Abstract
As the end product of the hypothalamus-pituitary-adrenal (HPA) axis, the glucocorticoid hormones cortisol and corticosterone coordinate circadian activities, stress-coping, and adaptation to change. For this purpose, the hormone promotes energy metabolism and controls defense reactions in the body and brain. This life-sustaining action exerted by glucocorticoids occurs in concert with the autonomic nervous and immune systems, transmitters, growth factors/cytokines, and neuropeptides. The current contribution will focus on the glucocorticoid feedback paradox in the HPA-axis: the phenomenon that stress responsivity remains resilient if preceded by stress-induced secretion of glucocorticoid hormone, but not if this hormone is previously administered. Furthermore, in animal studies, the mixed progesterone/glucocorticoid antagonist RU486 or mifepristone switches to an apparent partial agonist upon repeated administration. To address these enigmas several interesting phenomena are highlighted. These include the conditional nature of the excitation/inhibition balance in feedback regulation, the role of glucose as a determinant of stress responsivity, and the potential of glucocorticoids in resetting the stress response system. The analysis of the feedback paradox provides also a golden opportunity to review the progress in understanding the role of glucocorticoid hormone in resilience and vulnerability during stress, the science that was burned deeply in Mary Dallman’s emotions.
1. Introduction
My firstFootnote1 meeting with Mary Dallman was at the 1974 Endocrine Conference in Atlanta. That year my “dexamethasone” paper was published in the journal Endocrinology, but only after a thorough and in-depth editorial review process (de Kloet et al., Citation1974). While working on the various revisions I more and more suspected that only one person in the world could write such a meticulous review report that was longer than the paper itself. A year later, when we met in Atlanta, we discussed at length the implications of the finding that dexamethasone (Dex) targets the pituitary corticotrophs rather than the brain in the suppression of stress-induced HPA-axis activation. While discussing the data it became irrelevant who had been the reviewer. Our conversation was dynamic and inspiring and full of ideas. This early experience was imprinted in my memory for life and became reinforced in 1978 at my first visit paid to Mary’s lab in San Francisco.
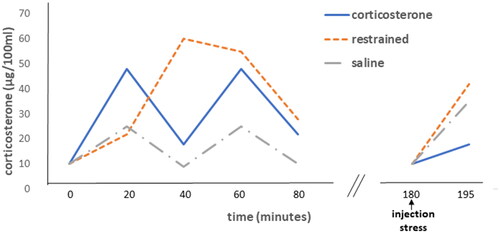
The intriguing concepts we also discussed in Atlanta were the “glucocorticoid feedback paradox” and the “variable setpoint” hypotheses. First published by Mary Dallman in 1961 (Yates et al., Citation1961), as a student member and last author of the famous Eugene Yates team, and later in the 1973 key paper with the legendary Mortyn Jones (Dallman & Jones, Citation1973). Here is the paradox: “An injection of corticosterone (B)1 suppressed subsequent stress-induced HPA-axis activation an hour later, while a stressor triggering the secretion of corticosterone toward a similar level did not ()”. Why?
Figure 1. Feedback paradox I: glucocorticoid feedback resistance evoked by a stressor but not by prior corticosterone. Left: Plasma corticosterone levels resulting from 60 min restraint, sc injections of corticosterone (60 μg) or, sc injection of saline at 0 and 40 min. Right: the responses to injection stress in the three groups are shown. After corticosterone treatment, the response to injection was significantly (p < .01) inhibited compared to either of the other groups. Figure adapted from (Dallman & Jones, Citation1973).
Fundamental for the line of reasoning to understand the “feedback paradox” and “variable setpoint hypothesis” was the “comparator” concept: the comparator was thought to detect a deficit (error signal) between the available and the required amount of corticosterone (Dallman et al., Citation1972; Dallman & Yates, Citation1969). The comparator could be reset throughout the day, accounting for circadian “variations on the theme of B”, which was also the title of one of Mary’s hallmark publications (Dallman et al., Citation1987). A deficit in B1 (corticosterone) was thought to trigger HPA-axis activation to satisfy the demand for corticosterone and its profile of actions, e.g. by providing metabolic substrates and controlling defense reactions. Apparently after a pharmacological corticosterone administration, there was no need for a second surge of corticosterone in response to the stressor was the idea.
In this contribution dedicated to Mary’s scientific life, I will first discuss the early pharmacology of glucocorticoid hormone in the regulation of the HPA-axis. Then focus on the discovery of the mineralocorticoid receptor (MR) and glucocorticoid receptor (GR), encoded by the NR3C2 and NR3C1 genes, respectively (Evans & Arriza, Citation1989), and how these receptors mediate the action of glucocorticoids during circadian- and stress-induced experiences (de Kloet & Joëls, Citation2023; de Kloet & Reul, Citation1987; Reul & de Kloet, Citation1985). Thus, there are sections on the role of glucocorticoids from perception to stress-coping and adaptation to change, and during chronic stress. Then, the puzzle of the feedback paradox is discussed, and how this knowledge may contribute to preventive and curative therapy for stress-induced affective disorders and neurodegenerative diseases.
2. Glucocorticoid feedback pharmacology: early days
Glucocorticoid feedback within the HPA-axis was first experimentally verified in a cutting-edge experiment by Dwight Ingle and Edward Kendall. In the late 1930s these two pioneers in glucocorticoid research first showed in rats that an adrenal extract containing corticosterone, called “cortin”, caused adrenal atrophy. However, cortin did not reduce adrenal weight in hypophysectomized animals replaced with ACTH suggesting that “cortin” feedback targets the pituitary rather than the adrenals. The experiment involved appropriate controls such as hypophysectomized animals receiving “cortin” or offered less food because animals without pituitaries eat less. The study is highlighted by Mary (Dallman, Citation2005). She emphasized that the use of four sets of controls for the two experimental groups was both necessary and sufficient. Thoroughly controlled experiments ever have been the trademark of Mary Dallman’s research.
Her 1984 Endocrine Review with Maureen Keller-Wood entitled “Corticosteroid inhibition of ACTH secretion” provides a complete overview of glucocorticoid feedback science at the time (Keller-Wood & Dallman, Citation1984). In this article, three-time domains of glucocorticoid feedback were defined. Firstly, the rate-limiting fast feedback from seconds to minutes was demonstrated when exogenous corticosterone was infused with a certain rate during stress-induced CRH and ACTH release. This feedback was thought to involve a putative receptor at the membrane level that could modulate some, at the time still to be discovered signal transduction pathway, engaged in rapid release of hypothalamic CRH, vasopressin, and pituitary ACTH. Secondly, intermediate feedback from 30 min to a few hours, regulating neural control at the PVN-CRH/AVP and pituitary POMC level, and its efficacy being dictated by the adaptive process. Thirdly, slow feedback taking several hours to days, which is involved in HPA-axis setpoint changes that characterize hypo- or hypercorticism in chronic stress and disease conditions. Today it seems that the three-time domains refer to the role of glucocorticoids in rapid coping, a slower adaptation, and at last a priming mechanism involved in preparation for the future.
Meanwhile, it appeared that the potent glucocorticoid Dex did poorly penetrate the brain, which supported the view that the pituitary is its prime target in the suppression of stress-induced HPA-axis activation. This Dex-targeting of the pituitary in stress-induced HPA-axis activation was the core of the Dex -suppression-test (DST) and Dex-CRH test (Carroll et al., Citation1981; Heuser et al., Citation1994). In humans, the tests were based on the escape of ACTH and cortisol from Dex suppression, which could be reinforced by an additional CRH challenge (Dex-CRH). Both tests seemed in the 1980s very promising in identifying an excessive drive from the brain to the pituitary in patients suffering from depression, pathological anxiety, and a host of other stress-related mental and physical disorders. However, the tests were too laborious, too many false positives were noted and the interpretation was felt too simplistic. Thus, the scientific community generally agreed with John Greden’s statement in the book “Endocrine Psychiatry: the riddle of Melancholia” (Shorter & Fink, Citation2010) (p81) that the DST informed about “what was going on in the brain” rather than being a laboratory test for depression.
3. Discovery of MR and GR
Meanwhile, the new era of stress-brain research already had started in 1968 when the late Bruce McEwen discovered that a tracer dose of 0.7 μg 3H-corticosterone administered to adrenalectomized (ADX) animals was accumulated and retained profoundly by nuclear receptors in neurons of the rat hippocampus and lateral septum, and to a lesser extent in other limbic neurons including those in the amygdala (McEwen et al., Citation1968). This was a surprising discovery since at the time the hypothalamic PVN and pituitary corticotrophs were thought to be the prime targets of corticosterone in HPA-axis feedback regulation. The second surprise was that the infusion of 3H-Dex in ADX animals labeled profoundly cell nuclei in pituitary corticotrophs, but little in brain cells (de Kloet et al., Citation1975).
Two factors did underlie this profound difference in corticosterone and Dex retention by nuclear receptors in the brain cells. Firstly, corticosterone freely enters the brain, but Dex entrance is hampered because it is a substrate of a multidrug resistance P-glycoprotein (mdr-Pgp) localized in the blood-brain barrier. Upon genetic deletion of the transporter, tracer 3H-Dex administered to ADX animals did penetrate the brain. In these Pgp mutants, the 3H-Dex labeled neurons resembled the cellular pattern of immunoreactive GR protein and GR mRNA expression (Meijer et al., Citation1998). Interestingly, cortisol is exogenous to rodents and also transported by Pgp (Karssen et al., Citation2001). GR is expressed in nearly every brain cell type, but unevenly and most in the hippocampus, PVN, pituitary corticotrophs, and ascending aminergic neurons (Box 1).
Secondly, Dex binds with a 10-fold higher affinity to GR than corticosterone. Alternatively, corticosterone shows a very high affinity to the mineralocorticoid receptor (MR). We realized that in vivo the 3H-corticosterone tracer used by McEwen was too low in concentration (0.7 μg) to visualize GR in brain autoradiograms. The low-affinity GR only becomes substantially occupied when circulating corticosterone concentrations did rise toward the circadian peak or after stress (Reul & de Kloet, Citation1985). MR could be either Aldo- or corticosterone-selective because of a colocalized 11β-hydroxy steroid-dehydrogenase (11HSD). If co-localized with the reductase 11HSD-type 1, MR is exposed to excess corticosterone or cortisol (human), and becomes a mostly glucocorticoid-responsive receptor: this is the case in most of the brain. If co-localized with the oxidase 11HSD-type 2, corticosterone and cortisol are converted to their bio-inactive 11-keto congeners, and MR becomes Aldo-specific. This Aldo specificity occurs in epithelial cells engaged in the maintenance of the Na/K balance such as e.g. in the kidney, and also in vascular endothelial cells and the n. tractus solitarii (NTS). In the NTS Aldo is engaged in the regulation of salt appetite and associated appraisal, emotional, and cognitive brain functions (Edwards et al., Citation1988; Funder et al., Citation1988; Gasparini et al., Citation2019) (Box 2).
MR and GR are nuclear receptors engaged in the regulation of gene expression as homodimers at very low or high concentrations of corticosterone, and heterodimers at intermediate levels of the hormone (Mifsud & Reul, Citation2016). Moreover, MR was found to amplify GR’s transcriptional response (Rivers et al., Citation2019). The MR- and GR activation occurs in coordination with numerous other proteins. Thus, there are the heat-shock proteins, particularly FKBP51, an MR-GR chaperone with a key role in the ultrashort feedback loop controlling receptor activity (Hartmann et al., Citation2021). Transcription factors, e.g. NFκB and NeuroD for GR and MR specificity (van Weert et al., Citation2019; Yang-Yen et al., Citation1990). Coregulators, for the contextual modulation of glucocorticoid action e.g. SRC1a potentiates GR-mediated action via nGRE in CRH-PVN neurons, while SRC1e amplifies GR function in the central amygdala (Zalachoras et al., Citation2016). The coregulators appear an important target for a new generation of compounds the “selective GR modulators”, SGRM (Meijer et al., Citation2018), or SMRM when it concerns MR (Fuller et al., Citation2019).
About 20 years ago, it was discovered that MR and GR also mediate rapid non-genomic actions (Di et al., Citation2003; Karst et al., Citation2005). MR activation promotes in the hippocampus the frequency rather than the amplitude of the miniature excitatory postsynaptic current (mEPSC), which signals an increased probability of presynaptic glutamate release. MR activation, thus, can rapidly enhance excitatory transmission in the hippocampus. However, in the infralimbic (il) PFC, the mEPSC frequency is suppressed by MR activation (Karst & Joëls, Citation2023). The Kd of the membrane MR-mediated effect seems about 10-fold lower than observed for the classical genomic actions of corticosterone on excitability. This suggests that the non-genomic MR may respond already to a small increment in basal corticosterone concentration (Karst et al., Citation2010). The magnitude of the Aldo effect on mEPSC is twice that of corticosterone suggesting perhaps a more prominent role of Aldo on the membrane level than in the nucleus. There is evidence that the MR-mediated effects involve a G protein receptor given the blockade of the rapid corticosterone effects in beta-arrestin knockout mice and following the application of G-15, an antagonist of the G-protein coupled estrogen receptor (Karst et al., Citation2022; Karst & Joëls, Citation2023).
GR activation stimulates the postsynaptic release of endocannabinoids that exert transsynaptic inhibitory control over the release of excitatory or inhibitory transmitters (Di et al., Citation2003). It was shown in hypothalamic neurons that glucocorticoids via the membrane could activate nuclear translocation of the unliganded GR to the nucleus. This was observed by exposure of neurons to a Dex-bovine serum albumin (BSA) complex that evoked a transcriptomic response distinct from exposure to solely Dex (Rainville et al., Citation2019). It cannot be excluded that membrane MR activation leaves a similar genomic footprint in concentrations that exceed the capacity of the nuclear receptor (Mifsud et al., Citation2021; Polman et al., Citation2013). Meanwhile, the rapid membrane GR signaling of endocannabinoid synthesis was found to involve the activation of various kinases, phospholipase C, and intracellular calcium mobilization (Harris et al., Citation2019). Thus, the membrane variants of MR and GR are important mediators of rapid corticosterone/cortisol action during stress, but how they act and/or connect with their nuclear versions needs to be further investigated (Groeneweg et al., Citation2012; Jiang et al., Citation2014; Joëls, Citation2018; Karst et al., Citation2022).
In hippocampal CA1 pyramidal neurons, MR and GR are colocalized in abundance and were found to mediate corticosterone action in a complementary manner. With rising corticosterone concentrations both the amplitude of depolarization-induced calcium currents and the hyperpolarization caused by serotonin-1A receptor activation display a clear U-shaped dose dependency. The CA1 neurons show high excitability and are thus vulnerable in the absence of corticosterone when no receptor is occupied, or at very high concentrations when both MR and GR are occupied. When corticosterone concentrations around the daily average of circulating levels are reached, gradually most MR and little GR are activated; this is a condition of stability and protection. The U-shaped curve in cellular excitability and long-term potentiation, thus, would depend on the level of circulating corticosterone and thus on the relative occupancy of MR and GR (Diamond et al., Citation1992; Joëls, Citation2006; Joëls & de Kloet, Citation1992).
