Abstract
Early-life attachment disruption appears to sensitize neuroinflammatory signaling to increase later vulnerability for stress-related mental disorders, including depression. How stress initiates this process is unknown, but studies with adult rats and mice suggest sympathetic nervous system activation and/or cortisol elevations during the early stress are key. Guinea pig pups isolated from their mothers exhibit an initial active behavioral phase characterized by anxiety-like vocalizing. This is followed by inflammatory-dependent depressive-like behavior and fever that sensitize on repeated isolation. Using strategies that have been successful in adult studies, we assessed whether sympathetic nervous system activity and cortisol contributed to the sensitization process in guinea pig pups. In Experiment 1, the adrenergic agonist ephedrine (3 or 10 mg/kg), either alone or with cortisol (2.5 mg/kg), did not increase depressive-like behavior or fever during initial isolation the following day as might have been expected to if this stimulation was sufficient to account for the sensitization process. In Experiment 2, both depressive-like behavior and fever sensitized with repeated isolation, but beta-adrenergic receptor blockade with propranolol (10 or 20 mg/kg) did not affect either of these responses or their sensitization. The high dose of propranolol did, however, reduce vocalizing. These results suggest sympathetic nervous system activation is neither necessary nor sufficient to induce the presumptive neuroinflammatory signaling underlying sensitization of depressive-like behavioral or febrile responses in developing guinea pigs. Thus, processes mediating sensitization of neuroinflammatory-based depressive-like behavior following early-life attachment disruption in this model appear to differ from those previously found to underlie neuroinflammatory priming in adults.
Introduction
A primary focal point of developmental psychobiology and behavioral neuroscience research over the past several decades has been the means by which early-life adversity exerts long-term effects on mental processes. Exposure in childhood to traumatic events, notably those involving some form of attachment disruption (e.g. abuse, neglect, or parental separation), increases vulnerability for a variety of stress-related mental disorders, including depression, anxiety, bipolar disorder, post-traumatic stress disorder (PTSD), and schizophrenia (Feigenson et al., Citation2014; Hammersley et al., Citation2003; Heim & Nemeroff, Citation2001; LeMoult et al., Citation2020). One potential mechanism for this phenomenon is an increase or sensitization of central neuroinflammatory signaling following the early event. The process has been conceptualized as inducing a “proinflammatory phenotype” that responds excessively to later stress, thereby triggering the mental disorder (Ehrlich et al., Citation2016; Slavich & Irwin, Citation2014). In support of this notion, both human and animal studies indicate attachment disruption elevates inflammatory markers at later ages, particularly in the context of later stress (Baumeister et al., Citation2016; Brown et al., Citation2021; Coplan et al., Citation2021; Danese & Lewis, Citation2017; Dutcher et al., Citation2020). Moreover, disruption of central inflammatory activity in rodent models has been found to prevent or reduce some of the pathology-like outcomes of early-life stress (Brenhouse & Andersen, Citation2011; Ganguly et al., Citation2019; Hennessy et al., Citation2022; Wieck et al., Citation2013). And in human populations, depression and schizophrenia have been associated with elevated inflammatory markers selectively in those individuals undergoing early adversity (Danese et al., Citation2008; Miller & Cole, Citation2012; Dennison et al., Citation2012).
One aspect of this process that is still poorly understood is how the early stress initiates the neuroinflammatory signaling and its subsequent sensitization. However, studies in adult rats and mice suggest the stress mediators of elevated sympathetic activity and hypothalamic-pituitary-adrenal (HPA) activation may play primary roles. For instance, adrenergic agonists both enhance the immediate central neuroinflammatory response to footshock (Blandino et al., Citation2006) and, when given alone, sensitize the response to lipopolysaccharide (LPS) 24 hr later through a mechanism involving neuroinflammatory (i.e. microglial) priming (Johnson et al., Citation2013). Further, beta-adrenergic blockade reduces microglial reactivity, proinflammatory cytokine release, and anxiety-like behavior induced by stress (Blandino et al., Citation2006; Wohleb et al., Citation2011; Lovelock & Deak, Citation2019). Thus, heightened sympathetic activity may set the stage for a sufficient stressor later in life to activate the primed microglia, leading to an inflammatory cascade and consequent psychopathology (Brenhouse & Schwarz, Citation2016; Mondelli & Vernon, Citation2019).
In addition, while glucocorticoids have widespread anti-inflammatory effects (Sapolsky et al., Citation2000), they also can underlie stress-induced sensitization of neuroinflammatory signaling. For example, prior exposure to stressors such as restraint or tail shock augments the neuroinflammatory response to LPS, but this increase can be blocked with a glucocorticoid receptor antagonist (de Pablos et al., Citation2014; Fonken et al., Citation2018; Frank et al., Citation2012). Conversely, glucocorticoid elevations can mimic the sensitizing effects of stress on neuroinflammatory signaling. In adult rats, administration of a dose of corticosterone that produces stress-like circulating levels is sufficient to enhance central as well as peripheral proinflammatory cytokine responses to LPS the following day (Frank et al., Citation2010). Thus, glucocorticoids, as well as sympathetic activity, can sensitize neuroinflammatory signaling in adult animals. Nonetheless, this work does not address effects during early development.
The guinea pig offers a well-suited model for addressing the effects of attachment disruption on sensitization of neuroinflammatory mechanisms that may underlie the development of depression. When a preweaning guinea pig is isolated from its mother and littermates in a novel environment, it exhibits both an increase in sympathetic nervous system activity and activation of the hypothalamic-pituitary-adrenal (HPA) axis (Hennessy & Moorman, Citation1989; Hennessy & Ritchey, Citation1987; Hennessy et al., Citation1989). Behaviorally, the initial isolation is characterized primarily by high-pitched whistle vocalizations. After about an hour, pups transition into a passive phase marked by depressive-like behaviors: an immobile crouched stance, extensive piloerection, and protracted eye-closure (Hennessy et al., Citation1995). These stress-induced depressive-like behaviors are not typical of healthy guinea pig pups and are reminiscent of the posture of macaque monkey infants during the “anaclitic depression” or “despair” stage of maternal separation (Kaufman & Rosenblum, Citation1967). Moreover, evidence indicates the depressive-like behaviors are mediated by proinflammatory signaling: injection of LPS produces the same behavioral reaction (Hennessy et al., Citation2011a); separation in a novel environment is accompanied by physiological signs of increased inflammatory activity, including fever (Hennessy et al., Citation2007a; Hennessy et al., Citation2010); and administration of anti-inflammatory compounds prior to separation reduce this behavioral response (Hennessy et al., Citation2007b; Hennessy et al., Citation2015; Perkeybile et al., Citation2009; Schiml-Webb et al., Citation2006). Particularly important in the context of early-life stress, these depressive-like behaviors and fever, but not vocalizations, sensitize on repeated isolations. That is, when guinea pig pups are isolated on two consecutive days, or even days later, they display more depressive-like behavior and a faster developing and greater peak in fever during later isolations than during the initial isolation (Hennessy et al., Citation2010; Schneider et al., Citation2012; Yusko et al., Citation2012). In addition to increasing depressive-like behaviors during the later isolation episodes, earlier isolation has also been found to increase the duration of immobility during the forced swim test (Hennessy et al., Citation2017). Reducing swim-test immobility is a widely used screen for antidepressant medications (Cryan et al., Citation2005; Czéh et al., Citation2016).
