45 month-old children (Send, Bardtke, Gilles, Wolf, Sütterlin, Wudy, et al., Citation2019). Various factors influencing the emotional well-being during the COVID-19 pandemic could lead to intraindividual changes, such as HPA axis reactivity. Interestingly, other studies found that loneliness during the COVID-19 pandemic had been associated with higher levels of cortisol (Haucke et al., Citation2022; Jopling et al., Citation2021). Pandemic related circumstances may influence diurnal cortisol patterns, which may be a reason why no effect of previous HPA axis activity was found. Including cortisol measurements in future assessments would allow the exploration of the development of the HPA axis reactivity during childhood and adolescence.
PRSs did not significantly predict the change in the child’s emotional well-being during the pandemic (H5). It has to be taken into account that the application of PRSs is limited to the underlying GWAS and that to date even large GWASs in psychiatric genetics are still underpowered (Wray et al., Citation2014). Also, environmental factors such as isolation and school closures could have a larger effect on well-being than genetic risk factors.
The present study has several limitations. First, the children did not answer the questions themselves. The main parent caregiver, in most cases the mother, answered the questions about the child, leading to possible bias as answers about the emotional well-being of the child are influenced by the caregiver’s perspective and his/her own mental distress (De Los Reyes & Kazdin, Citation2005). In particular, the observed association between the mothers’ and the children’s well-being could be partially driven by that bias. Second, the questionnaires assessing the three months prior to the pandemic were answered retrospectively, making that assessment prone to recall biases. Third, our sample had limited diversity (mostly high educational background and high socio-economic status) and may not be representative of the general population. In line with this, it has to be noted that the levels of PSS assessed at T1 and T4 indicate that the mothers did not experience high levels of stress on average. Fourth, while we investigated a set of variables of interest, and adjusted our analysis for relevant technical covariates, and age and sex of the children, further variables might have influenced the observed results, such as actual COVID-19 infections, having lost a job; or other measures on the child such as resilience, quality of attachment or cognitive aspects. In addition, it has to be considered that the time interval between T4 and T5 was relatively long, and therefore it is possible that adverse events prior and unrelated to the pandemic might also have had an influence on the results. Moreover, the PSS score of the mothers during the pandemic was not assessed and only previous assessed PSS measures could be included in the analysis. Additionally, as there was less data available from fathers than from mothers, the present study only included the mothers’ data.
The present part of the longitudinal birth cohort study assesses children’s emotional well-being during the COVID-19 pandemic and its association to stress linked predictors. The study contributes to the emerging evidence that mental health of children and adults is affected by the COVID-19 pandemic. Furthermore it indicates that the well-being of the children and their mothers was linked at each assessed time point. That underlines the importance of a strong parent-child relationship, especially during stressful times it can strengthen mental health and development of children. Therefore supporting parents’ mental health is really important and should be enhanced. Moreover, the results suggest that higher prenatal maternal stress is associated with a stronger decrease in children’s well-being at the age of 7 to 10 years. This is an important finding and provides a link for future studies investigating the influence of prenatal stress on the development of children. Due to the longitudinal study model, it is possible to assess various predictors and set findings in relation to previous assessments of this cohort. The results contribute to a more comprehensive understanding of stress. While in the present study, associations with chosen genetic and endocrine predictors were not significant, differential results might be observed in children and adolescents of different age groups. Additionally, while not significant, these results add to the literature, where significant results might be overrepresented, e.g. due to publication bias. Further, it is of interest to investigate whether the experience of the pandemic has an impact on the health and development of the children in the future, especially in regard to their stress perception and endocrine stress regulation.
Author contributions
Substantial contributions to the conception or design of the study, or the acquisition of data, or analysis and interpretation of data: T.N., L.Z, M.C., A.S.M.H., J.C.F., L.S., J.F., T.S.S., M.G., M.R., M.D., F.S, S.H.W.; Drafting the article or revising it critically for important intellectual content: T.N., L.Z., J.C.F., S.H.W., F.S; All authors approved the final version of the manuscript.