In conclusion, on different levels of biological complexity, from genome to cells, MR and GR mediate in a complementary manner the action of the naturally occurring glucocorticoids.
4. HPA-axis rhythm and responsivity
The identification of MR and GR initiated the next phase of glucocorticoid feedback studies. It was found in rodents that the MR antagonist spironolactone given either systemically, intracerebroventricularly (icv), or within the hippocampus elevates basal and stress-induced circulating ACTH and corticosterone (Ratka et al., Citation1989; van Haarst et al., Citation1997). This disinhibitory effect on HPA-axis activity can be explained by the MR blockade of the excitatory outflow from the hippocampus (Joëls, Citation2006). This hippocampal excitatory output enhances an inhibitory GABA-ergic network involving among others, neurons in the bed nucleus of the stria terminalis (BNST), that inhibit the PVN parvocellular neurons and block the secretion of CRH and vasopressin (Cole et al., Citation2022; Herman et al., Citation2003). Accordingly, hippocampal MR does suppress the HPA-axis during the circadian cycle and its “peak” activity following stress (Dallman et al., Citation1989; Ratka et al., Citation1989).
The role of GR in HPA-axis regulation is more complex. Firstly, during basal am conditions, GR shows only little occupancy and a GR antagonist (i.e. the mixed glucocorticoid/progesterone GR antagonist RU486 or mifepristone) would not be effective, which is indeed the case (Ratka et al., Citation1989). Secondly, GR activation together with MR controls the circadian amplitude in corticosterone secretion, which reflects Mary’s findings (Bradbury et al., Citation1991, Citation1994; Van Haarst et al., Citation1996). Thirdly, GR activation in the hippocampus counteracts the MR suppression of the stress-induced “peak” HPA-axis activity (Van Haarst et al., Citation1997), a finding that is predicted by the opposing MR- and GR-mediated effects on hippocampal neuronal excitability and connectivity (Joels et al., Citation2012; Joëls & de Kloet, Citation1992; Maggio & Segal, Citation2009). Finally, from a classical feedback perspective GR activation regulates in context-dependent fashion the “duration” of stress-induced HPA-axis activity (Ratka et al., Citation1989; Sapolsky et al., Citation2000).
The circadian pattern of corticosterone harbors, however, an ultradian rhythm. In rodents, this implies hourly pulses of corticosterone with the largest amplitude around the start of the active phase (Lightman et al., Citation2020). The hourly corticosterone pulses can be detected with microdialysis also in various tissues including the brain (Qian et al., Citation2012). The pulse interval of 1 hour still permits the high-affinity MR to remain in neuronal cell nuclei, as was observed in the hippocampus. However, the low-affinity GR nicely follows the hourly patterns in circulating corticosterone concentrations. The resulting so-called gene pulsing exerted by the hourly GR activation appears a prerequisite for glucocorticoid- and stress responsivity (Conway-Campbell et al., Citation2010).
A well-organized pulsatile/circadian rhythm appears essential for quality of life and health. If such a pattern becomes disorganized, as is the case during aging, or shows an altered frequency or amplitude during e.g. inflammation, this usually produces aberrant glucocorticoid signals and increased vulnerability to stress (Lightman & Conway-Campbell, Citation2010). During chronic stress and depression, GR resistance accompanies the flattened circadian rhythm. Furthermore, GR resistance develops if replacement with corticosterone is not pulsatile, but continuous, as is the case with the steady release of the steroid from a subcutaneously (sc)-implanted corticosterone pellet (den Boon & Sarabdjitsingh, Citation2017; Sarabdjitsingh et al., Citation2010).
Animal studies have provided the basis of an entirely new perspective for glucocorticoid replacement of adrenal insufficiency in patients. In a recent randomized, double-blind, placebo-controlled clinical trial, healthy individuals that had received metyrapone to block endogenous cortisol secretion served as a model. In comparison with the currently common oral cortisol replacement schedule, the group mimicking the naturally-occurring ultradian/circadian pattern with cortisol peak levels around awakening showed improved self-perceived vigor and less fatigue, and optimal signs of cognitive psychophysiology. The findings were supported by fMRI on the neuronal network level (Kalafatakis et al., Citation2018, Citation2021).
It has been known for more than 50 years that the absence or low levels of cortisol increases the detection or sensation of taste, smell, and sound at the expense of perception, as is the case in adrenally-deficient individuals (Addison patients) (Henkin & Daly, Citation1968). Sensory functions are also modulated by circadian glucocorticoid exposure and it was found that the daily GR activation at the start of the active period improves perception discrimination ability, i.e. the ability to interpret the meaning of sensory signals (Obleser et al., Citation2021). GR activation also affects nociception thresholds (Ratka et al., Citation1988). Alternatively, the enhanced detection of danger signals during low resting levels of glucocorticoids is of obvious ecological advantage in being alerted by potential predators (Box 3).
In conclusion, the dynamics and organization of the glucocorticoid secretory patterns appear determinants in the action of the glucocorticoid hormone from sensory sensation and perception to neuroendocrine and behavioral outcomes. MR and GR activity are both involved in the organization of the ultradian and circadian cycles of HPA-axis activity. During stress, hippocampal (limbic) MR activity affects the “peak” rather than the “duration” of HPA-axis activity, which is under negative feedback control of GR (Harris et al., Citation2013). Substitution therapy of adrenally-deficient individuals seems to work best if these endogenous ultradian/circadian and stress-induced glucocorticoid patterns are mimicked.
5. Sucrose can mimic glucocorticoid action under basal conditions
In the ADX rodent maintenance of basal am levels of ACTH required replacement with low levels of corticosterone in concentrations that matched MR saturation (Dallman et al., Citation1989). Additional corticosterone, that progressively occupied GR, was required for control of ACTH in the pm phase suggesting circadian changes in the HPA-axis setpoint (Bradbury et al., Citation1994). It came therefore as a surprise that Mary exclaimed at a meeting in Amsterdam in the mid-1990s that “glucocorticoid feedback in basal HPA-axis rhythms was of little significance (!)”. Indeed, in ADX animals sucrose administration as an energy supplement could replace the effect of glucocorticoid substitution in the suppression of ACTH release (Akana et al., Citation1994; Dallman et al., Citation1994; Hanson et al., Citation1994).
In rodents, energy supply directly regulates adrenal corticosterone secretion also during early life. For this purpose, we used the 24-h maternal deprivation paradigm: pups were removed from the dam for 24 h during the stress hyporesponsive period (SHRP). After 1–2 h of separation under basal conditions a gradual increase of ACTH release and corticosterone secretion occurs. At 8 h of separation it appeared that stroking the pups to replace maternal licking, normalized the central components of the enhanced HPA-axis activity, i.e. circulating ACTH and PVN-CRH- and cFos mRNA expression was back to baseline (van Oers et al., Citation1998).
However, the stroked maternally-deprived pups continued to secrete adrenal corticosterone at high levels. When the 8 h-deprived pups were additionally fed with milk, then also adrenal corticosterone secretion was suppressed. This finding demonstrates that direct control of basal corticosterone secretion by energy substrates also works in the pup. In a subsequent study, it was found during the 8-h separation that circulating glucose and leptin decreased in concentration, while ghrelin and corticosterone increased. All parameters, including the rise in corticosterone, were ameliorated after infusion of glucose or a ghrelin antagonist (Schmidt et al., Citation2006).
The powerful effects of feeding could also be demonstrated in schedule-induced behavior. It was found by Mary that hungry animals did reset their circadian pattern of glucocorticoid secretion and metabolic hormones toward peak levels at the new time of the day that feeding was expected (Dallman, Citation1999). Such reset of daily glucocorticoid secretion patterns in hungry animals is also apparent from a schedule-induced polydipsia protocol. Here, in anticipation of food at a fixed time point anytime during the day the animals engage in drinking. In parallel glucocorticoid secretion rises, but is very rapidly suppressed during the consummatory response when food is presented. Schedule-induced polydipsia is abolished in rodents after ADX but can be reinstated by corticosterone replacement rather than Dex. This finding suggests that MR activation may underly the appraisal process during anticipation of food (Cirulli et al., Citation1994).
In conclusion, the current studies suggest that under basal conditions (i) energy metabolites can replace glucocorticoids, (ii) starving may stimulate the adrenal secretion of glucocorticoids which by their catabolic action provide energy substrates, (iii) circadian glucocorticoid rhythms in starving animals are reset in anticipation of food. The above studies let Mary et al. suggest that the “HPA-axis is integral to a larger hypothalamic system that mediates energy flow” (Akana et al., Citation1994).
6. Coping with acute stress
What counts in coping with stress is that an individual can predict how to deal with an upcoming challenge. Such a sense of control promotes coping with and adaptation to change. But to what extent are MR and GR involved? And what is the role of energy metabolism in the stress-induced glucocorticoid response?
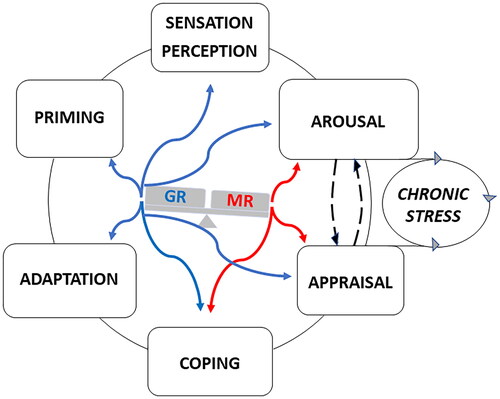
MR activation in limbic neurons promotes vigilance, attention, and sympathetic activity while suppressing HPA-axis activity. MR facilitates memory retrieval and appraisal processes and drives the selection of a low-cost habitual striatal coping style. Hence, an MR antagonist attenuates the sympathetic-driven pressor response, exerts anxiolytic and anti-aggressive effects, and promotes stress-coping toward costly hippocampal declarative processes (de Kloet et al., Citation2018; Joels et al., Citation2012; Schwabe et al., Citation2022; Schwabe & Wolf, Citation2013; Wirz et al., Citation2018) ().
Figure 2. MR- and GR-Mediated actions during information processing. MR and GR mediate in a complementary and balanced manner (see Box 4) the actions of glucocorticoids on the processing of information. MR is promiscuous and its activation by cortisol and corticosterone can be modulated by their 11 deoxy congeners, progesterone, and Aldo. GR is activated at higher concentrations of glucocorticoids after stress and toward/during the activity period in the circadian cycle. The actions exerted by glucocorticoids on information processing are conditional and time-dependent and should be considered in concert with CRH/vasopressin/POMC-driven HPA-axis activity, and sympathetic and cytokine responses together with multiple dedicated signaling cascades. Non-genomic actions rapidly proceed via the lower affinity membrane variants of MR and GR and thus may respond to a wide range of corticosterone concentrations. these rapid actions occur in the domains of arousal. appraisal processes and choice of coping style. The slow genomic responses mediated by MR and GR deal with energy metabolism, adaptation to change, and priming in preparation for the future (memory storage, inflammasome formation, and energy storage). However, the actions of glucocorticoids are also subject to meta-plasticity involving both genomic and non-genomic actions mediated by MR and GR. The actions are modified by receptor coregulator composition and are subject to ultrashort feedback loops via FKBP5. Perception discrimination ability and sensory detection threshold (nociception, taste, smell, hearing) are regulated via GR activation. The appraisal process may frustrate coping if the individual is unable to predict either the outcome of dealing with a threat or due to lack of sufficient energy as a source of motivation to pursue coping with e.g. fear or reward. If the prediction is uncertain the subject arrives in a state of lack of control: the ‘brain gets stuck’ as a consequence of chronic stress and useless energy expenditure (McEwen, 2017; McEwen & Akil, Citation2020; Sousa, Citation2016). The figure depicts an imbalance with excess MR- over GR-mediated actions. Such a positive “MR/GR balance” is characteristic of a sympathetic-driven dominant individual with low circulating corticosterone and a habitual coping style (de Boer et al., Citation2017). In a stable predictable context, this is a healthy resilient phenotype, but during lack of control the vulnerability to stressors is increased (Daskalakis et al., Citation2022; de Kloet et al., Citation1999, Citation2005, Citation2018; de Kloet & Joëls, Citation2023). Blue and red refer to GR- and MR-mediated actions.
The highest MR expression is in the hippocampal CA2 neurons, where MR is a so-called “terminal selector protein”. This implies that MR is a determinant of the CA2 neuronal phenotype and thus critical for the function of the hippocampal tri-synaptic circuit in the processing of contextual information. Hippocampal CA2 neurons and subiculum express abundant VP and oxytocin receptors which explains the relevance of MR also in social contexts (McCann et al., Citation2021; Oakley et al., Citation2021; ter Horst et al., Citation2014).
GR is expressed in nearly every cell type in the brain. Its activation would occur in response to a request for energy during daily activity, exercise, and real or anticipated stressors (see below). Regarding stressors, four domains of brain GR function can be distinguished. (i) GR-mediated actions dampen primary defense reactions by preventing these to overshoot and to become damaging (Sapolsky et al., Citation2000). (ii) GR promotes in a meta-plastic fashion with MR, NE, and CRH, initially emotional arousal and motivation to support behavioral coping (Karst & Joëls, Citation2016; Roozendaal, Citation2004). (iii) Subsequently, GR activation facilitates cognitive control over the stress experience to enable adaptation to change (Oitzl & de Kloet, Citation1992; Roozendaal & McGaugh, Citation2011). (iv) Contextualization of the selected coping style is required for memory storage (Schwabe et al., Citation2022). Such storage of coping context can be traced with c-fos activation in a sparsely distributed GR-positive hippocampal dentate gyrus network of neurons, as part of an engram (Reul et al., Citation2015) (Lesuis et al., Citation2021).
Memory storage can be viewed as an aspect of GR-mediated priming in preparation for the future involving epigenetic modifications. Immune/inflammatory defense reactions are also subject to priming. This includes in the brain the activated microglial network by the formation of a GR-dependent HMGB1/NFκB-driven NLPR3 inflammasome. Once damage occurs, the microglia inflammasome responds with pro-inflammatory MR-mediated actions, whereas GR activation has anti-inflammatory effects (de Kloet et al., Citation2005, Citation2018; De Nicola et al., Citation2020; Joëls et al., Citation2012; Sapolsky et al., Citation2000).