Therefore, the current study examined potential stress mediators contributing to depressive-like behavior, fever, and their sensitization in isolated guinea pig pups. Experiment 1 used a strategy like that of Frank et al. (Citation2010) and Johnson et al. (Citation2013). That is, we gave the adrenergic agonist ephedrine alone or in conjunction with cortisol (the primary glucocorticoid in the guinea pig) and examined the response to isolation the following day. In an earlier study, cortisol alone did not affect depressive-like behavior or fever of guinea pig pups the next day (Hennessy et al., Citation2018). Here, we asked if increased sympathetic activity, either alone or in conjunction with cortisol, would increase these measures as might be expected if increased sympathetic activity either alone or together with elevated cortisol concentrations was sufficient to account for the behavioral and febrile sensitization seen in repeatedly isolated guinea pig pups.
Experiment 2 used a strategy similar to that of Wohleb et al. (Citation2011), in which we administered the beta-adrenergic antagonist propranolol prior to an initial isolation to determine whether enhanced sympathetic activity was necessary for the emergence of depressive-like behavior and fever during the first isolation, or the sensitization of these responses during repeated isolations. Vocalizing was also of interest in Experiment 2. Because the ability to reduce the vocalizations of isolated guinea pig pups is a well-established screen for anxiolytic medications (Borsini et al., Citation2002; Groenink et al., Citation2015), and sympathetic activity is known to contribute to anxiety (Roth et al., Citation2008; Holwerda et al., Citation2018), we expected vocalizing to be reduced following propranolol administration.
General method
Subjects
Albino Harley guinea pigs (Cavia porcellus) were bred and housed in our laboratory. Each mother and her pups were maintained in opaque plastic cages (73 × 54 × 24 cm) with wire tops and sawdust bedding. Guinea pig chow and water were available ad libitum. Colony room lights were set to a 12:12 light/dark cycle, with lights on at 0700 hr. All pups remained with their mothers after birth (Day 0) and for the duration of experimentation, being removed only for surgery, post-operative checks, behavioral testing, and routine cage cleaning. Guinea pig litters typically consist of 2–5 pups. Thirteen to fourteen litters contributed pups to each condition in each experiment (1 pup/litter for each condition). All procedures were approved by the Wright State University Institutional Animal Care and Use Committee.
Surgery
Between Days 14–17, animals had telemetry probes (PD4000 Emitters from Starr Life Sciences, Oakmont, PA) surgically implanted in the abdominal cavity using aseptic surgical techniques. Pups were weighed, pretreated with atropine (0.05 mg/kg, IP), and then anesthetized with isoflurane (2%-5%). A small incision was made through the skin and abdominal muscle wall. The sterilized probe was then inserted into the abdominal cavity and sutured to the abdominal wall. Absorbable suture was used to close the abdominal wall, and sterile wound clips were used to close the skin incision. The analgesic buprenorphine (0.05 mg/ml, SC) was administered for post-operative pain management. Animals were weighed and inspected for signs of infection or hernia twice daily for the first five days and once daily thereafter. A recovery time of at least four days was allowed before experimental injections and testing. Only pups that showed steady weight gain and appeared healthy were used in the study (95.1%).
Drug preparation
The adrenergic receptor agonist ephedrine (Animal Pharmaceuticals LLC), cortisol (Santa Cruz Biotechnology), and beta-adrenergic antagonist propranolol (Sigma Aldrich), were compounded in saline vehicle. Drugs were placed in coded vials so that observers were blind to experimental conditions. All injections were SC.
Behavioral testing
For testing, guinea pig pups were gently removed from their home cage and carried in a small transport cage (<15 s) to the observation room. Pups were placed in a clear plastic cage (47 × 24 × 20 cm) on top of a telemetry receiver plate on a table under full room lighting. A cover of clear plastic grating was used to prevent escape from the observation cage. Trained observers (≥85% inter-observer reliability) scored behavior from behind one-way glass during minutes 0–30, 60–90, and 150–180 of the isolation. Anxiety-like whistle vocalizations were recorded using a hand-held tally counter. The presence or absence of passive, depressive-like behavior was scored in 1-min intervals. The individual depressive-like behaviors were a crouched posture with feet tucked beneath the body (which sometimes transitions to lying with the trunk supported by the cage floor), extensive piloerection (over at least half of the body surface), and closure of one or both eyes for at least 1 s. In addition to these individual behaviors, we also derived the measure of full passive behavior which is a 1-min interval in which the pup displayed all three depressive-like behaviors. Core temperature and movement were measured by the telemetry probe, which sent signals to the receiver plate beneath the observation cage. These data were collected in 3-min bins using VitalView Telemetry Data Acquisition Software throughout the 180 min isolation. All testing began between 1200 and 1500 hr to control for circadian variation. Consistent with related studies (e.g. Hennessy et al., Citation2011a, Citation2011b), testing was initiated at about 3 weeks of age, near the generally accepted age of weaning in the guinea pig (∼ Day 25), although the day of first test varied slightly as described below to accommodate scheduling of multiple 3-hr tests. It should be noted, however, that young guinea pigs continue to show a strong attraction to the mother, as well as physiological and behavioral reactions to isolation, including depressive-like responding, for weeks after the presumed day of weaning (Hennessy et al., Citation1996; Hennessy & Morris, Citation2005; Hennessy et al., Citation2003; Schneider et al., Citation2012).