Acknowledgements
We thank all parents and children for taking part in this study and our student employees and interns for their support with data acquisition and data entry.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Funding
Notes on contributors
Thao Nguyen
Thao Nguyen is a medical student with a clinical and research interest in mental health. As part of her doctoral thesis, she planned, carried out, and analyzed the COVID assessment in the POSEIDON study at the Central Institute of Mental Health (CIMH) in Mannheim.
Lea Zillich
Lea Zillich is a psychologist with a background in bioinformatics. Her research at the CIMH focuses on multi-omics studies in psychiatric disorders, particularly on substance use disorders. She planned and executed the COVID recruitment and analysis of the POSEIDON study together with Thao Nguyen.
Metin Cetin
Metin Cetin studies psychology (M.Sc.) at the University of Trier. His research interest is in the risk factors for mental disorders. He contributed to the COVID assessment waves in the POSEIDON study.
Alisha S. M. Hall
Alisha S. M. Hall is a PhD student at Aarhus University. Her background is in psychology and epidemiology, and her current research focuses on the genetic and environmental risk factors of personality disorders.
Jerome C. Foo
Jerome C. Foo has a background in psychology and neuroscience. His research focuses on psychiatric disorders and adolescent mental health. He is interested in employing various longitudinal ambulatory assessment methods to study disease dynamics.
Lea Sirignano
Lea Sirignano is a psychologist with a background in psychiatric genetics and ambulatory assessment. Within the POSEIDON birth cohort, she is investigating the genetic and epigenetic correlates of mental health.
Josef Frank
Josef Frank is a psychologist and biostatistician. His work encompasses the identification of genotype-phenotype associations in psychiatric diseases, epigenetics, and prediction modeling.
Tabea S. Send
Tabea S. Send is a psychological psychotherapist active in research and teaching. Her research focuses on stress and the development of mental disorders. In her seminars, trainings, and courses, she combines scientific findings with didactically appropriate concepts suitable for everyday life.
Maria Gilles
Maria Gilles is a senior physician at the Department of Psychiatry and Psychotherapy and co-head of the Stress-Related Disorders Research Group at the CIMH. She is a principal investigator of the POSEIDON study.
Marcella Rietschel
Marcella Rietschel is a psychiatrist and head of the Department of Genetic Epidemiology in Psychiatry at the CIMH Mannheim. Her research areas include the identification of genetic and environmental risk factors for psychiatric disease, phenotype-genotype correlations across diagnostic categories, as well as ethics in psychiatric-genetic research. She is a principal investigator of the POSEIDON study.
Michael Deuschle
Michael Deuschle is the senior physician at the Department of Psychiatry and Psychotherapy and head of the Stress-Related Disorder Research Group at the CIMH. Together with Marcella Rietschel and Maria Gilles, he initiated the POSEIDON study and is the principal investigator. He is currently also leading the Mental Health First Aid (MHFA) initiative in Mannheim.
Stephanie H. Witt
Stephanie H. Witt is a psychologist by training and molecular biologist. One of her research interests is the influence of environmental factors such as (early life) stress on disease vulnerability. In POSEIDON, she is responsible for the molecular genetic assessments, and plans and supervises the respective analyses.
Fabian Streit
Fabian Streit has a background in psychology, neuroscience, psychoneuroendocrinology, and psychiatric genetics. His aim is to apply methods from those fields to gain a better understanding of the factors influencing the risk of developing mental health disorders, with a focus on stress-related disorders. He is one of the main analysts in the POSEIDON study.