Using molecular-genetic-imaging technology the circuitry and networks underlying coping and adaptation are being unraveled. Thus, the emotional amygdala and motivational dopaminergic mesolimbic-cortical circuits appear constrained over time by mPFC-based top-down cognitive control (McKlveen et al., Citation2015, Citation2019; Ulrich-Lai & Herman, Citation2009) (Herman et al., Citation2020). This mPFC control is organized in specialized neuronal ensembles that project via various hubs (e.g. in the avBNST) to restrain hypothalamus-based sympathetic and neuroendocrine activity as well as the emotional/motivational drives. mPFC top-down control also directs the selected e.g. passive and active behavioral coping strategies via the periaqueductal motor outputs (Johnson et al., Citation2019, Citation2022; Lingg et al., Citation2020; Radley & Johnson, Citation2018; Wood et al., Citation2019). The bottom-up MR/GR-mediated feedback by glucocorticoids serves the coordination of these central functions with bodily needs (de Kloet et al., Citation2019). The underlying metabolic scenarios are coordinated by glucocorticoids from food intake, the choice of macronutrients, and the mobilization of energy metabolites from the liver, muscle, and fat. Then, the allocation and expenditure of glucose occur in the mitochondria of activated brain circuits (de Kloet & Herman, Citation2018; Picard et al., Citation2018) ().
The glucocorticoid-energy coupling during coping and adaptation is complex. Firstly, the teleological argument that a low level of circulating energy substrates (as occurs during fasting) would trigger a large glucocorticoid response to provide the necessary energy has not been supported by experiments in animals and humans. Firstly, there has been no relationship detected between glucose levels and the cortisol awakening curve in humans (Hucklebridge et al., Citation1999). Secondly, individuals who fasted for 8 hours before being subjected to the Trier Social Stress Test showed a lower stress-induced cortisol response than their colleagues that had received glucose one hour before testing (Kirschbaum et al., Citation1997). In rodents an analogous observation was made: after 24 h of starvation, exposure to a novel environment caused a higher glucose response, but a lower corticosterone response than with ad libitum feeding (Laugero, Citation2001). These findings suggest that if compared with the effect of starvation, the bio-availability of glucose is permissive to the stress-induced glucocorticoid response (Gonzalez-Bono et al., Citation2002).
Secondly, consumption (not expectation) of rewarding palatable comfort food and salty snacks rapidly blocks stress-induced corticosterone secretion. This seems an evolutionarily conserved mechanism that suppresses fear and stress in favor of the drive to feed and drink for the sake of survival, or collection of a reward. The suppression of a fearful response in the perspective of a reward can be abolished by lesioning the emotional basolateral amygdala (Dallman et al., Citation2003; Ulrich-Lai et al., Citation2010).
In conclusion, MR and GR-mediated actions are complementary in stress-coping and adaptation to change, in a process termed allostasis. Non-genomic MR activation rapidly promotes the selection of low-cost habitual coping styles at the expense of a costly declarative hippocampal mnemonic mechanism. GR activation makes energy available by catabolism for costly processes during adaptation to change. This includes appraisal processes concerned with the investment of energy to enable coping with stress. Consumption of high calory food can readily suppress stress-induced glucocorticoid secretion. Glucose is, however, permissive to the stress-induced glucocorticoid response if compared with the lack of hormone responsivity during the starved condition. It is complex and as Mary used to say “the whole brain and body participate in glucocorticoid feedback” (Dallman, Citation2011).
7. Coping with chronic stress: implications for disease
The most severe (psychological) stress is the inability to predict with an anxious premonition what’s coming up. This state is characterized by the lack of control, i.e. a condition of uncertainty that impairs stress-coping and adaptation. Such a chronic stress condition shows hyperactivity of the sympathetic nervous system, elevated basal, and prolonged excessive glucocorticoid secretion if confronted with an acute stressor. Bruce McEwen rated this stressed-out maladaptive state of high useless energy expenditure along the metric of “allostatic load”. He characterized the accompanying crashed information processing as the “brain gets stuck” (McEwen, Citation2017) ().
The highly active glucocorticoid-metabolic-brain axis under chronic stress would invite the consumption of energy-rich comfort food and salty snacks to replenish lost energy resources. Mary’s research has demonstrated that comfort food indeed dampens chronic stress-induced emotional and neuroendocrine reactions but at a price tag of increased propensity to develop obesity and hypertension. Such comfort food is also considered a reward, which engages the ventral tegmental area (VTA) dopaminergic system. Initially, motivational arousal is stimulated, but this may gradually culminate into “wanting” and risk for addiction (Dallman, Citation2010).
Chronic stress or glucocorticoid excess results in structural adaptations in the brain. Thus, the “emotional” e.g. amygdala and orbital frontal cortex show proliferating dendritic trees and an increased number of synaptic spines. At the same time, the “cognitive” e.g. hippocampus and to some extent mPFC atrophies. Neurogenesis in the hippocampal dentate gyrus is suppressed. These processes also result in a profound reorganization of neuronal network communication. In humans, fMRI supports the observations from animal studies: during chronic stress, the salient brain is proliferating at the expense of an atrophying executive brain. Circuits underlying low-cost habitual behaviors gain priority (Hermans et al., Citation2014; McEwen et al., Citation2015; Schwabe et al., Citation2022).
Thus, chronic stress-induced hypercorticism occurs because of a prolonged glucocorticoid secretion, which implies resistance to GR-mediated negative feedback. Indeed, such hypercorticism is characteristic of melancholic and psychotic depression, anxiety disorders, anorexia, and addiction as well as of inflammatory, immune, metabolic, and neurodegenerative disorders, which all show the GR-resistant phenotype. This energy-demanding disease state is labeled by George Chrousos et al. “acute stress syndrome” and is characterized besides hypercorticism, also by increased expression of pro-opiomelanocortin peptides (ACTH), CRH- and enhanced sympathetic nervous activity (Agorastos & Chrousos, Citation2022; Chrousos & Gold, Citation1992; Juruena et al., Citation2018).
Other chronic stress-related disorders such as myalgic encephalomyelitis (ME) or chronic fatigue syndrome and fibromyalgia characterized by chronic pain, a-typical depression, and post-traumatic stress syndrome (PTSD) as well as a variety of auto-immune disorders (e.g. rheumatoid arthritis, asthma) are characterized by hypocortisolism (Agorastos & Chrousos, Citation2022). This disease state was coined by Robert Dantzer as an “acute sickness syndrome” (Dantzer et al., Citation2008). It is characterized by impaired energy metabolism and fatigue next to a pro-inflammatory/hyperphagia phenotype. It is thought that over time (years) the acute stress syndrome may change into the sickness syndrome compromising energy resources and enhancing inflammasome functioning with increased release of cytokines. This view is in line with Selye’s concept of a General Adaptation Syndrome, where an initial alarm reaction is thought to culminate via a phase of resistance into exhaustion (Agorastos & Chrousos, Citation2022; Selye, Citation1946).
The switch of the hypercortisolemic “glucocorticoid resistant” phase of the acute stress syndrome to the hypercortisolemia state of “exhaustion” characteristic for sickness syndrome thus may depend on a degenerating mitochondrial function. Such an impaired mitochondrial function may result in reduced glucocorticoid synthesis, a phenomenon labeled “adrenal exhaustion or fatigue” by the lay press. This exhaustion phenomenon meets fierce rejection by the experts although it can be demonstrated by an impaired glucocorticoid stress response during starvation and thus lack of glucose (Dallman, Citation1999; Kirschbaum et al., Citation1997). In the catabolic state, mitochondrial DNA-encoded genes underlying oxidative phosphorylation indeed may become degenerated as was shown for instance in the dopaminergic mesolimbic-cortical circuitry (Picard et al., Citation2018; Weger et al., Citation2020). This would explain why chronic stress exposure may suppress pleasure and motivation. Markers for mitochondrial dysfunction in dopamine neurons were also observed in the postmortem brain of depressed patients (Scarpa et al., Citation2020).
But how are MR and GR involved in the chronic stress construct? In this respect, it is helpful to consider animal experiments by Carmen Sandi et al. based on a protocol of exposure to repeated variable stressors during puberty. While some rodents habituated and showed a diminished corticosterone peak response to the repeated stressor, others maintained “peak” levels. In adulthood, these animals showed a defect in spatial learning, which is a sign of impaired MR-mediated hippocampal function (Tzanoulinou et al., Citation2020). MR antagonists indeed interfere with the habituation of the corticosterone peak response to repeated daily stressors (Cole et al., Citation2000).
Other animals displayed a defect in HPA-axis shut-off suggesting GR feedback resistance. The prolonged” duration” of corticosterone secretion matches with impaired VTA-mediated social competence and enhanced amygdala-based emotional expression. Social competence and emotional expression were restored after a brief treatment with mifepristone at the time of selection during puberty. The GR antagonist appeared also effective beyond the pubertal selection context several weeks before testing of the impaired animals in young adulthood. Mifepristone may rapidly “reset” glucocorticoid-dependent behavioral and physiological responsivity (Papilloud et al., Citation2019).
Accordingly, chronic stress causes a profound reorganization of brain circuits in such a manner that the emotional brain remains in overdrive at the expense of cognitive control and flexibility. In animal models of the resistance phase during chronic stress, the stress response system shows enhanced responsivity. Indeed, exposure to an exogenous corticosterone challenge or an acute heterologous stressor revealed a dramatic change in the hippocampal dentate gyrus transcriptome of the chronically stressed animals. Gene pathways underlying chromatin structure remodeling, epigenetic changes, and mitochondrial function were overrepresented suggesting changes in vulnerability (Datson et al., Citation2013; Gray et al., Citation2014; Hunter et al., Citation2016). A prominent example of the corticosterone and stress effects is the mammalian target of the rapamycin (mTOR) signaling pathway which is known for its central role in translational control and regulation of hippocampal plasticity (Polman et al., Citation2012).
In conclusion, the highly demanding process of poor coping with chronic stress may deplete energy resources to such an extent that the resistance phase transforms toward exhaustion or sickness syndrome. The underlying mechanism of this apparent glucocorticoid-driven switch is, however, unknown, although deficits in mitochondrial function and lack of energy substrates may underlie decreased glucocorticoid secretion and action. But how can a state imposed by chronic stress be readily reset by glucocorticoid antagonists?
8. What about ‘reset’ by GR antagonists?
The reset of stress-induced behavioral and network changes with GR antagonists was observed in numerous other models. Thus, atrophy of hippocampal structure and its impaired function induced by either a single traumatic experience, chronic stress, early adversity or exogenous corticosterone can be rapidly restored with a brief (1–4 days) mifepristone treatment. This striking effect of the mixed progesterone/glucocorticoid antagonist is mimicked by more specific anti-glucocorticoids such as e.g. CORT 108297, Dazucorilant, or CORT 113176, a presumed SGRM (Ding et al., Citation2019; Hu et al., Citation2012; Loi et al., Citation2017; Mayer et al., Citation2006; Oomen et al., Citation2007). Dazucorilant was also found to reverse microgliosis, astrogliosis and pro-inflammatory mediators in the cervical spinal cord after chronic stress (Meyer et al., Citation2023). One study with frozen laser-dissected dentate gyrus tissue reported a mifepristone-dependent GR-CREB-BP interaction in parallel with recovery from chronic stress (Datson et al., Citation2012).
Models for neurodegenerative diseases, e.g. Alzheimer- and amyotrophic lateral sclerosis (ALS) show HPA-axis hyperactivity as do rodents with hyperglycemic metabolic syndrome and Type 1 or Type 2 diabetes (Canet et al., Citation2018; Revsin et al., Citation2005). In all these models GR-dependent mitochondrial function is compromised causing excitotoxicity in the face of an MR-driven pro-inflammatory phenotype dictated by the microglial inflammasome. Again mifepristone and related GR antagonists rapidly reverse neurodegeneration and excessive neuronal activation, restore neurogenesis and ameliorate hippocampal cognitive deficits (De Nicola et al., Citation2020; Lucassen et al., Citation2011; Meyer et al., Citation2020; Revsin et al., Citation2009). Interestingly, in the genetic non-obese type 1 diabetic (NOD) and the db/db type 2 mouse models, ADX and physiological corticosterone replacement also normalize the impaired hippocampal-dependent memory performance and synaptic plasticity. This finding suggests that attenuation of excessive corticosterone action indeed seems a necessary condition for improvement (Stranahan et al., Citation2008).
The rapid recovery of a deteriorated hippocampus after treatment with mifepristone or related antagonists raises the imminent question: what is the underlying mechanism of this rapid reset? Does GR antagonism (i) reverse the effect of excess glucocorticoid hormone? (ii) do the GR antagonists interfere with GR-mediated priming of activated microglial and overactive amygdala-based neuronal networks? (iii) do GR antagonists restore a metabolic imbalance? In addressing these questions it is essential to differentiate between the conditional and “out-of-context” action of GR antagonists.
Within context, the blockade of GR function in information processing has been examined in animal models for PTSD. It was found that mifepristone can prevent (re)consolidation of a fearful experience if administered systemically, icv, or in the central amygdala (Cohen et al., Citation2012; Gourley et al., Citation2009; Pitman et al., Citation2011; Taubenfeld et al., Citation2009). However, while animal data looked promising, clinical trials did not provide support for a GR antagonist-based therapy of PTSD, perhaps because the treatment was out of context and required interference with information processing for efficacy (Golier et al., Citation2023; Wood et al., Citation2015). Mifepristone is active, though, in the context of alcohol intake and dependence in both animal models and men (McGinn et al., Citation2021). Interestingly, most “reset” clinical and preclinical studies used an “out of context” design of mifepristone administration.
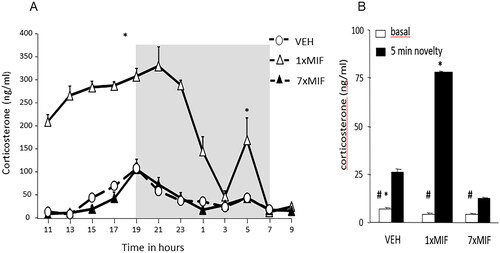
But what happens after mifepristone administration with HPA-axis regulation? Firstly, in rodents, administration of a high dose of mifepristone (200 mg/kg mouse, oral stress-free offering via oats) causes a prolonged elevation of basal circulating corticosterone levels, lasting up to 24 h. Corticosterone hypersecretion does not occur anymore for at least a week, however, if the daily administration of the antagonist was continued. Secondly, after the first mifepristone administration, the HPA-axis is sensitized: in response to 5 min of novelty exposure at 24 h after mifepristone, a strongly enhanced stress-induced corticosterone response occurs. In subsequent days of repeated mifepristone, this novelty-induced corticosterone response at 24 h after administration diminishes below the control level (Dalm et al., Citation2019) ().
Figure 3. Feedback paradox II: daily GR antagonist mifepristone reveals its apparent agonist action. (a) Circadian secretion of corticosterone in ng/ml measured every 2 h in the blood plasma of male mice C57BL/6J that received mifepristone (MIF) once (1 × 200mg/kg) or for seven days (7 × MIF). Oats + MIF or Oats + VEH were placed in the feeding cup at 0900 h, and consumed within 10 min. Mice were entrained in a 12–12-h light-dark cycle (the dark phase from 1900 to 0700 h represented by the gray-shaded area). (b) Basal and novelty (5 min exposure to the circular hole board)- induced corticosterone (ng/ml) was determined in mice, 24 h after the last administration of VEH, 1 × MIF, or 7 × MIF. Data are presented as mean ± SEM; p < .05* versus other groups, # within groups. Figure adapted from (Dalm et al., Citation2019).