Experimental design
Experiment 1
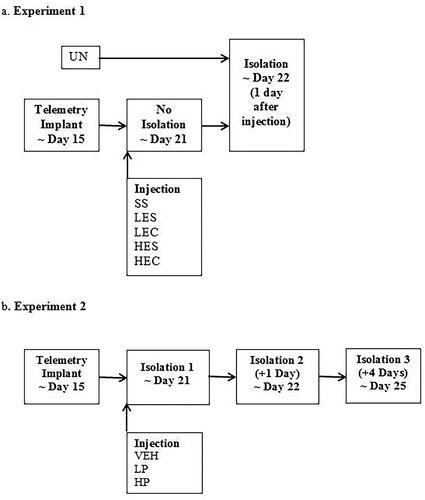
Guinea pig pups 19–25 days old were assigned to one of six conditions. In the first condition, pups were undisturbed, i.e. received no surgery or injection (UN; 6 males, 6 females). Pups in the other five conditions all had telemetry devices implanted and received two injections of either saline and saline (SS; 8 males, 4 females), a low dose of 3 mg/kg ephedrine and saline (LES; 6 males, 6 females), a high dose of 10 mg/kg ephedrine and saline (HES; 8 males, 4 females); 3 mg/kg ephedrine and 2.5 mg/kg cortisol (LEC; 7 males, 5 females), or 10 mg/kg ephedrine and 2.5 mg/kg cortisol (HEC; 9 males, 3 females). Pups then underwent a standard 3-hr isolation 24 hr later (). The 2.5 mg/kg dose of cortisol was chosen because it produces elevation of plasma cortisol in guinea pig pups of similar age that roughly approximates those seen during isolation (e.g. average across 1 and 3 hr = 94 μg/dl for isolation vs 122 μg/dl for cortisol injection; Hennessy et al., Citation2019; Perkeybile et al., Citation2009). For ephedrine, doses used here overlap those found to produce behavioral and thermoregulatory effects in rats (Carlisle, Frost, & Stock, Citation1999; Carlisle & Stock, Citation1996; Miller & Segert, Citation2005; Wellman et al., Citation1998). The mean age at isolation ranged from 20.6 (± 0.5) days (UN) to 22.4 (± 0.5) days (HEC). If adrenergic stimulation either alone or in conjunction with cortisol is sufficient to produce sensitization, we expected pups in one or more of the drug conditions to exhibit more depressive-like behavior and a faster rising and larger magnitude increase in core body temperature than shown by SS pups.
Figure 1. A Representation of experimental timeline. (A) Guinea pig pups received no surgery or injections (UN), or surgery to implant telemetry devices and two injections of saline and saline (SS), low dose ephedrine and saline (LES), low dose ephedrine and cortisol (LEC), high dose ephedrine and saline (HES), or high dose ephedrine and cortisol (HEC) prior to isolation on the day after injection and at a comparable age for non-injected pups. (B) Guinea pig pups were treated with saline vehicle (VEH), or low dose propranolol (LP), or high dose propranolol (HP), prior to first isolation. They were then isolated again without injection 1 and 4 days later.
Experiment 2
Guinea pig pups 19–22 days old with telemetry devices implanted were assigned to one of three conditions in which they were injected with either saline vehicle (VEH; 7 males, 7 females), a low dose of 10 mg/kg propranolol (LP; 7 males, 6 females), or a high dose of 20 mg/kg propranolol (HP; 7 males, 7 females) 20 min prior to testing. These doses have been found to inhibit stress-induced central neuroinflammatory signaling and behavior in mice and rats (Blandino et al., Citation2006; Wohleb et al., Citation2011). The pups were returned to the home cage and subsequent isolations occurred 24 hr and 4 days later (). If beta-adrenergic stimulation is necessary for depressive-like and anxiety-like behavior as well as fever to occur in isolated guinea pig pups, we expected these responses to be reduced by propranolol during the initial isolation. If beta-adrenergic stimulation is necessary for sensitization of depressive-like behavior and fever, we expected increases in behavior and fever from the first to the subsequent isolations to be reduced by propranolol. The mean age at first isolation ranged from 20.4 (± 0.3) days (VEH) to 21.3 (± 0.3) days (LP).
Statistical analysis
Our measures of depressive-like behavior were crouch, which of the three individual depressive-like behaviors appears the most specific to increased inflammatory activity and most sensitive to anti-inflammatory drug treatment (Hennessy et al., Citation2007b; Hennessy et al., Citation2015), and full passive which represents the most extreme form of depressive-like behavior. As in previous studies (e.g. Hennessy et al., Citation2022) activity counts were recorded and used as a visual control to assure any core temperature increases were a result of fever rather than increased physical exertion. Analysis of variance (ANOVA) was the primary method of analysis. All calculations were performed using IBM SPSS Version 26. For behavior, the factors in Experiment 1 were Condition, Sex, and Isolation, with Isolation treated as a repeated measure. In Experiment 2, the factors were Condition and Sex. For telemetry measures, data from 5 consecutive 3-min bins were combined (averaged for temperature, summed for activity) into 12, 15-min time blocks over the 3-hr test period. ANOVA factors for core temperature were Condition, Sex, Isolation, and Time Block, with Isolation and Time Block as repeated measures. For telemetry, three pups each were dropped from analysis of temperature and activity in Experiments 1 and 2 due to technical issues. Consistent with earlier work, our analysis revealed no sex differences, so they will not be discussed further. When sphericity was violated, as indicated by the Mauchly’s Test, the Huynh-Feldt correction factor was used and adjusted degrees of freedom are presented. Univariate ANOVAs were used to follow up significant interactions involving the factor of Condition. The Newman-Keuls test was used for paired comparisons. Results were considered significant at p ≤ 0.05. Effect sizes were estimated using partial eta squared (η2).
Results
Experiment 1
Vocalizations
For Experiment 1, ANOVA revealed no significant effect of Condition or Sex on vocalizations. That is, injection of ephedrine, with or without cortisol, had no effect on vocalizing during isolation the following day ().
Table 1. Mean (standard error) for vocalizations, depressive-like behavior, core temperature (°C), and activity for Experiment 1.
Depressive-like behavior
ANOVA revealed no main effects or interactions for either measure of depressive-like behavior (). We then looked at additional strategies to explore the questions of interest. Because there were no differences among conditions, UN and SAL groups were pooled to form a combined control group. Next, we combined low and high doses of ephedrine, regardless of cortisol (LES and LEC, HES and HEC) to examine effects of ephedrine. A one-way (Combined control, Low ephedrine, High ephedrine) ANOVA revealed no significant effects for crouch or full passive behavior. Finally, to increase power in examination of effects of cortisol, we ran a 2 (Ephedrine dose) × 2 (Presence or absence of cortisol) ANOVA for crouch and passive behavior. These analyses again resulted in no significant effects. Overall, we found neither dose of ephedrine, alone or with cortisol, increased depressive-like behavior during isolation the following day.
Telemetry
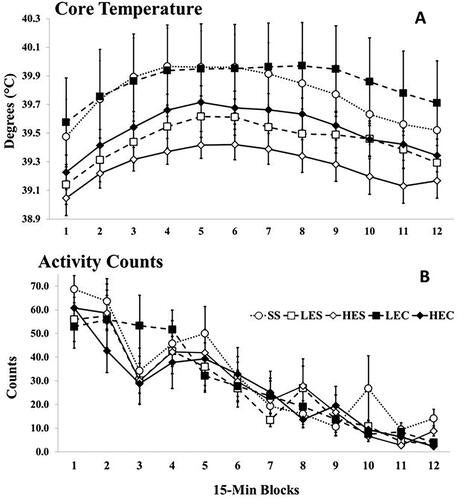
ANOVA of temperature yielded only a significant effect of Time Block F (2.50, 117.64) = 28.51, p < 0.001, η2= 0.38, with a rise and then decline of temperature, as in earlier studies (e.g. Schneider et al., Citation2012; ). That is, core temperature during isolation was unaffected by any drug treatment. We also tried the same additional strategies as used for depressive-like behavior, but as for behavior, there were no effects of treatment on core temperature. As seen in , heightened core temperature was not associated with increased activity.