References
- Achterberg, M., Dobbelaar, S., Boer, O. D., & Crone, E. A. (2021). Perceived stress as mediator for longitudinal effects of the COVID-19 lockdown on wellbeing of parents and children. Scientific Reports, 11(1), 1. https://doi.org/10.1038/s41598-021-81720-8
- Adams, E. L., Smith, D., Caccavale, L. J., & Bean, M. K. (2021). Parents are stressed! Patterns of parent stress across COVID-19. Frontiers in Psychiatry, 12, 626456. https://doi.org/10.3389/fpsyt.2021.626456
- Agorastos, A., & Chrousos, G. P. (2022). The neuroendocrinology of stress: The stress-related continuum of chronic disease development. Molecular Psychiatry, 27(1), 502–10. https://doi.org/10.1038/s41380-021-01224-9
- Ahrens, K. F., Neumann, R. J., von Werthern, N. M., Kranz, T. M., Kollmann, B., Mattes, B., Puhlmann, L. M. C., Weichert, D., Lutz, B., Basten, U., Fiebach, C. J., Wessa, M., Kalisch, R., Lieb, K., Chiocchetti, A. G., Tüscher, O., Reif, A., & Plichta, M. M. (2022). Association of polygenic risk scores and hair cortisol with mental health trajectories during COVID lockdown. Translational Psychiatry, 12(1), 1–10. https://doi.org/10.1038/s41398-022-02165-9
- Bignardi, G., Dalmaijer, E. S., Anwyl-Irvine, A. L., Smith, T. A., Siugzdaite, R., Uh, S., & Astle, D. E. (2020). Longitudinal increases in childhood depression symptoms during the COVID-19 lockdown. Archives of Disease in Childhood, 106(8), 791–797. https://doi.org/10.1136/archdischild-2020-320372
- Bussières, E. L., Malboeuf-Hurtubise, C., Meilleur, A., Mastine, T., Hérault, E., Chadi, N., Montreuil, M., Généreux, M., & Camden, C. (2021). Consequences of the COVID-19 pandemic on children’s mental health: A meta-analysis. Frontiers in Psychiatry, 12, 691659. https://doi.org/10.3389/fpsyt.2021.691659
- Buthmann, J. L., Miller, J. G., & Gotlib, I. H. (2022). Maternal–prenatal stress and depression predict infant temperament during the COVID-19 pandemic. Development and Psychopathology, 2022, 1–9. https://doi.org/10.1017/S0954579422001055
- Chang, C. C., Chow, C. C., Tellier, L. C., Vattikuti, S., Purcell, S. M., & Lee, J. J. (2015). Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience, 4(1), 8. https://doi.org/10.1186/s13742-015-0047-8
- Choi, S. W., & O’Reilly, P. F. (2019). PRSice-2: Polygenic Risk Score software for biobank-scale data. GigaScience, 8(7), giz082. https://doi.org/10.1093/gigascience/giz082
- Cobham, V. E., McDermott, B., Haslam, D., & Sanders, M. R. (2016). The role of parents, parenting and the family environment in children’s post-disaster mental health. Current Psychiatry Reports, 18(6), 53. https://doi.org/10.1007/s11920-016-0691-4
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. https://doi.org/10.2307/2136404
- Cusinato, M., Iannattone, S., Spoto, A., Poli, M., Moretti, C., Gatta, M., & Miscioscia, M. (2020). Stress, resilience, and well-being in italian children and their parents during the COVID-19 pandemic. International Journal of Environmental Research and Public Health, 17(22), 8297. https://doi.org/10.3390/ijerph17228297
- Daly, M., Sutin, A. R., & Robinson, E. (2022). Longitudinal changes in mental health and the COVID-19 pandemic: Evidence from the UK Household Longitudinal Study. Psychological Medicine, 52(13), 2549–2558. https://doi.org/10.1017/S0033291720004432