Furthermore, after daily mifepristone, the exploration of a novel environment changed from initial hyperactivity the first day to serial (habitual) search patterns later on, a phenomenon that matches a predicted functional implication of the observed increased hippocampal MR expression. This finding suggests that the reset of HPA-axis activity may be related to altered MR- rather than GR-mediated actions (Dalm et al., Citation2019). Support for this notion comes from the effect of tricyclic antidepressants, which have been known for a long time to enhance the expression of particularly MR before the attenuation of enhanced HPA-axis activity (Reul et al., Citation1993; Seckl & Fink, Citation1992).
Other studies also showed after repeated administration of mifepristone an apparent agonist effect in the suppression of stress-induced corticosterone secretion. Havel, Dallman et al reported after two weeks of administration of mifepristone (30 mg/kg sc) to obese Zucker rats increased adrenal weight and decreased fat weight indicating some antagonism. However, thymus weight was reduced, and circulating glucose increased, but corticosterone and ACTH levels were also reduced, indicating an apparent agonist effect (Havel et al., Citation1996). The Jim Herman lab showed that by using stress-induced c-fos expression as a criterion repeated administration of mifepristone enhanced inhibitory signaling via mPFC and ventral hippocampus, while it suppressed excitatory input in the amygdala (Nguyen et al., Citation2017; Solomon et al., Citation2014; Wulsin et al., Citation2010).
Reul et al. using a series of selective GR antagonists (ORG 34850 and ORG 34116) elicited episodic increases in HPA-axis activity upon repeated administration suggesting that these compounds act distinct from mifepristone (Bachmann et al., Citation2003). Viho et al reproduced the profound initial HPA-axis disinhibition by mifepristone (60 mg/kg mouse orally). A single lower dose of the selective GR antagonist relacorilant (60 mg/kg orally) also disinhibited corticosterone secretion while blocking corticosterone-induced insulin resistance and many other certified GR targets. Notably, relacorilant showed a similar GR interactome as mifepristone, except for recruitment of the NCOR1 and NCOR2 corepressors. In this detailed pharmacokinetic- and dynamic study, relacorilant lacked however in vivo antagonism for GR-regulated brain genes and was less effective in disinhibition of the HPA-axis (Viho et al., Citation2022). In a phase 2 trial relacorilant reduced hyperglycemia, hypertension, and other symptoms of Cushing’s disease while improving mood (Pivonello et al., Citation2021).
In conclusion, in the rodent hippocampus, mifepristone and related more selective GR antagonists can rapidly reset markers for pro-inflammatory activity, excitotoxicity, neuronal activation, and structural features of hippocampal atrophy. This striking effect is observed in several models for chronic stress and neurodegeneration, including those for Alzheimer’s disease and ALS. How this rapid reversal occurs is currently not known. It is remarkable though that in the rodent repeated GR blockade with mifepristone results in the dampening of stress-induced corticosterone secretion rather than the expected disinhibition. How this paradoxical change from antagonism to an apparent agonism can be explained is unclear, but the role of glucose bio-availability, prevalence of MR-mediated central action, and change in excitatory vs inhibitory input to the PVN cannot be excluded.
9. Feedback paradox
The knowledge on stress summarized in the previous sections does not explain yet the glucocorticoid feedback paradox: the phenomenon that stress-induced HPA-axis responsivity is maintained if preceded by a stressor, but not by prior exogenous corticosterone exposure. As an explanation, however, one could maintain the position that the feedback paradox occurs because of glucocorticoid resistance evoked by the initial stress experience. The initial stress-induced glucocorticoid rise feeds back on a variety of inhibitory and excitatory circuits impinging on the hypothalamic CRH neurons. The resulting excitability would depend on the context of the stress experience and the ability of the animal to cope. This position receives some support by the observation that blockade of corticosterone synthesis by cyanoketone before the second stressor, increases subsequent stress-induced corticosterone secretion (Akana et al., Citation1992). In this line of reasoning, exogenous corticosterone would just only activate inhibitory signals preventing activation of the HPA-axis.
A role in the bioavailability of energy substrates cannot be excluded as well, since stress-induced glucocorticoid secretion in starved individuals only occurs if replenished with glucose before the second stressor (Gonzalez-Bono et al., Citation2002; Laugero, Citation2001). Such a role of the bioavailability of energy substrates in the feedback paradox can be verified in real-time glucose and glucocorticoid measurements. In the clinical realm, one can envision biosensors monitoring the need for cortisol and energy substrates during rest, stress, and exercise that is combined with a bio-device releasing the hormone on demand. Such a technology at least would greatly advance the quality of life of the adrenally deficient individual (Lightman et al., Citation2020).
The role of energy substrates also highlights glucocorticoids as “mitokines”. The synthesis, as well as the action of glucocorticoids, depends on energy substrates. Moreover, glucocorticoids coordinate the entire process from macronutrient selection and food intake to energy mobilization, allocation, expenditure, and storage (Dallman, Citation2010; Picard et al., Citation2018; Tempel et al., Citation1993). How glucocorticoids act on energy metabolism is dependent on context, e.g. the need for energy in e.g. (anticipation of) coping with a threat, expectancy of a reward, or during exercise. The selected coping style relies on the interaction between top-down mPFC-mediated cognitive control and amygdala/VTA-generated emotional/motivational arousal with bottom-up glucocorticoid feedback coordinating metabolically-demanding body and brain functions (de Kloet et al., Citation2019; Koorneef et al., Citation2018). The outcome of this interaction depends on context. For instance, glucocorticoids have positive effects on cognitive control and motivation in the prospect of a reward, successful coping, or during exercise (Chen et al., Citation2017; Collins et al., Citation2009; Dallman, Citation2010; Douma & de Kloet, Citation2020). In contrast, as pointed out before glucocorticoids have negative effects during chronic stress, which can be ameliorated by mifepristone.
The mechanism underlying the apparent antagonist/agonist switch after mifepristone application in rodents upon repeated administration has not been resolved yet. Could it be inflicted by the initial long-lasting rebound glucocorticoid response after the GR antagonist? Does the blockade of amygdala GR -as previously observed with antagonists or knockout of co-activators- result in attenuation of the amygdala emotional hyperdrive as Jim Herman et al. c-fos study suggests? Does the rapid reduction of bio-available energy substrates by the antagonist play a role, since lack of glucose was found to abolish the acute glucocorticoid stress response in the Trier Social Stress Test? Is it a striking example of the still-debated phenomenon of adrenal exhaustion or pituitary ACTH depletion? Or is the balance in MR vs GR-mediated actions involved and thus a glucocorticoid-dependent resilience switch turned on? (Box 4).
In humans an 8-d treatment with 200 mg mifepristone orally daily causes elevated HPA-axis activity without clinical symptoms, which is reversed to baseline at 4 d after discontinuation of the anti-glucocorticoid treatment (Bertagna, Citation1994). It was found that 7 d of treatment with mifepristone can reduce the psychotic symptoms of psychotic depression (Belanoff et al., Citation2002; Blasey et al., Citation2009). A positive result in the cognitive performance of patients suffering from bipolar depression was reported (Watson et al., Citation2012).
Furthermore, in a pooled analysis of five studies, it appeared that a dose of 1200 mg mifepristone corresponding with a mifepristone plasma level of 1637 ng/ml had an optimal therapeutical outcome in psychotic depression which lasted for a period of up to 8 weeks (Block et al., Citation2017; Block et al., Citation2018). Baseline cortisol and ACTH were threefold increased after a week, but it seemed that the effect of mifepristone is beyond the HPA-axis, thus directed at the brain (Lombardo et al., Citation2019). Fasting glucose levels appeared to decrease after 4 weeks of mifepristone (Roat-Shumway et al., Citation2018). Meanwhile, mifepristone appeared an effective therapy for Cushing symptoms (Fleseriu et al., Citation2012).
Thus, there is a profound difference in HPA-axis responsivity to mifepristone between rodents and humans. The question then is what the underlying reason for this difference could be. One species difference is the biological half-life. In humans, at high doses half-life is about three days because mifepristone is protected from degradation by its high affinity binding to circulating ɑ1-glycoprotein (Heikinheimo et al., Citation1987). In rodents, mifepristone is not bound to circulating proteins and is depleted after 90 min from the circulation after an oral dose of 50 mg/kg. Mifepristone is a Pgp substrate and is retained in the brain for at least 3 h in similar concentrations as corticosterone (Dalm et al., Citation2019).
10. Perspective
This tribute to Mary Dallman summarizes highlights over the past half-century in glucocorticoid research. Starting with her early contributions to the “comparator” and “variable set point” concepts as an inroad to allostasis, the story further unfolded from the discovery of MR and GR to their complementary role in processing acute and chronic stressors.
Key to her contributions is the recognition that the bioavailability of energy substrates, and the appraisal of the cost of an effort to cope are critical determinants of HPA-axis activation to make glucocorticoids available. She also realized that the transcriptional regulation by glucocorticoids in negative feedback was too time-consuming for rapid stress coping and defense reactions, as has been demonstrated by Alan Watts and experimentally verified by Kim et al. (Kim et al., Citation2019; Watts, Citation2005). The discovery of non-genomic glucocorticoid effects which were effective in the time constraints of the coping and appraisal processes, therefore, received a warm welcome (Dallman, Citation2003). This refers to the GR-endocannabinoid action in decreasing energy expenditure - as well as to the rapid energy-saving MR-mediated switch in coping style. How the latter switch from a costly declarative to a less demanding sensory-motor coupled habitual mode proceeds still presents a conundrum (Schwabe et al., Citation2022). One could envision that such an MR-mediated switch is located in neuronal ensembles in the limbic/mPFC that divert connectivity to the dorsolateral striatal sensory-motor pathway (Colelli et al., Citation2014; Dias-Ferreira et al., Citation2009; Karst & Joëls, Citation2023).
This article does not address the profound gender differences in coping styles and stress vulnerability (Taylor et al., Citation2000). These gender differences develop during fetal and early life and have their roots in the organizational role of sex steroids (Kroon et al., Citation2020). In addition, more recent studies also point to a sexual dimorphic role of glucocorticoids in the immune system and the placenta that contributes to the masculinization of the brain (Duma et al., Citation2010; Galbally et al., Citation2022; McCarthy, Citation2020).
Not discussed also is the discipline of epigenomics which is fundamental to understanding the priming of the glucocorticoid/stress response system by early life experiences. Key to understanding the role of glucocorticoids during development is the epigenetic modulation of GR function which is dependent on “the control and predictability of energy-related upcoming events” as is demonstrated already by the newborn (Daskalakis et al., Citation2013; Galbally et al., Citation2020; Provençal et al., Citation2020; Turecki & Meaney, Citation2016). Neither addressed is the role of gene variants of GR-, FKBP5, and MR in resilience and vulnerability to psychopathology (Joëls et al., Citation2008; Klok et al., Citation2011; Matosin et al., Citation2018). How these genetically and epigenetically regulated receptor variants dictate cell-type-specific transcriptomes and what these signatures imply for stress-related neuropsychiatric symptoms is currently being investigated (Chatzinakos et al., Citation2021; Daskalakis et al., Citation2022).
As long as the riddles of the “feedback paradox” are not explained one could wonder whether glucocorticoid substitution therapy of adrenally deficient individuals precisely mimics the naturally-occurring glucocorticoid rhythms (Lightman et al., Citation2020). This is exemplified by Mary’s Eureka moment when she noted that the percentage of fat in rat chow is a determinant in the corticosterone secretion pattern (Dallman, Citation2011). This moment of serendipity culminated in the finding that the consummatory response modulates the valuation of a stressor and the selection of a coping style.
Alternatively, the symptomatic nature of glucocorticoid pharmacotherapy has demanded for almost a century more selective glucocorticoids targeting solely e.g. inflammation or auto-immune responses, while excluding unwanted neuroendocrine and metabolic side effects. The new generation of SGRM and SMRM seem to fulfill this promise by exploiting the different coregulator cocktails colocalized with the receptors (Meijer et al., Citation2018). At the same time, disentangling glucocorticoid action on defense reactions from energy metabolism may be a challenge. An alternative in this respect is the supplementation of the catabolic glucocorticoid action with anabolic adrenal dehydroepiandrosterone (DHEA) to the benefit of its therapeutic focus (Ji et al., Citation2021).
Altogether, Mary Dallman’s careful dissection of crucial aspects of HPA-axis functioning has been a source of inspiration for numerous researchers. To fully appreciate Mary’s take on life and research please consult her anecdotal account on the occasion of receiving the Distinguished Research Award from the “Society for the Study of Ingestive Behavior” (Dallman, Citation2011). Her Opus Deum on stress says: “It is the whole brain, not just a small piece of it, that manages stress responses and tries its best to integrate the capacities of the entire mind-body of the organism.” It is a great story of 50 years of science by a great scientist.
Acknowledgment
The input of my colleagues at the former Division of Medical Pharmacology and the current Division of Endocrinology led by Onno Meijer at Leiden University Medical Center is greatly acknowledged. A great inspiration for our research came from the collaboration with Marian Joëls in the various configurations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Box 1. Dexamethasone depletes endogenous glucocorticoids from the body and brain.
There are about 50 members ATP-binding cassette (ABC) family of transporters, but the most important for glucocorticoids are ABCB1 or p-glycoprotein or MDR-1 (multiple drug resistance protein 1) and MRP-1 (multidrug resistance-associated protein 1). ABCB1 does transport cortisol and synthetic glucocorticoids, but not corticosterone (Devine et al., Citation2023; Karssen et al., Citation2001; Meijer et al., Citation1998). Accordingly, Dex depletes the brain and body of these glucocorticoids because it suppresses pituitary ACTH release, and thus adrenal glucocorticoid secretion. Back in the 1970s we called this glucocorticoid depletion by Dex ‘chemical ADX’ (de Kloet et al., Citation1975). Indeed low doses of Dex which affected peripheral processes and suppressed pituitary ACTH release caused a disinhibition of PVN – CRH neuronal activation (Karssen et al., Citation2005). The idea is that deficits caused by Dex perhaps can be ameliorated by corticosterone replacement and there is indeed some support for this notion (Warris et al., Citation2016). However, caution should be exercised in generalization over various tissues since for instance in adipose tissue corticosterone entrance is hampered by an ABCC1 transporter where cortisol penetration is inhibited by the brain ABCB1 (Devine et al., Citation2023).
Box 2. Mineralocorticoid receptors.
MR and GR descend from an ancestral corticosteroid receptor (CR) which appeared first in jawless fish (lamprey and hagfish) some 530 million years ago. Then, at 480 million years MR and GR appear in cartilaginous skates and sharks. Aldo appeared ‘only’ 380 million years ago in lobe-finned fish, i.e. lungfish, which is a forerunner of terrestrial vertebrates (Baker & Katsu, Citation2019).