Figure 2. Mean (A) core temperature and (B) activity across the 12, 15-min Time blocks for Experiment 1. Vertical lines indicated standard errors of the means. N’s = 10-12/Condition. The effect of Time Block was significant for core temperature, p < 0.001.
Experiment 2
Vocalizations
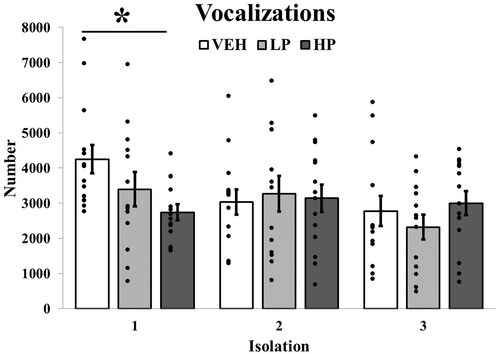
For vocalizing in Experiment 2, ANOVA revealed a main effect of Isolation F (2, 70) = 5.82, p = 0.005, η2= 0.14. This effect was qualified by a significant Condition × Isolation interaction, F (4, 70) = 3.80, p < 0.01, η2= 0.18. Follow-up tests were significant only for the first isolation F (2, 38) = 3.99, p < 0.05, η2= 0.17. There was a dose-related effect with paired comparisons showing the high dose of propranolol significantly reducing vocalizations (p < 0.05; ). Thus, beta-adrenergic blockade reduced the anxiety-like behavior of vocalizing.
Figure 3. Mean number of vocalizations across isolations in Experiment 2. Guinea pig pups treated with high dose propranolol (HP) showed significantly reduced vocalizing during the first isolation. N’s = 13 or 14/Condition. Vertical lines indicate standard errors and dots illustrate individual values. * p < 0.05 vs VEH.
Depressive-like behavior
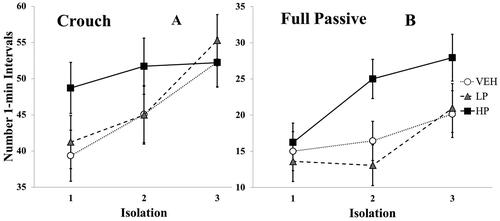
ANOVA for crouch yielded only a main effect of Isolation, F (2, 70) = 13.75, p < 0.001, η 2= 0.28, indicating sensitization of crouching with repeated isolations (). Similarly, for full passive behavior ANOVA also yielded only a main effect of Isolation, F (2, 70) = 10.70, p < 0.001, η2= 0.23, reflecting sensitization, though the effect of Condition approached significance, F (2, 35) = 2.66, p = 0.084, η2= 0.13. As seen in , there was a tendency for the high dose of propranolol to increase, rather than decrease, the full passive response.
Figure 4. Mean number of 1-min intervals in which the depressive-like measures of (A) crouch and (B) full passive were Observed across isolations. Vertical lines indicate standard errors. N’s = 13 or 14/condition. The overall pattern of increasing behavior with repeated isolations was significant for both measures, p’s < 0.001.
Telemetry
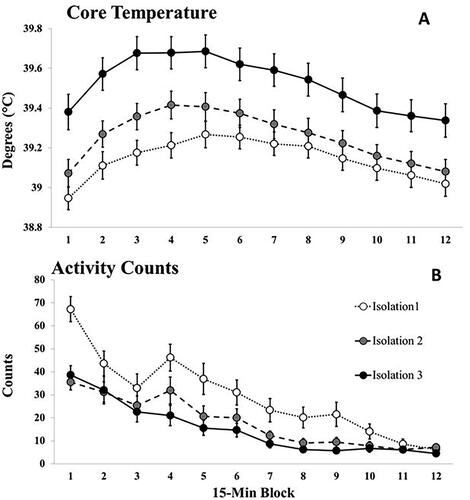
ANOVA revealed no significant effect of Condition (VEH = 39.39 ± 0.11; LP = 39.27 ± 0.12; HP = 39.26 ± 0.10, p = 0.62. Interactions involving the factor of Condition similarly were all nonsignificant. However, there were significant main effects for Isolation, F (1.92, 59.37) = 39.93, p < 0.001, η2= 0.56 and Time Block F (4.23, 131.18) = 52.42, p < 0.001, η2= 0.63 as well as a significant interaction of Isolation × Time block F (10.42, 322.99) = 3.05, p = 0.001, η2= 0.09. As seen in , this reflects sensitization of the febrile response, with higher and more rapid increases in core temperature with repeated isolations. As is clear in the comparisons of , increased core temperature was not associated with increased activity.
Figure 5. Mean (A) core temperature and (B) activity counts across the 12, 15-min Time blocks for the three isolations of Experiment 2. Data points represent averages across drug conditions (N’s = 41/isolation). Vertical lines indicate standard errors of the means. The Isolation × Time Block interaction was significant for core temperature, p < 0.001.
Discussion
Our studies with guinea pigs appear to be the first to directly examine the signal by which early-life attachment disruption can initiate sensitization of neuroinflammatory-based, depressive-like behavior. Utilizing a strategy similar to that of Johnson et al. (Citation2013) with adult rats, who found beta-adrenergic stimulation was sufficient to increase response to later administration of LPS, Experiment 1 found, in contrast, that activation of adrenergic receptors with ephedrine did not induce greater depressive-like behavior or fever in response to initial isolation of infant guinea pigs the following day. Previously, Frank et al. (Citation2010) found that prior injection of glucocorticoids enhanced the proinflammatory response to LPS the next day. However, Experiment 1 found that cortisol with ephedrine did not increase depressive-like behavior of young guinea pigs during the later isolation. These results, together with our earlier finding that cortisol alone was ineffective (Hennessy et al., Citation2019), suggest sympathetic activation or glucocorticoids, alone or together, are not sufficient to induce sensitization of depressive-like behaviors in isolated guinea pig pups.
Adopting a design like that of Wohleb et al. (Citation2011), who found beta-adrenergic blockade reduced inflammatory activity in mice exposed to repeated social defeat, Experiment 2 found that although high-dose propranolol was sufficient to reduce anxiety-like behavior, there was no effect whatsoever on the emergence of depressive-like behaviors or fever, or their sensitization. These findings suggest that beta-adrenergic stimulation is not necessary to induce sensitization of depressive-like behavior in developing guinea pig pups.