- Day, F. R., Ong, K. K., & Perry, J. R. B. (2018). Elucidating the genetic basis of social interaction and isolation. Nature Communications, 9(1), 2457. https://doi.org/10.1038/s41467-018-04930-1
- De Los Reyes, A., & Kazdin, A. E. (2005). Informant discrepancies in the assessment of childhood psychopathology: A critical review, theoretical framework, and recommendations for further study. Psychological Bulletin, 131(4), 483–509. https://doi.org/10.1037/0033-2909.131.4.483
- Duguay, G., Garon-Bissonnette, J., Lemieux, R., Dubois-Comtois, K., Mayrand, K., & Berthelot, N. (2022). Socioemotional development in infants of pregnant women during the COVID-19 pandemic: the role of prenatal and postnatal maternal distress. Child and Adolescent Psychiatry and Mental Health, 16(1), 28. https://doi.org/10.1186/s13034-022-00458-x
- Fancourt, D., Steptoe, A., & Bu, F. (2021). Trajectories of anxiety and depressive symptoms during enforced isolation due to COVID-19 in England: A longitudinal observational study. The Lancet. Psychiatry, 8(2), 141–149. https://doi.org/10.1016/s2215-0366(20)30482-x
- Faul, F., Erdfelder, E., Buchner, A., & Lang, A.-G. (2009). Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. https://doi.org/10.3758/BRM.41.4.1149
- Harris, P. A., Taylor, R., Minor, B. L., Elliott, V., Fernandez, M., O’Neal, L., McLeod, L., Delacqua, G., Delacqua, F., Kirby, J., & Duda, S. N. (2019). The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics, 95, 103208. https://doi.org/10.1016/j.jbi.2019.103208
- Haucke, M., Golde, S., Saft, S., Hellweg, R., Liu, S., & Heinzel, S. (2022). The effects of momentary loneliness and COVID-19 stressors on hypothalamic–pituitary adrenal (HPA) axis functioning: A lockdown stage changes the association between loneliness and salivary cortisol. Psychoneuroendocrinology, 145, 105894. https://doi.org/10.1016/j.psyneuen.2022.105894
- Howard, D. M., Adams, M. J., Clarke, T.-K., Hafferty, J. D., Gibson, J., Shirali, M., Coleman, J. R. I., Hagenaars, S. P., Ward, J., Wigmore, E. M., Alloza, C., Shen, X., Barbu, M. C., Xu, E. Y., Whalley, H. C., Marioni, R. E., Porteous, D. J., Davies, G., Deary, I. J., … McIntosh, A. M. (2019). Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nature Neuroscience, 22(3), 343–352. https://doi.org/10.1038/s41593-018-0326-7
- Jopling, E., Rnic, K., Tracy, A., & LeMoult, J. (2021). Impact of loneliness on diurnal cortisol in youth. Psychoneuroendocrinology, 132, 105345. https://doi.org/10.1016/j.psyneuen.2021.105345
- Kudielka, B. M., Hellhammer, D. H., & Wüst, S. (2009). Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology, 34(1), 2–18. https://doi.org/10.1016/j.psyneuen.2008.10.004
- Kwong, A. S. F., Morris, T. T., Pearson, R. M., Timpson, N. J., Rice, F., Stergiakouli, E., & Tilling, K. (2021). Polygenic risk for depression, anxiety and neuroticism are associated with the severity and rate of change in depressive symptoms across adolescence. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 62(12), 1462–1474. https://doi.org/10.1111/jcpp.13422
- Larsen, R., & Diener, E. (1992). Review of Personality and Social Psychology, No. 13. Emotion.