In vertebrates MR is an Aldo-specific receptor in epithelial cells of e.g. kidney, intestinal and salivary glands, and in NTS neurons where it regulates the Na/K balance, including salt appetite (Gasparini et al., Citation2019). The Aldo specificity of MR is because corticosterone and cortisol are inactivated by the oxidase activity of the enzyme 11β-hydroxysteroid dehydrogenase (11-HSD2) that converts the bioactive 11-OH to the inactive 11 = O (keto) group (Edwards et al., Citation1988; Funder et al., Citation1988). However, in most of the brain, this enzyme operates as a reductase (11-HSD-1) which regenerates bioactive corticosterone and cortisol (Chapman et al., Citation2013). This assigns to MR a glucocorticoid-preferring property. As a consequence in rat hippocampal cell nuclei, the corticosterone concentration is 10–100 fold higher than that of Aldo (Yongue & Roy, Citation1987). The glucocorticoid-preferring MR is found in the brain, and also in e.g. cardiomyocytes, macrophages, skin, and retina, and it is found to drive a pro-inflammatory signaling cascade (Behar-Cohen & Zhao, Citation2022; Jaisser & Farman, Citation2016; Joëls et al., Citation2008).
Excess Aldo as the end product of the renin-angiotensin system may participate in processes that are under MR-mediated control of glucocorticoids in non-epithelial tissues, however. This is because Aldo has larger transactivational activity in gene expression and a larger non-genomic action than observed for the natural glucocorticoids, even though their binding affinities are not different (Karst et al., Citation2005, Citation2010; Karst & Joëls, Citation2016). Thus, particularly during hyperaldosteronism (Syndrome of Conn) or affective disorders characterized by hypocortisolism (e.g. a-typical depression, chronic fatigue), the (relative) excess aldosterone may contribute to a pathological role of the glucocorticoid-preferring MR (Murck et al., Citation2023).
Box 3. Actions mediated by MR and GR are dependent on the brain state.
Born and colleagues demonstrated that at the circadian HPA-axis trough, during slow-wave sleep (SWS), predominant MR occupancy was found to promote recapitulation and fidelity of declarative memory storage rather than facilitating daytime memory retrieval (Groch et al., Citation2013; Klinzing et al., Citation2019). Liston et al discovered that during sleep MR-mediated actions promote the pruning of older cortical spines allowing the optimization of previous daytime GR-promoted memory (Liston et al., Citation2013). GR activation before sleep onset disrupts this SWS-linked mnemonic recapitulation process. In addition, during REM sleep GR activation of the hippocampus and dopaminergic VTA somehow causes memory reorganization, a phenomenon thought to be important for dreaming (Kelemen et al., Citation2014). Dopamine’s role in the processing of reward expectancy and motivation, therefore seems an important ingredient for anecdotal reports on the creative and predictive power of dreaming: “Desire is the motor of dreaming” is one of the unsurprising Freud citations (Sidarto Ribeiro, Citation2021).
Box 4. The MR/GR balance hypothesis.
Selye’s pendulum hypothesis was based on the pro-inflammatory action of mineralocorticoids vs the anti-inflammatory action of glucocorticoids: “An absolute or relative excess or deficiency of mineralocorticoids vs glucocorticoids could set disease susceptibility at different levels” (Selye, Citation1950). With the advent of MR and GR such opposing action actually could be achieved by solely cortisol and corticosterone (de Kloet & Reul, Citation1987; Evans & Arriza, Citation1989).
Based on cellular studies and observations in animal studies on neuroendocrine and autonomic regulations and behavioral performance it was discovered that MR and GR coordinate in a complementary manner the action of endogenous glucocorticoids in the brain and body (De Kloet et al., Citation1998; Harris et al., Citation2013; Joëls & de Kloet, Citation1992). If both receptors are expressed they operate as MR-MR and GR-GR homodimers or tetramers in response to either low or high glucocorticoid concentrations, respectively. At intermediate levels of glucocorticoid preferentially MR-GR heterodimers may occur (Mifsud & Reul, Citation2016; Oakley et al., Citation2021).
The work has led to the balance hypothesis: “Upon imbalance of MR and GR-mediated actions, the initiation and/or management of the stress response becomes compromised. At a certain threshold, this may lead to a condition of neuroendocrine dysregulation and impaired behavioral adaptation, which potentially can aggravate stress-related deterioration and promote vulnerability.” (de Kloet et al., Citation2005).
Notes on contributor
Edo Ronald (Ron) de Kloet is emeritus professor of medical pharmacology at Leiden University and Leiden University Medical Center. He is fascinated by the question how stress acts on the brain. For this purpose he investigates with many collaborators the action of the glucocorticoid hormone from a neuroendocrine perspective. In particular he wants to know how this master regulator of the stress system can switch in action from protective to harmful. Understanding the underlying mechanism will advance knowledge in preventive and curative treatment of stress-related disorders.
Additional information
Funding
Notes
1 Mary preferred the use of B = corticosterone and F = cortisol to the other common abbreviation, “cort”, which does not distinguish between the two active adrenal glucocorticoids (Dallman, Citation2005). The letters B and F refer to the respective bands labeled by Kendall and Reichstein in the chromatographic separation of the steroids from adrenal extracts.
References
- Agorastos, A., & Chrousos, G. P. (2022). The neuroendocrinology of stress: the stress-related continuum of chronic disease development. Molecular Psychiatry, 27(1), 1–19. https://doi.org/10.1038/s41380-021-01224-9
- Akana, S. F., Dallman, M. F., Bradbury, M. J., Scribner, K. A., Strack, A. M., & Walker, C. D. (1992). Feedback and facilitation in the adrenocortical system: unmasking facilitation by partial inhibition of the glucocorticoid response to prior stress. Endocrinology, 131(1), 57–68. https://doi.org/10.1210/en.131.1.57
- Akana, S. F., Strack, A. M., Hanson, E. S., & Dallman, M. F. (1994). Regulation of activity in the hypothalamo-pituitary-adrenal axis is integral to a larger hypothalamic system that determines caloric flow. Endocrinology, 135(3), 1125–1134. https://doi.org/10.1210/ENDO.135.3.8070356
- Bachmann, C. G., Linthorst, A. C. E., Holsboer, F., & Reul, J. M. H. M. (2003). Effect of chronic administration of selective glucocorticoid receptor antagonists on the rat hypothalamic-pituitary-adrenocortical axis. Neuropsychopharmacology, 28(6), 1056–1067. https://doi.org/10.1038/sj.npp.1300158
- Baker, M. E., & Katsu, Y. (2019). Evolution of the mineralocorticoid receptor. Vitamins and Hormones, 109, 17–36. https://doi.org/10.1016/bs.vh.2018.10.009
- Behar-Cohen, F., & Zhao, M. (2022). Mineralocorticoid pathway in retinal health and diseases. British Journal of Pharmacology, 179(13), 3190–3204. https://doi.org/10.1111/BPH.15770
- Belanoff, J. K., Rothschild, A. J., Cassidy, F., DeBattista, C., Baulieu, E.-E., Schold, C., & Schatzberg, A. F. (2002). An open label trial of C-1073 (mifepristone) for psychotic major depression. Biological Psychiatry, 52(5), 386–392. https://doi.org/10.1016/S0006-3223(02)01432-4
- Bertagna, X. (1994). Administration of RU 486 for 8 days in normal volunteers: antiglucocorticoid effect with no evidence of peripheral cortisol deprivation. Journal of Clinical Endocrinology & Metabolism, 78(2), 375–380. https://doi.org/10.1210/jc.78.2.375
- Blasey, C. M., Debattista, C., Roe, R., Block, T., & Belanoff, J. K. (2009). A multisite trial of mifepristone for the treatment of psychotic depression: a site-by-treatment interaction. Contemporary Clinical Trials, 30(4), 284–288. https://doi.org/10.1016/j.cct.2009.03.001
- Block, T., Petrides, G., Kushner, H., Kalin, N., Belanoff, J., & Schatzberg, A. (2017). Mifepristone plasma level and glucocorticoid receptor antagonism associated with response in patients with psychotic depression. Journal of Clinical Psychopharmacology, 37(5), 505–511. https://doi.org/10.1097/JCP.0000000000000744
- Block, T. S., Kushner, H., Kalin, N., Nelson, C., Belanoff, J., & Schatzberg, A. (2018). Combined analysis of mifepristone for psychotic depression: plasma levels associated with clinical response. Biological Psychiatry, 84(1), 46–54. https://doi.org/10.1016/j.biopsych.2018.01.008
- Bradbury, M. J., Akana, S. F., Cascio, C. S., Levin, N., Jacobson, L., & Dallman, M. F. (1991). Regulation of basal ACTH secretion by corticosterone is mediated by both type I (MR) and type II (GR) receptors in rat brain. The Journal of Steroid Biochemistry and Molecular Biology, 40(1–3), 133–142. https://doi.org/10.1016/0960-0760(91)90176-6
- Bradbury, M. J., Akana, S. F., & Dallman, M. F. (1994). Roles of type I and II corticosteroid receptors in regulation of basal activity in the hypothalamo-pituitary-adrenal axis during the diurnal trough and the peak: evidence for a nonadditive effect of combined receptor occupation. Endocrinology, 134(3), 1286–1296. https://doi.org/10.1210/ENDO.134.3.8119168
- Canet, G., Chevallier, N., Zussy, C., Desrumaux, C., & Givalois, L. (2018). Central role of glucocorticoid receptors in Alzheimer’s disease and depression. Frontiers in Neuroscience, 12, 739. https://doi.org/10.3389/fnins.2018.00739
- Carroll, B. J., Feinberg, M., Greden, J. F., Tarika, J., Albala, A. A., Haskett, R. F., James, N. M., Kronfol, Z., Lohr, N., Steiner, M., de Vigne, J. P., & Young, E. (1981). A specific laboratory test for the diagnosis of melancholia: Standardization, validation, and clinical utility. Archives of General Psychiatry, 38(1), 15–22. https://doi.org/10.1001/archpsyc.1981.01780260017001
- Chapman, K., Holmes, M., & Seckl, J. (2013). 11 -Hydroxysteroid dehydrogenases: Intracellular gate-keepers of tissue glucocorticoid action. Physiological Reviews, 93(3), 1139–1206. https://doi.org/10.1152/physrev.00020.2012
- Chatzinakos, C., Georgiadis, F., & Daskalakis, N. P. (2021). GWAS meets transcriptomics: From genetic letters to transcriptomic words of neuropsychiatric risk. Neuropsychopharmacology, 46(1), 255–256. https://doi.org/10.1038/s41386-020-00835-0
- Chen, C., Nakagawa, S., An, Y., Ito, K., Kitaichi, Y., & Kusumi, I. (2017). The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Frontiers in Neuroendocrinology, 44, 83–102. https://doi.org/10.1016/j.yfrne.2016.12.001
- Chrousos, G. P., & Gold, P. W. (1992). The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA, 267(9), 1244–1252. https://doi.org/10.1001/jama.1992.03480090092034
- Cirulli, F., van Oers, H., De Kloet, E. R., & Levine, S. (1994). Differential influence of corticosterone and dexamethasone on schedule-induced polydipsia in adrenalectomized rats. Behavioural Brain Research, 65(1), 33–39. https://doi.org/10.1016/0166-4328(94)90070-1
- Cohen, S., Kozlovsky, N., Matar, M. A., Kaplan, Z., Zohar, J., & Cohen, H. (2012). Post-exposure sleep deprivation facilitates correctly timed interactions between glucocorticoid and adrenergic systems, which attenuate traumatic stress responses. Neuropsychopharmacology, 37(11), 2388–2404. https://doi.org/10.1038/NPP.2012.94
- Cole, A. B., Montgomery, K., Bale, T. L., & Thompson, S. M. (2022). What the hippocampus tells the HPA axis: Hippocampal output attenuates acute stress responses via disynaptic inhibition of CRF + PVN neurons. Neurobiology of Stress, 20, 100473. https://doi.org/10.1016/J.YNSTR.2022.100473
- Cole, M. A., Kalman, B. A., Pace, T. W., Topczewski, F., Lowrey, M. J., & Spencer, R. L. (2000). Selective blockade of the mineralocorticoid receptor impairs hypothalamic-pituitary-adrenal axis expression of habituation. Journal of Neuroendocrinology, 12(10), 1034–1042. https://doi.org/10.1046/j.1365-2826.2000.00555.x
- Colelli, V., Campus, P., Conversi, D., Orsini, C., & Cabib, S. (2014). Either the dorsal hippocampus or the dorsolateral striatum is selectively involved in consolidation of forced swim-induced immobility depending on genetic background. Neurobiology of Learning and Memory, 111, 49–55. https://doi.org/10.1016/j.nlm.2014.03.004
- Collins, A., Hill, L. E., Chandramohan, Y., Whitcomb, D., Droste, S. K., & Reul, J. M. H. M. (2009). Exercise improves cognitive responses to psychological stress through enhancement of epigenetic mechanisms and gene expression in the dentate gyrus. PLOS One, 4(1), e4330. https://doi.org/10.1371/JOURNAL.PONE.0004330
- Conway-Campbell, B. L., Sarabdjitsingh, R. A., McKenna, M. A., Pooley, J. R., Kershaw, Y. M., Meijer, O. C., de Kloet, E. R., & Lightman, S. L. (2010). Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. Journal of Neuroendocrinology, 22(10), 1093–1100. https://doi.org/10.1111/j.1365-2826.2010.02051.x
- Dallman, M. F., Akana, S. F., Bhatnagar, S., Bell, M. E., Choi, S., Chu, A., Horsley, C., Levin, N., Meijer, O., Soriano, L. R., Strack, A. M., & Viau, V. (1999). Starvation: Early signals, sensors, and sequelae. Endocrinology, 140(9), 4015–4023. https://doi.org/10.1210/en.140.9.4015