These differences in results might be due to a variety of differences between our and earlier studies. One obvious possibility is that we used guinea pigs, whereas previous studies examined rats and mice (Frank et al., Citation2010; Johnson et al., Citation2013; Wohleb et al., Citation2011). There are, for instance, clear differences in localization and number of beta-adrenergic receptors in rats and guinea pigs (Booze et al., Citation1989). Another important distinction is the nature of the stressors used in these experiments. While earlier results have been demonstrated for the physical stressors of chronic social defeat (Wohleb et al., Citation2011) and footshock (Lovelock & Deak, Citation2019), as well as direct immune challenge with LPS (Frank et al., Citation2010; Johnson et al., Citation2013), our study examined the purely psychological stressor of separation from an attachment figure in a threatening environment. The divergent results might also be due to changes with development. That is, the underlying mechanism accounting for stress-induced inflammatory signaling may change with age. In rats, for instance, the number and morphology of microglia, suspected elements of the priming mechanism in adults, vary greatly across development (Schwarz et al., Citation2012). Further, adolescent rats show significantly reduced induction of neuroimmune genes in the CNS after either LPS (Doremus-Fitzwater et al., Citation2015) or acute footshock (Marsland et al., Citation2022) when compared to adults. Thus, age-related differences in the mechanisms underlying stress-induced neuroinflammatory signaling may help account for the greater vulnerability during early life than at later ages, to the long-term effects of stress on psychopathology and other later outcomes (Jaaro-Peled & Sawa, Citation2020; Opendak et al., Citation2017).
The mechanism by which early-life stress induces sensitization of neuroinflammatory signaling and depressive-like behavior in young guinea pigs remains unclear. While the nonspecific adrenergic agonist ephedrine was injected in Experiment 1, and Experiment 2 examined blockade of beta-adrenergic receptors, it remains possible that alpha-adrenergic receptors play a role. For example, alpha-adrenergic blockade has been found to inhibit neuroinflammatory signaling as a result of chronic sleep fragmentation (Wheeler et al., Citation2021), while alpha-1- adrenergic – but not beta-adrenergic – blockade inhibited neuroinflammatory signaling underlying demyelinating disease in rats (Brosnan et al., Citation1985). Similarly, we did not antagonize cortisol effects in Experiment 2, so it is possible that cortisol elevations are necessary for sensitization in our model.
Another potential mechanism is corticotropin releasing factor (CRF) acting in the periphery. Endogenous peripheral CRF has a number of proinflammatory influences, notably in stress-induced disorders such as inflammatory bowel syndrome (Guilarte et al., Citation2020). Moreover, endogenous peripheral CRF has been found to mediate the increase in plasma levels of the proinflammatory cytokine IL-6 following the stress of immobilization (Ando et al., Citation1998). In young guinea pigs, peripheral CRF injection, but not intracerebroventricular infusion, potently increases depressive-like behavior during isolation via a CRF-receptor mediated mechanism (Hennessy et al., Citation1995; Hennessy et al., Citation2011b). Importantly here, peripheral injection of the anti-inflammatory cytokine IL-10 attenuates the effect of peripheral CRF administration on guinea pig depressive-like behavior, indicating exogenous peripheral CRF induces depressive-like behavior via a neuroinflammatory mechanism (Hennessy et al., Citation2011a). Increased peripheral inflammation can increase central inflammatory signaling through various pathways (e.g. migration of active immune cells into the brain, afferent vagal stimulation), which potentially may then promote the onset of depressive-like behavior (Peirce & Alviña, Citation2019). However, whether endogenous CRF induces inflammatory activity underlying depressive-like behavior and its sensitization in our model remains to be determined.
The lack of sensitization of vocalizing by guinea pig pups seen here is entirely consistent with many past findings (e.g. Schneider et al., Citation2012). Nor have we ever found association between vocalizing and inflammatory activity. These findings stand in contrast to those of Wohleb et al. (Citation2011) who found anxiety-like behavior of adult mice in the light-dark box to increase in conjunction with inflammatory signaling during repeated social defeat. The same distinctions in species, type of stressor, and age that were cited above, as well as different measures of anxiety, could have played a role in these differences in results. Increased inflammatory signaling is only one of a number of hypothesized mediators of stress-induced anxiety (Daviu et al., Citation2019; Dunsmoor & Paz, Citation2015; Michopoulos et al., Citation2017) so that the two studies may well be modeling differing neurobiological mechanisms.
Limitations of the current experiments include absence of a means of assessing the role of alpha-adrenergic stimulation or of glucocorticoid receptor blockade. In addition, although doses of adrenergic drugs were chosen based on their effects in previous work, and we found the high dose of propranolol reduce anxiety-like vocalizations, we cannot be sure that the doses were sufficient to engage neuroinflammatory mechanisms underlying depressive-like behavior and fever. Additional studies on this topic that simultaneously monitor proinflammatory signaling and sympathetic activity could address this issue.
In sum, while studies in adult rats and mice have revealed a clear role for sympathetic activity in stress-induced neuroinflammatory priming, our results indicate that sympathetic nervous system activation does not appear to play a similar major role in the sensitization of neuroinflammatory-based depressive-like behavior of guinea pig pups repeatedly isolated from their mother in a threatening environment. Further studies both aimed at identifying the mechanism in young guinea pigs, as well as testing the generalization of our findings to other – particularly primate – models may have significant translational value for understanding the means by which early-life stress promotes the later onset of depression and other forms of psychopathology in human populations.
Disclosure statement
The authors have no conflicts of interest to report.
Data availability statement
The data that support the findings of this study are available from the corresponding author, MBH, upon reasonable request.
Additional information
Funding
References
- Ando, T., Rivier, J., Yanaihara, H., & Arimura, A. (1998). Peripheral corticotropin-releasing factor mediates the elevation of plasma IL-6 by immobilization stress in rats. The American Journal of Physiology, 275(5), 1–10. https://doi.org/10.1152/ajpregu.1998.275.5.R1461
- Baumeister, D., Akhtar, R., Ciufolini, S., Pariante, C. M., & Mondelli, V. (2016). Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C-reactive protein, interleukin-6 and tumor necrosis factor-α. Molecular Psychiatry, 21(5), 642–649. https://doi.org/10.1038/mp.2015.67
- Blandino, P., Jr, Barnum, C. J., & Deak, T. (2006). The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. Journal of Neuroimmunology, 173(1–2), 87–95. https://doi.org/10.1016/j.jneuroim.2005.11.021
- Booze, R. M., Crisostomo, E., & Davis, J. N. (1989). Species differences in the localization and number of CNS beta adrenergic receptors: rat versus guinea pig. The Journal of Pharmacology and Experimental Therapeutics, 249(3), 911–920.