- Levinson, D. F. (2006). The genetics of depression: A review. Biological Psychiatry, 60(2), 84–92. https://doi.org/10.1016/j.biopsych.2005.08.024
- Masten, A. S., & Motti-Stefanidi, F. (2020). Multisystem resilience for children and youth in disaster: Reflections in the context of COVID-19. Adversity and Resilience Science, 1(2), 95–106. https://doi.org/10.1007/s42844-020-00010-w
- McIntosh, A. M., Sullivan, P. F., & Lewis, C. M. (2019). Uncovering the genetic architecture of major depression. Neuron, 102(1), 91–103. https://doi.org/10.1016/j.neuron.2019.03.022
- Meesters, Y., & Gordijn, M. (2016). Seasonal affective disorder, winter type: Current insights and treatment options. Psychology Research and Behavior Management, 9, 317–327. https://doi.org/10.2147/PRBM.S114906
- Merikangas, K., Milham, M., & Stringaris, A. (2020). The CoRonavIrus Health Impact Survey (CRISIS). Retrieved November 17, 2021 from http://www.crisissurvey.org/download/
- Nikolaidis, A., Paksarian, D., Alexander, L., Derosa, J., Dunn, J., Nielson, D. M., Droney, I., Kang, M., Douka, I., Bromet, E., Milham, M., Stringaris, A., & Merikangas, K. R. (2021). The Coronavirus Health and Impact Survey (CRISIS) reveals reproducible correlates of pandemic-related mood states across the Atlantic. Scientific Reports, 11(1), 8139. https://doi.org/10.1038/s41598-021-87270-3
- O’Connor, D. B., Thayer, J. F., & Vedhara, K. (2021). Stress and health: A review of psychobiological processes. Annual Review of Psychology, 72(1), 663–688. https://doi.org/10.1146/annurev-psych-062520-122331
- Okbay, A., Baselmans, B. M., De Neve, J. E., Turley, P., Nivard, M. G., Fontana, M. A., Meddens, S. F., Linnér, R. K., Rietveld, C. A., Derringer, J., Gratten, J., Lee, J. J., Liu, J. Z., de Vlaming, R., Ahluwalia, T. S., Buchwald, J., Cavadino, A., Frazier-Wood, A. C., Furlotte, N. A., … Cesarini, D. (2016). Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nature Genetics, 48(6), 624–633. https://doi.org/10.1038/ng.3552
- Perry, N. B., Donzella, B., Troy, M. F., & Barnes, A. J. (2022). Mother and child hair cortisol during the COVID-19 pandemic: Associations among physiological stress, pandemic-related behaviors, and child emotional-behavioral health. Psychoneuroendocrinology, 137, 105656. https://doi.org/10.1016/j.psyneuen.2021.105656
- Posner, J., Russell, J. A., & Peterson, B. S. (2005). The circumplex model of affect: An integrative approach to affective neuroscience, cognitive development, and psychopathology. Development and Psychopathology, 17(3), 715–734. https://doi.org/10.1017/s0954579405050340
- Provenzi, L., Grumi, S., Altieri, L., Bensi, G., Bertazzoli, E., Biasucci, G., Cavallini, A., Decembrino, L., Falcone, R., Freddi, A., Gardella, B., Giacchero, R., Giorda, R., Grossi, E., Guerini, P., Magnani, M. L., Martelli, P., Motta, M., Nacinovich, R., … Borgatti, R. (2023). Prenatal maternal stress during the COVID-19 pandemic and infant regulatory capacity at 3 months: A longitudinal study. Development and Psychopathology, 35(1), 35–43. https://doi.org/10.1017/S0954579421000766
- Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. https://doi.org/10.1016/s0306-4530(02)00108-7
- Racine, N., Hetherington, E., McArthur, B. A., McDonald, S., Edwards, S., Tough, S., & Madigan, S. (2021). Maternal depressive and anxiety symptoms before and during the COVID-19 pandemic in Canada: A longitudinal analysis. The Lancet. Psychiatry, 8(5), 405–415. https://doi.org/10.1016/S2215-0366(21)00074-2
- Ravens-Sieberer, U., Kaman, A., Otto, C., Adedeji, A., Devine, J., Erhart, M., Napp, A. K., Becker, M., Blanck-Stellmacher, U., Löffler, C., Schlack, R., & Hurrelmann, K. (2020). Mental health and quality of life in children and adolescents during the COVID-19 pandemic-results of the Copsy Study. Deutsches Arzteblatt International, 117(48), 828–829. https://doi.org/10.3238/arztebl.2020.0828