- Dallman, M. F. (2003). Fast glucocorticoid feedback favors ‘the munchies. Trends in Endocrinology and Metabolism, 14(9), 394–396. https://doi.org/10.1016/J.TEM.2003.09.005
- Dallman, M. F. (2005). Adrenocortical function, feedback, and alphabet soup. American Journal of Physiology, Endocrinology and Metabolism, 289(3), E361–E362. https://doi.org/10.1152/classicessays.00033.2005
- Dallman, M. F. (2010). Stress-induced obesity and the emotional nervous system. Trends in Endocrinology and Metabolism, 21(3), 159–165. https://doi.org/10.1016/J.TEM.2009.10.004
- Dallman, M. F. (2011). Retrospective and perspective on the occasion of receiving the SSIBs distinguished research award. Physiology & Behavior, 104(4), 530–534. https://doi.org/10.1016/j.physbeh.2011.04.047
- Dallman, M. F., Akana, S. F., Cascio, C. S., Darlington, D. N., Jacobson, L., & Levin, N. (1987). Regulation of ACTH secretion: Variations on a theme of B. Recent Progress in Hormone Research, 43, 113–173. https://doi.org/10.1016/B978-0-12-571143-2.50010-1
- Dallman, M. F., Akana, S. F., Levin, N., Walker, C. D., Bradbury, M. J., Suemaru, S., & Scribner, K. S. (1994). Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Annals of the New York Academy of Sciences, 746, 22–31. https://doi.org/10.1111/J.1749-6632.1994.TB39206.X
- Dallman, M. F., & Jones, M. T. (1973). Corticosteroid feedback control of ACTH secretion: Effect of stress-induced corticosterone secretion on subsequent stress responses in the rat. Endocrinology, 92(5), 1367–1375. https://doi.org/10.1210/ENDO-92-5-1367
- Dallman, M. F., Jones, M. T., Vernikos-Danellis, J., & Ganong, W. F. (1972). Corticosteroid feedback control of ACTH secretion: Rapid effects of bilateral adrenalectomy on plasma ACTH in the rat. Endocrinology, 91(4), 961–968. https://doi.org/10.1210/ENDO-91-4-961
- Dallman, M. F., Levin, N., Cascio, C. S., Akana, S. F., Jacobson, L., & Kuhn, R. W. (1989). Pharmacological evidence that the inhibition of diurnal adrenocorticotropin secretion by corticosteroids is mediated via type i corticosterone-preferring receptors. Endocrinology, 124(6), 2844–2850. https://doi.org/10.1210/endo-124-6-2844
- Dallman, M. F., Pecoraro, N., Akana, S. F., La Fleur, S. E., Gomez, F., Houshyar, H., Bell, M. E., Bhatnagar, S., Laugero, K. D., & Manalo, S. (2003). Chronic stress and obesity: A new view of “comfort food”. Proceedings of the National Academy of Sciences of the United States of America, 100(20), 11696–11701. https://doi.org/10.1073/PNAS.1934666100
- Dallman, M. F., & Yates, F. E. (1969). Dynamic asymmetries in the corticosteroid feedback path and distribution metabolism-binding elements of the adrenocortical system. Annals of the New York Academy of Sciences, 156(2), 696–721. https://doi.org/10.1111/J.1749-6632.1969.TB14008.X
- Dalm, S., Karssen, A. M., Meijer, O. C., Belanoff, J. K., & de Kloet, E. R. (2019). Resetting the stress system with a mifepristone challenge. Cellular and Molecular Neurobiology, 39(4), 503–522. https://doi.org/10.1007/S10571-018-0614-5
- Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W., & Kelley, K. W. (2008). From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience, 9(1), 46–56. https://doi.org/10.1038/nrn2297
- Daskalakis, N. P., Bagot, R. C., Parker, K. J., Vinkers, C. H., & de Kloet, E. R. (2013). The three-hit concept of vulnerability and resilience: Toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology, 38(9), 1858–1873. https://doi.org/10.1016/j.psyneuen.2013.06.008
- Daskalakis, N. P., Meijer, O. C., & de Kloet, E. R. (2022). Mineralocorticoid receptor and glucocorticoid receptor work alone and together in cell-type-specific manner: Implications for resilience prediction and targeted therapy. Neurobiology of Stress, 18, 100455. https://doi.org/10.1016/j.ynstr.2022.100455
- Datson, N. A., Speksnijder, N., Mayer, J. L., Steenbergen, P. J., Korobko, O., Goeman, J., de Kloet, E. R., Joëls, M., & Lucassen, P. J. (2012). The transcriptional response to chronic stress and glucocorticoid receptor blockade in the hippocampal dentate gyrus. Hippocampus, 22(2), 359–371. https://doi.org/10.1002/hipo.20905
- Datson, N. A., Van Den Oever, J. M. E., Korobko, O. B., Magarinos, A. M., De Kloet, E. R., & McEwen, B. S. (2013). Previous history of chronic stress changes the transcriptional response to glucocorticoid challenge in the dentate gyrus region of the male rat hippocampus. Endocrinology, 154(9), 3261–3272. https://doi.org/10.1210/en.2012-2233
- de Boer, S. F., Buwalda, B., & Koolhaas, J. M. (2017). Untangling the neurobiology of coping styles in rodents: Towards neural mechanisms underlying individual differences in disease susceptibility. Neuroscience and Biobehavioral Reviews, 74(Pt B), 401–422. https://doi.org/10.1016/j.neubiorev.2016.07.008
- de Kloet, A. D., & Herman, J. P. (2018). Fat-brain connections: Adipocyte glucocorticoid control of stress and metabolism. Frontiers in Neuroendocrinology, 48, 50–57. https://doi.org/10.1016/J.YFRNE.2017.10.005
- de Kloet, E. R., de Kloet, S. F., de Kloet, C. S., & de Kloet, A. D. (2019). Top-down and bottom-up control of stress-coping. Journal of Neuroendocrinology, 31(3), e12675. https://doi.org/10.1111/jne.12675
- de Kloet, E. R., & Joëls, M. (2023). The cortisol switch between vulnerability and resilience. Molecular Psychiatry. Advance online publication. https://doi.org/10.1038/s41380-022-01934-8
- de Kloet, E. R., Joëls, M., & Holsboer, F. (2005). Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience, 6(6), 463–475. https://doi.org/10.1038/nrn1683
- de Kloet, E. R., Meijer, O. C., de Nicola, A. F., de Rijk, R. H., & Joëls, M. (2018). Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Frontiers in Neuroendocrinology, 49, 124–145. https://doi.org/10.1016/j.yfrne.2018.02.003
- de Kloet, E. R., Oitzl, M. S., & Joëls, M. (1999). Stress and cognition: are corticosteroids good or bad guys? Trends in Neurosciences, 22(10), 422–426. https://doi.org/10.1016/S0166-2236(99)01438-1
- de Kloet, E. R., & Reul, J. M. H. M. (1987). Feedback action and tonic influence of corticosteroids on brain function: A concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology, 12(2), 83–105. https://doi.org/10.1016/0306-4530(87)90040-0
- de Kloet, E. R., van der Vies, J., & de Wied, D. (1974). The site of the suppressive action of dexamethasone on pituitary-adrenal activity. Endocrinology, 94(1), 61–73. https://doi.org/10.1210/endo-94-1-61
- de Kloet, E. R., Vreugdenhil, E., Oitzl, M. S., & Joëls, M. (1998). Brain corticosteroid receptor balance in health and disease. Endocrine Reviews, 19(3), 269–301. (Issue https://doi.org/10.1210/edrv.19.3.0331
- de Kloet, R., Wallach, G., & McEwen, B. S. (1975). Differences in corticosterone and dexamethasone binding to rat brain and pituitary. Endocrinology, 96(3), 598–609. https://doi.org/10.1210/endo-96-3-598
- De Nicola, A. F., Meyer, M., Guennoun, R., Schumacher, M., Hunt, H., Belanoff, J., de Kloet, E. R., & Gonzalez Deniselle, M. C. (2020). Insights into the therapeutic potential of glucocorticoid receptor modulators for neurodegenerative diseases. International Journal of Molecular Sciences, 21(6), 2137. https://doi.org/10.3390/ijms21062137
- den Boon, F. S., & Sarabdjitsingh, R. A. (2017). Circadian and ultradian patterns of HPA-axis activity in rodents: Significance for brain functionality. Best Practice & Research Clinical Endocrinology & Metabolism, 31(5), 445–457. https://doi.org/10.1016/J.BEEM.2017.09.001
- Devine, K., Villalobos, E., Kyle, C. J., Andrew, R., Reynolds, R. M., Stimson, R. H., Nixon, M., & Walker, B. R. (2023). The ATP-binding cassette proteins ABCB1 and ABCC1 as modulators of glucocorticoid action. Nature Reviews Endocrinology, 19(2), 112–124. https://doi.org/10.1038/S41574-022-00745-9
- Di, S., Malcher-Lopes, R., Halmos, K. C., & Tasker, J. G. (2003). Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. The Journal of Neuroscience, 23(12), 4850–4857. 10.1523/JNEUROSCI.23-12-04850.2003
- Diamond, D. M., Bennett, M. C., Fleshner, M., & Rose, G. M. (1992). Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus, 2(4), 421–430. https://doi.org/10.1002/HIPO.450020409
- Dias-Ferreira, E., Sousa, J. C., Melo, I., Morgado, P., Mesquita, A. R., Cerqueira, J. J., Costa, R. M., & Sousa, N. (2009). Chronic stress causes frontostriatal reorganization and affects decision-making. Science, 325(5940), 621–625. https://doi.org/10.1126/SCIENCE.1171203
- Ding, J., da Silva, M. S., Lingeman, J., Chen, X., Shi, Y., Han, F., & Meijer, O. C. (2019). Late glucocorticoid receptor antagonism changes the outcome of adult life stress. Psychoneuroendocrinology, 107, 169–178. https://doi.org/10.1016/j.psyneuen.2019.05.014
- Douma, E. H., & de Kloet, E. R. (2020). Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neuroscience and Biobehavioral Reviews, 108, 48–77. https://doi.org/10.1016/j.neubiorev.2019.10.015
- Duma, D., Collins, J. B., Chou, J. W., & Cidlowski, J. A. (2010). Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Science Signaling, 3(143), ra74. https://doi.org/10.1126/scisignal.2001077
- Edwards, C. R., Stewart, P. M., Burt, D., Brett, L., McIntyre, M. A., Sutanto, W. S., de Kloet, E. R., & Monder, C. (1988). Localisation of 11 beta-hydroxysteroid dehydrogenase–tissue specific protector of the mineralocorticoid receptor. Lancet, 2(8618), 986–989. https://doi.org/10.1016/S0140-6736(88)90742-8
- Evans, R. M., & Arriza, J. L. (1989). A molecular framework for the actions of glucocorticoid hormones in the nervous system. Neuron, 2(2), 1105–1112. https://doi.org/10.1016/0896-6273(89)90177-3
- Fleseriu, M., Biller, B. M. K., Findling, J. W., Molitch, M. E., Schteingart, D. E., & Gross, C. (2012). Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. The Journal of Clinical Endocrinology and Metabolism, 97(6), 2039–2049. https://doi.org/10.1210/JC.2011-3350
- Fuller, P. J., Yao, Y. Z., Jin, R., He, S., Martín-Fernández, B., Young, M. J., & Smith, B. J. (2019). Molecular evolution of the switch for progesterone and spironolactone from mineralocorticoid receptor agonist to antagonist. Proceedings of the National Academy of Sciences of the United States of America, 116(37), 18578–18583. https://doi.org/10.1073/PNAS.1903172116
- Funder, J. W., Pearce, P. T., Smith, R., & Smith, A. I. (1988). Mineralocorticoid action: Target tissue specificity is enzyme, not receptor, mediated. Science, 242(4878), 583–585. https://doi.org/10.1126/science.2845584
- Galbally, M., Watson, S. J., Lappas, M., de Kloet, E. R., Wyrwoll, C. S., Mark, P. J., & Lewis, A. J. (2022). Exploring sex differences in fetal programming for childhood emotional disorders. Psychoneuroendocrinology, 141, 105764. https://doi.org/10.1016/j.psyneuen.2022.105764
- Galbally, M., Watson, S. J., van Ijzendoorn, M., Saffery, R., Ryan, J., de Kloet, E. R., Oberlander, T. F., Lappas, M., & Lewis, A. J. (2020). The role of glucocorticoid and mineralocorticoid receptor DNA methylation in antenatal depression and infant stress regulation. Psychoneuroendocrinology, 115, 104611. https://doi.org/10.1016/J.PSYNEUEN.2020.104611
- Gasparini, S., Resch, J. M., Narayan, S. V., Peltekian, L., Iverson, G. N., Karthik, S., & Geerling, J. C. (2019). Aldosterone-sensitive HSD2 neurons in mice. Brain Structure & Function, 224(1), 387–417. https://doi.org/10.1007/S00429-018-1778-Y
- Golier, J. A., Li, X., Bizien, M., Hurley, R. A., Bechard, B. W., Kimbrell, T., Flory, J. D., Baker, D. G., Yehuda, R., & Reda, D. J. (2023). Efficacy and safety of mifepristone in the treatment of male US veterans with posttraumatic stress disorder: A phase 2a randomized clinical trial. JAMA Network Open, 6(5), e2310223. https://doi.org/10.1001/JAMANETWORKOPEN.2023.10223
- Gonzalez-Bono, E., Rohleder, N., Hellhammer, D. H., Salvador, A., & Kirschbaum, C. (2002). Glucose but not protein or fat load amplifies the cortisol response to psychosocial stress. Hormones and Behavior, 41(3), 328–333. https://doi.org/10.1006/hbeh.2002.1766
- Gourley, S. L., Kedves, A. T., Olausson, P., & Taylor, J. R. (2009). A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology, 34(3), 707–716. https://doi.org/10.1038/NPP.2008.123
- Gray, J. D., Rubin, T. G., Hunter, R. G., & McEwen, B. S. (2014). Hippocampal gene expression changes underlying stress sensitization and recovery. Molecular Psychiatry, 19(11), 1171–1178. https://doi.org/10.1038/mp.2013.175
- Groch, S., Wilhelm, I., Lange, T., & Born, J. (2013). Differential contribution of mineralocorticoid and glucocorticoid receptors to memory formation during sleep. Psychoneuroendocrinology, 38(12), 2962–2972. https://doi.org/10.1016/j.psyneuen.2013.08.006
- Groeneweg, F. L., Karst, H., de Kloet, E. R., & Joëls, M. (2012). Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Molecular and Cellular Endocrinology, 350(2), 299–309. https://doi.org/10.1016/j.mce.2011.06.020
- Hanson, E. S., Bradbury, M. J., Akana, S. F., Scribner, K. S., Strack, A. M., & Dallman, M. F. (1994). The diurnal rhythm in adrenocorticotropin responses to restraint in adrenalectomized rats is determined by caloric intake. Endocrinology, 134(5), 2214–2220. https://doi.org/10.1210/ENDO.134.5.8156924