- Borsini, F., Podhorna, J., & Marazziti, D. (2002). Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology, 163(2), 121–141. https://doi.org/10.1007/s00213-002-1155-6
- Brenhouse, H., & Andersen, S. L. (2011). Nonsteroidal anti-inflammatory treatment prevents delayed effects of early life stress. Biological Psychiatry, 70(5), 434–440. https://doi.org/10.1016/j.biopsych.2011.05.006
- Brenhouse, H. C., & Schwarz, J. M. (2016). Immunoadolescence: Neuroimmune development and adolescent behavior. Neuroscience and Biobehavioral Reviews, 70, 288–299. https://doi.org/10.1016/j.neubiorev.2016.05.035
- Brosnan, C. F., Goldmuntz, E. A., Cammer, W., Factor, S. M., Bloom, B. R., & Norton, W. T. (1985). Prazosin, an alpha 1-adrenergic receptor antagonist, suppresses experimental autoimmune encephalomyelitis in the Lewis rat. Proceedings of the National Academy of Sciences of the United States of America, 82(17), 5915–5919. https://doi.org/10.1073/pnas.82.17.5915
- Brown, M., Worrell, C., & Pariante, C. M. (2021). Inflammation and early life stress: An updated review of childhood trauma and inflammatory markers in adulthood. Pharmacology, Biochemistry, and Behavior, 211Article, 173291. https://doi.org/10.1016/j.pbb.2021.173291
- Carlisle, H. J., & Stock, M. J. (1996). Temperature-dependent effects of ephedrine in the cold. Physiology & Behavior, 60, 1147–1150. https://doi.org/10.1016/0031-9384(96)00217-X
- Carlisle, H. J., Frost, T. S., & Stock, M. J. (1999). Thermal preference behavior following clonidine, norepinephrine, isoproterenol, and ephedrine. Physiology & Behavior, 66, 585–589. https://doi.org/10.1016/S0031-9384(98)00328-X
- Coplan, J. D., George, R., Syed, S. A., Rozenboym, A. V., Tang, J. E., Fulton, S. L., & Perera, T. D. (2021). Early Life Stress and the Fate of Kynurenine Pathway Metabolites. Frontiers in Human Neuroscience, 15, 636144. Article 636144. https://doi.org/10.3389/fnhum.2021.636144
- Cryan, J. F., Valentino, R. J., & Lucki, I. (2005). Assessing substrates underlying the behavioral effects of antidepressants using the modified forced swim test. Neuroscience and Biobehavioral Reviews, 29(4-5), 547–569. https://doi.org/10.1016/j.neubiorev.2005.03.008
- Czéh, B., Fuchs, E., Wiborg, O., & Simon, M. (2016). Animal models of major depression and their clinical implications. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 64, 293–310. https://doi.org/10.1016/j.neubiorev.2005.03.008
- Danese, A., & Lewis, S. J. (2017). Psychoneuroimmunology of Early-Life Stress: The Hidden Wounds of Childhood Trauma? Neuropsychopharmacology: official Publication of the American College of Neuropsychopharmacology, 42(1), 99–114. https://doi.org/10.1038/npp.2016.198
- Danese, A., Moffitt, T. E., Pariante, C. M., Ambler, A., Poulton, R., & Caspi, A. (2008). Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Archives of General Psychiatry, 65(4), 409–415. https://doi.org/10.1001/archpsyc.65.4.409
- Daviu, N., Bruchas, M. R., Moghaddam, B., Sandi, C., & Beyeler, A. (2019). Neurobiological links between stress and anxiety. Neurobiology of Stress, 11, 100191. Article 100191. https://doi.org/10.1016/j.ynstr.2019.100191
- de Pablos, R. M., Herrera, A. J., Espinosa-Oliva, A. M., Sarmiento, M., Muñoz, M. F., Machado, A., & Venero, J. L. (2014). Chronic stress enhances microglia activation and exacerbates death of nigral dopaminergic neurons under conditions of inflammation. Journal of Neuroinflammation, 11(1), 34. Article 34. https://doi.org/10.1186/1742-2094-11-34
- Dennison, U., McKernan, D., Cryan, J., & Dinan, T. (2012). Schizophrenia patients with a history of childhood trauma have a pro-inflammatory phenotype. Psychological Medicine, 42(9), 1865–1871. https://doi.org/10.1017/S0033291712000074
- Doremus-Fitzwater, T. L., Gano, A., Paniccia, J. E., & Deak, T. (2015). Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiology & Behavior, 148, 131–144. https://doi.org/10.1016/j.physbeh.2015.02.032
- Dunsmoor, J. E., & Paz, R. (2015). Fear Generalization and Anxiety: Behavioral and Neural Mechanisms. Biological Psychiatry, 78(5), 336–343. https://doi.org/10.1016/j.biopsych.2015.04.010
- Dutcher, E. G., Pama, E. A. C., Lynall, M.-E., Khan, S., Clatworthy, M. R., Robbins, T. W., Bullmore, E. T., & Dalley, J. W. (2020). Early-life stress and inflammation: A systematic review of a key experimental approach in rodents. Brain and Neuroscience Advances, 4, 2398212820978049. https://doi.org/10.1177/2398212820978049
- Ehrlich, K. B., Ross, K. M., Chen, E., & Miller, G. E. (2016). Testing the biological embedding hypothesis: Is early life adversity associated with a later proinflammatory phenotype? Development and Psychopathology, 28(4pt2), 1273–1283. https://doi.org/10.1017/S0954579416000845
- Feigenson, K. A., Kusnecov, A. W., & Silverstein, S. M. (2014). Inflammation and the two-hit hypothesis of schizophrenia. Neuroscience and Biobehavioral Reviews, 38, 72–93. https://doi.org/10.1016/j.neubiorev.2013.11.006
- Fonken, L. K., Frank, M. G., Gaudet, A. D., D’Angelo, H. M., Daut, R. A., Hampson, E. C., Ayala, M. T., Watkins, L. R., & Maier, S. F. (2018). Neuroinflammatorypriming to stress is deferentially regulated in male and female rats. Brain, Behavior, and Immunity, 70, 257–267. https://doi.org/10.1016/j.bbi.2018.03.005
- Frank, M. G., Miguel, Z. D., Watkins, L. R., & Maier, S. F. (2010). Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain, Behavior, and Immunity, 24(1), 19–30. https://doi.org/10.1016/j.bbi.2009.07.008
- Frank, M. G., Thompson, B. M., Watkins, L. R., & Maier, S. F. (2012). Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain, Behavior, and Immunity, 26(2), 337–345. https://doi.org/10.1016/j.bbi.2011.10.005
- Ganguly, P., Honeycutt, J. A., Rowe, J. R., Demaestri, C., & Brenhouse, H. C. (2019). Effects of early life stress on cocaine conditioning and AMPA receptor composition are sex-specific and driven by TNF. Brain, Behavior, and Immunity, 78, 41–51. https://doi.org/10.1016/j.bbi.2019.01.006
- Groenink, L., Verdouw, P. M., Bakker, B., & Wever, K. E. (2015). Pharmacological and methodological aspects of the separation-induced vocalization test in guinea pig pups; a systematic review and meta-analysis. European Journal of Pharmacology, 753, 191–208. https://doi.org/10.1016/j.ejphar.2014.10.062
- Guilarte, M., Vicario, M., Martínez, C., de Torres, I., Lobo, B., Pigrau, M., González-Castro, A., Rodiño-Janeiro, B. K., Salvo-Romero, E., Fortea, M., Pardo-Camacho, C., Antolín, M., Saperas, E., Azpiroz, F., Santos, J., & Alonso-Cotoner, C. (2020). Peripheral corticotropin-releasing factor triggers jejunal mast cell activation and abdominal pain in patients with diarrhea-predominant irritable bowel syndrome. The American Journal of Gastroenterology, 115(12), 2047–2059. https://doi.org/10.14309/ajg.0000000000000789
- Hammersley, P., Dias, A., Todd, G., Bowen-Jones, K., Reilly, B., & Bentall, R. P. (2003). Childhood trauma and hallucinations in bipolar affective disorder: Preliminary investigation. The British Journal of Psychiatry: The Journal of Mental Science, 182(6), 543–547. https://doi.org/10.1192/bjp.182.6.543
- Heim, C., & Nemeroff, C. B. (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry, 49(12), 1023–1039. https://doi.org/10.1016/s0006-3223(01)01157-x
- Hennessy, M. B., Deak, T., Schiml-Webb, P. A., & Barnum, C. J. (2007a). Immune influences on behavior and endocrine activity in early-experience and maternal separation paradigms. In M.T. Czerbska (Ed.), Psychoneuroendocrinology Research Trends., (pp. 293–319). Nova Science Publishers.