- Raw, J. A. L., Waite, P., Pearcey, S., Shum, A., Patalay, P., & Creswell, C. (2021). Examining changes in parent-reported child and adolescent mental health throughout the UK’s first COVID-19 national lockdown. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 62(12), 1391–1401. https://doi.org/10.1111/jcpp.13490
- Ripke, S., Neale, B. M., Corvin, A., Walters, J. T., Farh, K.-H., Holmans, P. A., Lee, P., Bulik-Sullivan, B., Collier, D. A., & Huang, H. (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature, 511(7510), 421–427. https://doi.org/10.1038/nature13595
- Robinson, E., Sutin, A. R., Daly, M., & Jones, A. (2022). A systematic review and meta-analysis of longitudinal cohort studies comparing mental health before versus during the COVID-19 pandemic in 2020. Journal of Affective Disorders, 296, 567–576. https://doi.org/10.1016/j.jad.2021.09.098
- Russell, B. S., Hutchison, M., Tambling, R., Tomkunas, A. J., & Horton, A. L. (2020). Initial challenges of caregiving during COVID-19: Caregiver burden, mental health, and the parent-child relationship. Child Psychiatry and Human Development, 51(5), 671–682. https://doi.org/10.1007/s10578-020-01037-x
- Send, T. S., Bardtke, S., Gilles, M., Wolf, I. A. C., Sütterlin, M. W., Kirschbaum, C., Laucht, M., Witt, S. H., Rietschel, M., Streit, F., & Deuschle, M. (2019). Stress reactivity in preschool-aged children: Evaluation of a social stress paradigm and investigation of the impact of prenatal maternal stress. Psychoneuroendocrinology, 101, 223–231. https://doi.org/10.1016/j.psyneuen.2018.11.002
- Send, T. S., Bardtke, S., Gilles, M., Wolf, I. A. C., Sütterlin, M. W., Wudy, S. A., Wang, R., Laucht, M., Witt, S. H., Rietschel, M., Streit, F., & Deuschle, M. (2019). Prenatal maternal stress is associated with lower cortisol and cortisone levels in the first morning urine of 45-month-old children. Psychoneuroendocrinology, 103, 219–224. https://doi.org/10.1016/j.psyneuen.2019.01.017
- Send, T. S., Gilles, M., Codd, V., Wolf, I., Bardtke, S., Streit, F., Strohmaier, J., Frank, J., Schendel, D., Sütterlin, M. W., Denniff, M., Laucht, M., Samani, N. J., Deuschle, M., Rietschel, M., & Witt, S. H. (2017). Telomere length in newborns is related to maternal stress during pregnancy. Neuropsychopharmacology , 42(12), 2407–2413. https://doi.org/10.1038/npp.2017.73
- Spinelli, M., Lionetti, F., Pastore, M., & Fasolo, M. (2020). Parents’ stress and children’s psychological problems in families facing the COVID-19 outbreak in Italy. Frontiers in Psychology, 11, 1713. https://doi.org/10.3389/fpsyg.2020.01713
- Turner, S., Armstrong, L. L., Bradford, Y., Carlson, C. S., Crawford, D. C., Crenshaw, A. T., de Andrade, M., Doheny, K. F., Haines, J. L., Hayes, G., Jarvik, G., Jiang, L., Kullo, I. J., Li, R., Ling, H., Manolio, T. A., Matsumoto, M., McCarty, C. A., McDavid, A. N., … Ritchie, M. D. (2011). Quality control procedures for genome-wide association studies. Current Protocols in Human Genetics, 68(1), 1.19.11–11.19.18. https://doi.org/10.1002/0471142905.hg0119s68
- van den Bergh, B. R. H., van den Heuvel, M. I., Lahti, M., Braeken, M., de Rooij, S. R., Entringer, S., Hoyer, D., Roseboom, T., Räikkönen, K., King, S., & Schwab, M. (2020). Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neuroscience and Biobehavioral Reviews, 117, 26–64. https://doi.org/10.1016/j.neubiorev.2017.07.003
- Wray, N. R., Lee, S. H., Mehta, D., Vinkhuyzen, A. A., Dudbridge, F., & Middeldorp, C. M. (2014). Research review: Polygenic methods and their application to psychiatric traits. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 55(10), 1068–1087. https://doi.org/10.1111/jcpp.12295