- Harris, A. P., Holmes, M. C., de Kloet, E. R., Chapman, K. E., & Seckl, J. R. (2013). Mineralocorticoid and glucocorticoid receptor balance in control of HPA axis and behaviour. Psychoneuroendocrinology, 38(5), 648–658. https://doi.org/10.1016/j.psyneuen.2012.08.007
- Harris, C., Weiss, G. L., Di, S., & Tasker, J. G. (2019). Cell signaling dependence of rapid glucocorticoid-induced endocannabinoid synthesis in hypothalamic neuroendocrine cells. Neurobiology of Stress, 10, 100158. https://doi.org/10.1016/j.ynstr.2019.100158
- Hartmann, J., Bajaj, T., Klengel, C., Chatzinakos, C., Ebert, T., Dedic, N., McCullough, K. M., Lardenoije, R., Joëls, M., Meijer, O. C., McCann, K. E., Dudek, S. M., Sarabdjitsingh, R. A., Daskalakis, N. P., Klengel, T., Gassen, N. C., Schmidt, M. V., & Ressler, K. J. (2021). Mineralocorticoid receptors dampen glucocorticoid receptor sensitivity to stress via regulation of FKBP5. Cell Reports, 35(9), 109185. https://doi.org/10.1016/j.celrep.2021.109185
- Havel, P. J., Busch, B. L., Curry, D. L., Johnson, P. R., Dallman, M. F., & Stern, J. S. (1996). Predominately glucocorticoid agonist actions of RU-486 in young specific-pathogen-free Zucker rats. The American Journal of Physiology, 271(3 Pt 2), R710–R717. https://doi.org/10.1152/ajpregu.1996.271.3.R710
- Heikinheimo, O., Kontula, K., Croxatto, H., Spitz, I., Luukkainen, T., & Lähteenmäki, P. (1987). Plasma concentrations and receptor binding of RU 486 and its metabolites in humans. Journal of Steroid Biochemistry, 26(2), 279–284. https://doi.org/10.1016/0022-4731(87)90083-5
- Henkin, R. I., & Daly, R. L. (1968). Auditory detection and perception in normal man and in patients with adrenal cortical insufficiency: effect of adrenal cortical steroids. The Journal of Clinical Investigation, 47(6), 1269–1280. https://doi.org/10.1172/JCI105819
- Herman, J. P., Figueiredo, H., Mueller, N. K., Ulrich-Lai, Y., Ostrander, M. M., Choi, D. C., & Cullinan, W. E. (2003). Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology, 24(3), 151–180. https://doi.org/10.1016/j.yfrne.2003.07.001
- Herman, J. P., Nawreen, N., Smail, M. A., & Cotella, E. M. (2020). Brain mechanisms of HPA axis regulation: neurocircuitry and feedback in context Richard Kvetnansky lecture. Stress, 23(6), 617–632. https://doi.org/10.1080/10253890.2020.1859475
- Hermans, E. J., Henckens, M. J., Joels, M., & Fernandez, G. (2014). Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends in Neurosciences. 37(6), 304–314. https://doi.org/10.1016/j.tins.2014.03.006
- Heuser, I., Yassouridis, A., & Holsboer, F. (1994). The combined dexamethasone/CRH test: A refined laboratory test for psychiatric disorders. Journal of Psychiatric Research, 28(4), 341–356. https://doi.org/10.1016/0022-3956(94)90017-5
- Hu, P., Oomen, C., van Dam, A.-M., Wester, J., Zhou, J.-N., Joëls, M., & Lucassen, P. J. (2012). A single-day treatment with mifepristone is sufficient to normalize chronic glucocorticoid induced suppression of hippocampal cell proliferation. PLOS One, 7(9), e46224. https://doi.org/10.1371/journal.pone.0046224
- Hucklebridge, F. H., Clow, A., Abeyguneratne, T., Huezo-Diaz, P., & Evans, P. (1999). The awakening cortisol response and blood glucose levels. Life Sciences, 64(11), 931–937. https://doi.org/10.1016/S0024-3205(99)00019-3
- Hunter, R. G., Seligsohn, M., Rubin, T. G., Griffiths, B. B., Ozdemir, Y., Pfaff, D. W., Datson, N. A., & McEwen, B. S. (2016). Stress and corticosteroids regulate rat hippocampal mitochondrial DNA gene expression via the glucocorticoid receptor. Proceedings of the National Academy of Sciences of the United States of America, 113(32), 9099–9104. https://doi.org/10.1073/PNAS.1602185113
- Jaisser, F., & Farman, N. (2016). Emerging roles of the mineralocorticoid receptor in pathology: Toward new paradigms in clinical pharmacology. Pharmacological Reviews, 68(1), 49–75. https://doi.org/10.1124/PR.115.011106
- Ji, E., Weickert, C. S., Purves-Tyson, T., White, C., Handelsman, D. J., Desai, R., O’Donnell, M., Liu, D., Galletly, C., Lenroot, R., & Weickert, T. W. (2021). Cortisol-dehydroepiandrosterone ratios are inversely associated with hippocampal and prefrontal brain volume in schizophrenia. Psychoneuroendocrinology, 123, 104916. https://doi.org/10.1016/J.PSYNEUEN.2020.104916
- Jiang, C. L., Liu, L., & Tasker, J. G. (2014). Why do we need nongenomic glucocorticoid mechanismsα. Frontiers in Neuroendocrinology, 35(1), 72–75. (Issue https://doi.org/10.1016/j.yfrne.2013.09.005
- Joëls, M. (2006). Corticosteroid effects in the brain: U-shape it. Trends in Pharmacological Sciences, 27(5), 244–250. https://doi.org/10.1016/j.tips.2006.03.007
- Joëls, M. (2018). Corticosteroids and the brain. Journal of Endocrinology, 238(3), R121–R130. https://doi.org/10.1530/JOE-18-0226
- Joëls, M., & de Kloet, E. R. (1992). Control of neuronal excitability by corticosteroid hormones. Trends in Neurosciences, 15(1), 25–30. https://doi.org/10.1016/0166-2236(92)90345-9
- Joëls, M., Karst, H., DeRijk, R., & de Kloet, E. R. (2008). The coming out of the brain mineralocorticoid receptor. Trends in Neurosciences, 31(1), 1–7. https://doi.org/10.1016/j.tins.2007.10.005
- Joels, M., Sarabdjitsingh, R. A., & Karst, H. (2012). Unraveling the time domains of corticosteroid hormone influences on brain activity: Rapid, slow, and chronic modes. Pharmacological Reviews, 64(4), 901–938. https://doi.org/10.1124/pr.112.005892
- Johnson, S. B., Emmons, E. B., Lingg, R. T., Anderson, R. M., Romig-Martin, S. A., Lalumiere, R. T., Narayanan, N. S., Viau, V., & Radley, J. J. (2019). Prefrontal-bed nucleus circuit modulation of a passive coping response set. The Journal of Neuroscience, 39(8), 1405–1419. https://doi.org/10.1523/JNEUROSCI.1421-18.2018
- Johnson, S. B., Lingg, R. T., Skog, T. D., Hinz, D. C., Romig-Martin, S. A., Viau, V., Narayanan, N. S., & Radley, J. J. (2022). Activity in a prefrontal-periaqueductal gray circuit overcomes behavioral and endocrine features of the passive coping stress response. Proceedings of the National Academy of Sciences of the United States of America, 119(44), e2210783119. https://doi.org/10.1073/PNAS.2210783119
- Juruena, M. F., Bocharova, M., Agustini, B., & Young, A. H. (2018). Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review. Journal of Affective Disorders, 233, 45–67. https://doi.org/10.1016/J.JAD.2017.09.052
- Kalafatakis, K., Russell, G. M., Ferguson, S. G., Grabski, M., Harmer, C. J., Munafò, M. R., Marchant, N., Wilson, A., Brooks, J. C., Thakrar, J., Murphy, P., Thai, N. J., & Lightman, S. L. (2021). Glucocorticoid ultradian rhythmicity differentially regulates mood and resting state networks in the human brain: A randomised controlled clinical trial. Psychoneuroendocrinology, 124, 105096. https://doi.org/10.1016/J.PSYNEUEN.2020.105096
- Kalafatakis, K., Russell, G. M., Harmer, C. J., Munafo, M. R., Marchant, N., Wilson, A., Brooks, J. C., Durant, C., Thakrar, J., Murphy, P., Thai, N. J., & Lightman, S. L. (2018). Ultradian rhythmicity of plasma cortisol is necessary for normal emotional and cognitive responses in man. Proceedings of the National Academy of Sciences of the United States of America, 115(17), E4091–E4100. https://doi.org/10.1073/PNAS.1714239115/SUPPL_FILE/PNAS.1714239115.SAPP.PDF
- Karssen, A. M., Meijer, O. C., Berry, A., Sanjuan Piñol, R., & De Kloet, E. R. (2005). Low doses of dexamethasone can produce a hypocorticosteroid state in the brain. Endocrinology, 146(12), 5587–5595. https://doi.org/10.1210/en.2005-0501
- Karssen, A. M., Meijer, O. C., van der Sandt, I. C., Lucassen, P. J., de Lange, E. C., de Boer, A. G., & de Kloet, E. R. (2001). Multidrug resistance P-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology, 142(6), 2686–2694. https://doi.org/10.1210/endo.142.6.8213
- Karst, H., Berger, S., Erdmann, G., Schütz, G., & Joëls, M. (2010). Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proceedings of the National Academy of Sciences of the United States of America, 107(32), 14449–14454. https://doi.org/10.1073/pnas.0914381107
- Karst, H., Berger, S., Turiault, M., Tronche, F., Schutz, G., & Joels, M. (2005). Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proceedings of the National Academy of Sciences of the United States of America, 102(52), 19204–19207. https://doi.org/10.1073/pnas.0507572102
- Karst, H., den Boon, F. S., Vervoort, N., Adrian, M., Kapitein, L. C., & Joëls, M. (2022). Non-genomic steroid signaling through the mineralocorticoid receptor: Involvement of a membrane-associated receptor? Molecular and Cellular Endocrinology, 541, 111501. https://doi.org/10.1016/J.MCE.2021.111501
- Karst, H., & Joëls, M. (2016). Severe stress hormone conditions cause an extended window of excitability in the mouse basolateral amygdala. Neuropharmacology, 110(Pt A), 175–180. https://doi.org/10.1016/j.neuropharm.2016.07.027
- Karst, H., & Joëls, M. (2023). Corticosterone rapidly reduces glutamatergic but not GABAergic transmission in the infralimbic prefrontal cortex of male mice. Steroids, 198, 109283. https://doi.org/10.1016/J.STEROIDS.2023.109283
- Kelemen, E., Bahrendt, M., Born, J., & Inostroza, M. (2014). Hippocampal corticosterone impairs memory consolidation during sleep but improves consolidation in the wake state. Hippocampus, 24(5), 510–515. https://doi.org/10.1002/hipo.22266
- Keller-Wood, M. E., & Dallman, M. F. (1984). Corticosteroid inhibition of ACTH secretion. Endocrine Reviews, 5(1), 1–24. https://doi.org/10.1210/EDRV-5-1-1
- Kim, J. S., Han, S. Y., & Iremonger, K. J. (2019). Stress experience and hormone feedback tune distinct components of hypothalamic CRH neuron activity. Nature Communications, 10(1), 5696. https://doi.org/10.1038/s41467-019-13639-8
- Kirschbaum, C., Bono, E. G., Rohleder, N., Gessner, C., Pirke, K. M., Salvador, A., & Hellhammer, D. H. (1997). Effects of fasting and glucose load on free cortisol responses to stress and nicotine. The Journal of Clinical Endocrinology and Metabolism, 82(4), 1101–1105. https://doi.org/10.1210/JCEM.82.4.3882
- Klinzing, J. G., Niethard, N., & Born, J. (2019). Mechanisms of systems memory consolidation during sleep. Nature Neuroscience, 22(10), 1743–1744. https://doi.org/10.1038/s41593-019-0467-3
- Klok, M. D., Giltay, E. J., Van der Does, A. J. W., Geleijnse, J. M., Antypa, N., Penninx, B. W. J. H., de Geus, E. J. C., Willemsen, G., Boomsma, D. I., van Leeuwen, N., Zitman, F. G., de Kloet, E. R., & DeRijk, R. H. (2011). A common and functional mineralocorticoid receptor haplotype enhances optimism and protects against depression in females. Translational Psychiatry, 1(12), e62. https://doi.org/10.1038/tp.2011.59
- Koorneef, L. L., Bogaards, M., Reinders, M. J. T., Meijer, O. C., & Mahfouz, A. (2018). How metabolic state may regulate fear: Presence of metabolic receptors in the fear circuitry. Frontiers in Neuroscience, 12, 594. https://doi.org/10.3389/fnins.2018.00594
- Kroon, J., Pereira, A. M., & Meijer, O. C. (2020). Glucocorticoid sexual dimorphism in metabolism: Dissecting the role of sex hormones. Trends in Endocrinology and Metabolism, 31(5), 357–367. https://doi.org/10.1016/j.tem.2020.01.010
- Laugero, K. D. (2001). A new perspective on glucocorticoid feedback: relation to stress, carbohydrate feeding and feeling better. Journal of Neuroendocrinology, 13(9), 827–835. https://doi.org/10.1046/J.1365-2826.2001.00706.X
- Lesuis, S. L., Brosens, N., Immerzeel, N., van der Loo, R. J., Mitrić, M., Bielefeld, P., Fitzsimons, C. P., Lucassen, P. J., Kushner, S. A., van den Oever, M. C., & Krugers, H. J. (2021). Glucocorticoids promote fear generalization by increasing the size of a dentate gyrus engram cell population. Biological Psychiatry, 90(7), 494–504. https://doi.org/10.1016/J.BIOPSYCH.2021.04.010
- Lightman, S. L., Birnie, M. T., & Conway-Campbell, B. L. (2020). Dynamics of ACTH and cortisol secretion and implications for disease. Endocrine Reviews, 41(3), 470–490. https://doi.org/10.1210/ENDREV/BNAA002
- Lightman, S. L., & Conway-Campbell, B. L. (2010). The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nature Reviews. Neuroscience, 11(10), 710–718. https://doi.org/10.1038/nrn2914
- Lingg, R. T., Johnson, S. B., Emmons, E. B., Anderson, R. M., Romig-Martin, S. A., Narayanan, N. S., McGaugh, J. L., LaLumiere, R. T., & Radley, J. J. (2020). Bed nuclei of the stria terminalis modulate memory consolidation via glucocorticoid-dependent and -independent circuits. Proceedings of the National Academy of Sciences of the United States of America, 117(14), 8104–8114. https://doi.org/10.1073/pnas.1915501117
- Liston, C., Cichon, J. M., Jeanneteau, F., Jia, Z., Chao, M. V., & Gan, W.-B. (2013). Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nature Neuroscience, 16(6), 698–705. https://doi.org/10.1038/nn.3387
- Loi, M., Sarabdjitsingh, R. A., Tsouli, A., Trinh, S., Arp, M., Krugers, H. J., Karst, H., Van Den Bos, R., & Joëls, M. (2017). Transient prepubertal mifepristone treatment normalizes deficits in contextual memory and neuronal activity of adult male rats exposed to maternal deprivation. eNeuro, 4(5), ENEURO.0253-17.2017. https://doi.org/10.1523/ENEURO.0253-17.2017