- Hennessy, M. B., Deak, T., Schiml-Webb, P. A., Carlisle, C. W., & O’Brien, E. (2010). Maternal separation produces, and a second separation enhances, core temperature and passive behavioral responses in guinea pig pups. Physiology & Behavior, 100(4), 305–310. https://doi.org/10.1016/j.physbeh.2010.02.024
- Hennessy, M. B., Fitch, C., Jacobs, S., Deak, T., & Schiml, P. A. (2011b). Behavioral effects of peripheral corticotropin-releasing factor during maternal separation maybe mediated by proinflammatory activity. Psychoneuroendocrinology, 36(7), 996–1004. https://doi.org/10.1016/j.psyneuen.2010.12.011
- Hennessy, M. B., Long, S. J., Nigh, C. K., Williams, M. T., & Nolan, D. J. (1995). Effects of peripherally administered corticotropin-releasing factor (CRF) and a CRF antagonist: does peripheral CRE activity mediate behavior of guinea pig pups during isolation? Behavioral Neuroscience, 109(6), 1137–1145. https://doi.org/10.1037//07357044.109.6.1137
- Hennessy, M. B., Mazzei, S. J., & McInturf, S. M. (1996). The fate of filial attachment in juvenile guinea pigs housed apart from the mother. Developmental Psychobiology, 29(8), 641–651. https://doi.org/10.1002/(SICI)1098-2302(199612)29:8<641::AID-DEV1>3.0.CO;2-T
- Hennessy, M. B., Miller, J. A., Carter, K. A., Molina, A. L., Schiml, P. A., & Deak, T. (2022). Sensitization of depressive-like behavior is attenuated by disruption of prostaglandin synthesis days following brief early attachment-figure isolation. Developmental Psychobiology, 64(2)Article, e22237. https://doi.org/10.1002/dev.22237
- Hennessy, M. B., & Moorman, L. (1989). Factors influencing cortisol and behavioral responses to maternal separation in guinea pigs. Behavioral Neuroscience, 103(2), 378–385. https://doi.org/10.1037//0735-7044.103.2.378
- Hennessy, M. B., & Morris, A. (2005). Passive responses of young guinea pigs during exposure to a novel environment: influences of social partners and age. Developmental Psychobiology, 46(2), 86–96. https://doi.org/10.1002/dev.20045
- Hennessy, M. B., Paik, K. D., Caraway, J. D., Schiml, P. A., & Deak, T. (2011a). Proinflammatory activity and the sensitization of depressive-like behavior during maternal separation. Behavioral Neuroscience, 125(3), 426–433. https://doi.org/10.1037/a0023559
- Hennessy, M. B., & Ritchey, R. L. (1987). Hormonal and behavioral attachment responses in infant guinea pigs. Developmental Psychobiology, 20(6), 613–625. https://doi.org/10.1002/dev.420200607
- Hennessy, M. B., Schiml, P. A., Berberich, K., Beasley, N. L., & Deak, T. (2019). Early attachment disruption, inflammation, and vulnerability for depression in rodent and primate models. Frontiers in Behavioral Neuroscience, 12Article, 314. https://doi.org/10.3389/fnbeh.2018.00314
- Hennessy, M. B., Schiml-Webb, P. A., Miller, E. E., Maken, D. S., Bullinger, K. L., & Deak, T. (2007b). Anti-inflammatory agents attenuate the passive responses of guinea pig pups: evidence for stress-induced sickness behavior during maternal separation. Psychoneuroendocrinology, 32(5), 508–515. https://doi.org/10.1016/j.psyneuen.2007.03.004
- Hennessy, M. B., Schreibeis, A. D., Schiml, P. A., & Deak, T. (2017). Maternal separation increases later immobility during forced swim in guinea pig pups: evidence for sensitization of a depressive-like state. Developmental Psychobiology, 59(1), 128–132. https://doi.org/10.1002/dev.21444
- Hennessy, M. B., Stafford, N. P., Yusko-Osborne, B., Schiml, P. A., Xanthos, E. D., & Deak, T. (2015). Naproxen attenuates sensitization of depressive-like behavior and fever during maternal separation. Physiology & Behavior, 139, 34–40. https://doi.org/10.1016/j.physbeh.2014.11.030
- Hennessy, M. B., Tamborski, N. P., Schiml, P., & Lucot, J. (1989). The influence of maternal separation on plasma concentrations of ACTh, epinephrine, and norephinephrine in guinea pig pups. Physiology & Behavior, 45(6), 1147–1152. https://doi.org/10.1016/0031-9384(89)90101-7
- Hennessy, M. B., Young, T. L., O’Leary, S. K., & Maken, D. S. (2003). Social preferences of developing guinea pigs (Cavia porcellus) from the preweaning to the periadolescent period. Journal of Comparative Psychology (Washington, D.C.: 1983), 117(4), 406–413. https://doi.org/10.1037/0735-7036.117.4.406
- Holwerda, S. W., Luehrs, R. E., Gremaud, A. L., Wooldridge, N. A., Stroud, A. K., Fiedorowicz, J. G., Abboud, F. M., & Pierce, G. L. (2018). Relative burst amplitude of muscle sympathetic nerve activity is an indicator of altered sympathetic outflow in chronic anxiety. Journal of Neurophysiology, 120(1), 11–22. https://doi.org/10.1152/jn.00064.2018
- Jaaro-Peled, H., & Sawa, A. (2020). Neurodevelopmental Factors in Schizophrenia. The Psychiatric Clinics of North America, 43(2), 263–274. https://doi.org/10.1016/j.psc.2020.02.010
- Johnson, J. D., Zimomra, Z. R., & Stewart, L. T. (2013). Beta-adrenergic receptor activation primes microglia cytokine production. Journal of Neuroimmunology, 254(1-2), 161–164. https://doi.org/10.1016/j.jneuroim.2012.08.007
- Kaufman, I. C., & Rosenblum, L. A. (1967). The reaction to separation in infant monkeys: Anaclitic depression and conservation withdrawal. Psychosomatic Medicine, 29(6), 648–675. https://doi.org/10.1097/00006842-196711000-00010
- LeMoult, J., Humphreys, K. L., Tracy, A., Hoffmeister, J. A., Ip, E., & Gotlib, I. H. (2020). Meta-analysis: Exposure to early life stress and risk for depression in childhood and adolescence. Journal of the American Academy of Child and Adolescent Psychiatry, 59(7), 842–855. https://doi.org/10.1016/j.jaac.2019.10.011
- Lovelock, D. F., & Deak, T. (2019). Acute stress imposed during adolescence yields heightened anxiety in Sprague-Dawley rats that persists into adulthood: Sex differences and potential involvement of the medial amygdala. Brain Research, 1723, 146392. https://doi.org/10.1016/j.brainres.2019.146392
- Marsland, P., Parrella, A., Orlofsky, M., Lovelock, D. F., Vore, A. S., Varlinskaya, E. I., & Deak, T. (2022). Neuroendocrine and neuroimmune responses in male and female rats: evidence for functional immaturity of the neuroimmune system during early adolescence. The European Journal of Neuroscience, 55(9-10), 2311–2325. https://doi.org/10.1111/ejn.15118
- Michopoulos, V., Powers, A., Gillespie, C. F., Ressler, K. J., & Jovanovic, T. (2017). Inflammation in fear-and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology: official Publication of the American College of Neuropsychopharmacology, 42(1), 254–270. https://doi.org/10.1038/npp.2016.146
- Miller, D. K., & Segert, I. L. (2005). Mecamylamine attenuates ephedrine-induced hyperactivity in rats. Pharmacology, Biochemistry, and Behavior, 81(1), 165–169. https://doi.org/10.1016/j.pbb.2005.03.008
- Miller, G. E., & Cole, S. W. (2012). Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry, 72(1), 34–40. https://doi.org/10.1016/j.biopsych.2012.02.034
- Mondelli, V., & Vernon, A. C. (2019). From early adversities to immune activation in psychiatric disorders: The role of the sympathetic nervous system. Clinical and Experimental Immunology, 197(3), 319–328. https://doi.org/10.1111/cei.13351
- Opendak, M., Gould, E., & Sullivan, R. (2017). Early life adversity during the infant sensitive period for attachment: Programming of behavioral neurobiology of threat processing and social behavior. Developmental Cognitive Neuroscience, 25, 145–159. https://doi.org/10.1016/j.dcn.2017.02.002
- Peirce, J. M., & Alviña, K. (2019). The role of inflammation and the gut microbiome in depression and anxiety. Journal of Neuroscience Research, 97(10), 1223–1241. https://doi.org/10.1002/jnr.24476
- Perkeybile, A. M., Schiml-Webb, P. A., O’Brien, E., Deak, T., & Hennessy, M. B. (2009). Anti-inflammatory influences on behavioral, but not cortisol, responses during maternal separation. Psychoneuroendocrinology, 34(7), 1101–1108. https://doi.org/10.1016/j.psyneuen.2009.02.014
- Roth, W. T., Doberenz, S., Dietel, A., Conrad, A., Mueller, A., Wollburg, E., Meuret, A. E., Taylor, C. B., & Kim, S. (2008). Sympathetic activation in broadly defined generalized anxiety disorder. Journal of Psychiatric Research, 42(3), 205–212. https://doi.org/10.1016/j.jpsychires.2006.12.003
- Sapolsky, R. M., Romero, L. M., & Munck, A. U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 21(1), 55–89. https://doi.org/10.1210/edrv.21.1.0389
- Schiml-Webb, P. A., Deak, T., Greenlee, T., Maken, D. S., & Hennessy, M. B. (2006). Alpha melanocyte stimulating hormone reduces putative stress-induced sickness behaviors in isolated guinea pig pups. Behavioural Brain Research, 168(2), 326–330. https://doi.org/10.1016/j.bbr.2005.08.022
- Schneider, R. L., Schiml, P. A., Deak, T., & Hennessy, M. B. (2012). Persistent sensitization of depressive-like behavior and thermogenic response during maternal separation in pre- and post-weaning guinea pigs. Developmental Psychobiology, 54(5), 514–522. https://doi.org/10.1002/dev.20609
- Schwarz, J. M., Sholar, P. W., & Bilbo, S. D. (2012). Sex differences in microglial colonization of the developing rat brain. Journal of Neurochemistry, 120(6), 948–963. https://doi.org/10.1111/j.1471-4159.2011.07630.x
- Slavich, G. M., & Irwin, M. R. (2014). From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin, 140(3), 774–815. https://doi.org/10.1037/a0035302
- Wellman, P. J., Miller, D. K., Livermore, C. L., Green, T. A., McMahon, L. R., & Nation, J. R. (1998). Effects of (-) ephedrine on locomotion, feeding, and nucleus accumbens dopamine in rats. Psychopharmacology, 135(2), 133–140. https://doi.org/10.1007/s002130050494
- Wheeler, N. D., Ensminger, D. C., Rowe, M. M., Wriedt, Z. S., & Ashley, N. T. (2021). Alpha- and beta- adrenergic receptors regulate inflammatory responses to acute and chronic sleep fragmentation in mice. PeerJ. 9Article, e11616. https://doi.org/10.7717/peerj.11616
- Wieck, A., Andersen, S. L., & Brenhouse, H. C. (2013). Evidence for a neuroinflammatory mechanism in delayed effects of early life adversity in rats: Relationship to cortical NMDA receptor expression. Brain, Behavior, and Immunity, 28, 218–226. https://doi.org/10.1016/j.bbi.2012.11.012
- Wohleb, E. S., Hanke, M. L., Corona, A. W., Powell, N. D., Stiner, L. M., Bailey, M. T., Nelson, R. J., Godbout, J. P., & Sheridan, J. F. (2011). β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(17), 6277–6288. https://doi.org/10.1523/JNEUROSCI.0450-11.2011
- Yusko, B., Hawk, K., Schiml, P. A., Deak, T., & Hennessy, M. B. (2012). Sensitization of depressive-like behavior during repeated maternal separation is associated with more-rapid increase in core body temperature and reduced plasma cortisol levels. Physiology & Behavior, 105(3), 861–867. https://doi.org/10.1016/j.physbeh.2011.10.026