- Lombardo, G., Enache, D., Gianotti, L., Schatzberg, A. F., Young, A. H., Pariante, C. M., & Mondelli, V. (2019). Baseline cortisol and the efficacy of antiglucocorticoid treatment in mood disorders: A meta-analysis. Psychoneuroendocrinology, 110, 104420. https://doi.org/10.1016/J.PSYNEUEN.2019.104420
- Lucassen, P. J., Fitzsimons, C. P., Vreugdenhil, E., Hu, P., Oomen, C., Revsin, Y., Joëls, M., & de Kloet, E. R. (2011). Regulation of structural plasticity and neurogenesis during stress and diabetes; protective effects of glucocorticoid receptor antagonists. In Hormones in Neurodegeneration, Neuroprotection, and Neurogenesis (pp. 103–120). Wiley-VCH Verlag GmbH & Co. KgaA. https://doi.org/10.1002/9783527633968.ch6
- Maggio, N., & Segal, M. (2009). Differential corticosteroid modulation of inhibitory synaptic currents in the dorsal and ventral hippocampus. The Journal of Neuroscience, 29(9), 2857–2866. https://doi.org/10.1523/JNEUROSCI.4399-08.2009
- Matosin, N., Halldorsdottir, T., & Binder, E. B. (2018). Understanding the molecular mechanisms underpinning gene by environment interactions in psychiatric disorders: The FKBP5 model. Biological Psychiatry, 83(10), 821–830. https://doi.org/10.1016/j.biopsych.2018.01.021
- Mayer, J. L., Klumpers, L., Maslam, S., de Kloet, E. R., Joëls, M., & Lucassen, P. J. (2006). Brief treatment with the glucocorticoid receptor antagonist mifepristone normalises the corticosterone-induced reduction of adult hippocampal neurogenesis. Journal of Neuroendocrinology, 18(8), 629–631. https://doi.org/10.1111/j.1365-2826.2006.01455.x
- McCann, K. E., Lustberg, D. J., Shaughnessy, E. K., Carstens, K. E., Farris, S., Alexander, G. M., Radzicki, D., Zhao, M., & Dudek, S. M. (2021). Novel role for mineralocorticoid receptors in control of a neuronal phenotype. Molecular Psychiatry, 26(1), 350–364. https://doi.org/10.1038/s41380-019-0598-7
- McCarthy, M. M. (2020). A new view of sexual differentiation of mammalian brain. Journal of Comparative Physiology A, 206(3), 369–378. https://doi.org/10.1007/S00359-019-01376-8
- McEwen, B. S. (2017). Neurobiological and systemic effects of chronic stress. Chronic Stress, 1, 247054701769232. https://doi.org/10.1177/2470547017692328
- McEwen, B. S., & Akil, H. (2020). Revisiting the stress concept: Implications for affective disorders. The Journal of Neuroscience, 40(1), 12–21. https://doi.org/10.1523/JNEUROSCI.0733-19.2019
- McEwen, B. S., Bowles, N. P., Gray, J. D., Hill, M. N., Hunter, R. G., Karatsoreos, I. N., & Nasca, C. (2015). Mechanisms of stress in the brain. Nature Neuroscience, 18(10), 1353–1363. https://doi.org/10.1038/nn.4086
- McEwen, B. S., Weiss, J. M., & Schwartz, L. S. (1968). Selective retention of corticosterone by limbic structures in rat brain. Nature, 220(5170), 911–912. https://doi.org/10.1038/220911a0
- McGinn, M. A., Tunstall, B. J., Schlosburg, J. E., Gregory-Flores, A., George, O., de Guglielmo, G., Mason, B. J., Hunt, H. J., Koob, G. F., & Vendruscolo, L. F. (2021). Glucocorticoid receptor modulators decrease alcohol self-administration in male rats. Neuropharmacology, 188, 108510. https://doi.org/10.1016/j.neuropharm.2021.108510
- McKlveen, J. M., Moloney, R. D., Scheimann, J. R., Myers, B., & Herman, J. P. (2019). “Braking” the prefrontal cortex: The role of glucocorticoids and interneurons in stress adaptation and pathology. Biological Psychiatry, 86(9), 669–681. https://doi.org/10.1016/J.BIOPSYCH.2019.04.032
- McKlveen, J. M., Myers, B., & Herman, J. P. (2015). The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. Journal of Neuroendocrinology, 27(6), 446–456. https://doi.org/10.1111/jne.12272
- Meijer, O. C., De Lange, E. C. M., Breimer, D. D., De Boer, A. G., Workel, J. O., & De Kloet, E. R. (1998). Penetration of dexamethasone into brain glucocorticoid targets is enhanced in mdr1A P-glycoprotein knockout mice. Endocrinology, 139(4), 1789–1793. https://doi.org/http://www.scopus.com/inward/record.url?eid=2-s2.0-0031732606&partnerID=MN8TOARS https://doi.org/10.1210/endo.139.4.5917
- Meijer, O. C., Koorneef, L. L., & Kroon, J. (2018). Glucocorticoid receptor modulators. Annales D’endocrinologie, 79(3), 107–111. https://doi.org/10.1016/j.ando.2018.03.004
- Meyer, M., Meijer, O., Hunt, H., Belanoff, J., Lima, A., De Kloet, E. R., Gonzalez Deniselle, M. C., & De Nicola, A. F. (2023). Stress-induced Neuroinflammation of the spinal cord is restrained by Cort113176 (Dazucorilant), a specific glucocorticoid receptor modulator. Molecular Neurobiology. Advance online publication. https://doi.org/10.1007/s12035-023-03554-x 37566177
- Meyer, M., Kruse, M. S., Garay, L., Lima, A., Roig, P., Hunt, H., Belanoff, J., de Kloet, E. R., Deniselle, M. C. G., & De Nicola, A. F. (2020). Long-term effects of the glucocorticoid receptor modulator CORT113176 in murine motoneuron degeneration. Brain Research, 1727, 146551. https://doi.org/10.1016/j.brainres.2019.146551
- Mifsud, K. R., Kennedy, C. L. M., Salatino, S., Sharma, E., Price, E. M., Haque, S. N., Gialeli, A., Goss, H. M., Panchenko, P. E., Broxholme, J., Engledow, S., Lockstone, H., Cordero Llana, O., & Reul, J. M. H. M. (2021). Distinct regulation of hippocampal neuroplasticity and ciliary genes by corticosteroid receptors. Nature Communications, 12(1), 4737. https://doi.org/10.1038/s41467-021-24967-z
- Mifsud, K. R., & Reul, J. M. H. M. (2016). Acute stress enhances heterodimerization and binding of corticosteroid receptors at glucocorticoid target genes in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 113(40), 11336–11341. https://doi.org/10.1073/pnas.1605246113
- Murck, H., Lehr, L., & Jezova, D. (2023). A viewpoint on aldosterone and BMI related brain morphology in relation to treatment outcome in patients with major depression. Journal of Neuroendocrinology, 35(2), e13219. https://doi.org/10.1111/JNE.13219
- Nguyen, E. T., Streicher, J., Berman, S., Caldwell, J. L., Ghisays, V., Estrada, C. M., Wulsin, A. C., & Solomon, M. B. (2017). A mixed glucocorticoid/mineralocorticoid receptor modulator dampens endocrine and hippocampal stress responsivity in male rats. Physiology & Behavior, 178, 82–92. https://doi.org/10.1016/j.physbeh.2017.01.020
- Oakley, R. H., Whirledge, S. D., Petrillo, M. G., Riddick, N. V., Xu, X., Moy, S. S., & Cidlowski, J. A. (2021). Combinatorial actions of glucocorticoid and mineralocorticoid stress hormone receptors are required for preventing neurodegeneration of the mouse hippocampus. Neurobiology of Stress, 15, 100369. https://doi.org/10.1016/j.ynstr.2021.100369
- Obleser, J., Kreitewolf, J., Vielhauer, R., Lindner, F., David, C., Oster, H., & Tune, S. (2021). Circadian fluctuations in glucocorticoid level predict perceptual discrimination sensitivity. iScience, 24(4), 102345. https://doi.org/10.1016/j.isci.2021.102345
- Oitzl, M. S., & de Kloet, E. R. (1992). Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behavioral Neuroscience, 106(1), 62–71. https://doi.org/10.1037/0735-7044.106.1.62
- Oomen, C. A., Mayer, J. L., De Kloet, E. R., Joëls, M., & Lucassen, P. J. (2007). Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. The European Journal of Neuroscience, 26(12), 3395–3401. https://doi.org/10.1111/j.1460-9568.2007.05972.x
- Papilloud, A., Veenit, V., Tzanoulinou, S., Riccio, O., Zanoletti, O., Guillot de Suduiraut, I., Grosse, J., & Sandi, C. (2019). Peripubertal stress-induced heightened aggression: modulation of the glucocorticoid receptor in the central amygdala and normalization by mifepristone treatment. Neuropsychopharmacology, 44(4), 674–682. https://doi.org/10.1038/s41386-018-0110-0
- Picard, M., McEwen, B. S., Epel, E. S., & Sandi, C. (2018). An energetic view of stress: Focus on mitochondria. Frontiers in Neuroendocrinology, 49, 72–85. https://doi.org/10.1016/j.yfrne.2018.01.001
- Pitman, R. K., Milad, M. R., Igoe, S. A., Vangel, M. G., Orr, S. P., Tsareva, A., Gamache, K., & Nader, K. (2011). Systemic mifepristone blocks reconsolidation of cue-conditioned fear; propranolol prevents this effect. Behavioral Neuroscience, 125(4), 632–638. https://doi.org/10.1037/A0024364
- Pivonello, R., Bancos, I., Feelders, R. A., Kargi, A. Y., Kerr, J. M., Gordon, M. B., Mariash, C. N., Terzolo, M., Ellison, N., & Moraitis, A. G. (2021). Relacorilant, a selective glucocorticoid receptor modulator, induces clinical improvements in patients with cushing syndrome: Results from a prospective, open-label phase 2 study. Frontiers in Endocrinology, 12, 662865. https://doi.org/10.3389/FENDO.2021.662865
- Polman, J. A. E., De Kloet, E. R., & Datson, N. A. (2013). Two populations of glucocorticoid receptor-binding sites in the male rat hippocampal genome. Endocrinology, 154(5), 1832–1844. https://doi.org/10.1210/en.2012-2187 23525215
- Polman, J. A. E., Hunter, R. G., Speksnijder, N., Van Den Oever, J. M. E., Korobko, O. B., McEwen, B. S., De Kloet, E. R., & Datson, N. A. (2012). Glucocorticoids modulate the mtor pathway in the hippocampus: Differential effects depending on stress history. Endocrinology, 153(9), 4317–4327. https://doi.org/10.1210/en.2012-1255
- Provençal, N., Arloth, J., Cattaneo, A., Anacker, C., Cattane, N., Wiechmann, T., Röh, S., Ködel, M., Klengel, T., Czamara, D., Müller, N. S., Lahti, J., Räikkönen, K., Pariante, C. M., & Binder, E. B. (2020). Glucocorticoid exposure during hippocampal neurogenesis primes future stress response by inducing changes in DNA methylation. Proceedings of the National Academy of Sciences of the United States of America, 117(38), 23280–23285. https://doi.org/10.1073/pnas.1820842116
- Qian, X., Droste, S. K., Lightman, S. L., Reul, J. M. H. M., & Linthorst, A. C. E. (2012). Circadian and ultradian rhythms of free glucocorticoid hormone are highly synchronized between the blood, the subcutaneous tissue, and the brain. Endocrinology, 153(9), 4346–4353. https://doi.org/10.1210/en.2012-1484
- Radley, J. J., & Johnson, S. B. (2018). Anteroventral bed nuclei of the stria terminalis neurocircuitry: Towards an integration of HPA axis modulation with coping behaviors – Curt Richter Award Paper 2017. Psychoneuroendocrinology, 89, 239–249. https://doi.org/10.1016/j.psyneuen.2017.12.005
- Rainville, J. R., Weiss, G. L., Evanson, N., Herman, J. P., Vasudevan, N., & Tasker, J. G. (2019). Membrane-initiated nuclear trafficking of the glucocorticoid receptor in hypothalamic neurons. Steroids, 142, 55–64. https://doi.org/10.1016/J.STEROIDS.2017.12.005
- Ratka, A., Sutanto, W., Bloemers, M., & de Kloet, E. R. (1989). On the role of brain mineralocorticoid (type I) and glucocorticoid (type II) receptors in neuroendocrine regulation. Neuroendocrinology, 50(2), 117–123. https://doi.org/10.1159/000125210
- Ratka, A., Sutanto, W., & De Kloet, R. (1988). Long-lasting glucocorticoid suppression of opioid-induced antinociception. Neuroendocrinology, 48(4), 439–444. https://doi.org/10.1159/000125046
- Reul, J. M., & de Kloet, E. R. (1985). Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology, 117(6), 2505–2511. https://doi.org/10.1210/endo-117-6-2505
- Reul, J. M. H. M., Collins, A., Saliba, R. S., Mifsud, K. R., Carter, S. D., Gutierrez-Mecinas, M., Qian, X., & Linthorst, A. C. E. (2015). Glucocorticoids, epigenetic control and stress resilience. Neurobiology of Stress, 1(1), 44–59. https://doi.org/10.1016/j.ynstr.2014.10.001
- Reul, J. M. H. M., Stec, I., Soder, M., & Holsboer, F. (1993). Chronic treatment of rats with the antidepressant amitriptyline attenuates the activity of the hypothalamic-pituitary-adrenocortical system. Endocrinology, 133(1), 312–320. https://doi.org/10.1210/ENDO.133.1.8391426
- Revsin, Y., Rekers, N. V., Louwe, M. C., Saravia, F. E., De Nicola, A. F., De Kloet, E. R., & Oitzl, M. S. (2009). Glucocorticoid receptor blockade normalizes hippocampal alterations and cognitive impairment in streptozotocin-induced type 1 diabetes mice. Neuropsychopharmacology, 34(3), 747–758. https://doi.org/10.1038/NPP.2008.136
- Revsin, Y., Saravia, F., Roig, P., Lima, A., De Kloet, E. R., Homo-Delarche, F., & De Nicola, A. F. (2005). Neuronal and astroglial alterations in the hippocampus of a mouse model for type 1 diabetes. Brain Research, 1038(1), 22–31. https://doi.org/10.1016/j.brainres.2004.12.032
- Rivers, C. A., Rogers, M. F., Stubbs, F. E., Conway-Campbell, B. L., Lightman, S. L., & Pooley, J. R. (2019). Glucocorticoid receptor-tethered mineralocorticoid receptors increase glucocorticoid-induced transcriptional responses. Endocrinology, 160(5), 1044–1056. https://doi.org/10.1210/EN.2018-00819
- Roat-Shumway, S., Wroolie, T. E., Watson, K., Schatzberg, A. F., & Rasgon, N. L. (2018). Cognitive effects of mifepristone in overweight, euthymic adults with depressive disorders. Journal of Affective Disorders, 239, 242–246. https://doi.org/10.1016/J.JAD.2018.07.014
- Roozendaal, B., Hahn, E. L., Nathan, S. V., de Quervain, D. J.-F., & McGaugh, J. L. (2004). Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. The Journal of Neuroscience, 24(37), 8161–8169. https://doi.org/10.1523/JNEUROSCI.2574-04.2004
- Roozendaal, B., & McGaugh, J. L. (2011). Memory modulation. Behavioral Neuroscience, 125(6), 797–824. https://doi.org/10.1037/a0026187
- Sapolsky, R. M., Romero, L. M., & Munck, A. U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 21(1), 55–89. https://doi.org/10.1210/er.21.1.55
- Sarabdjitsingh, R. A., Isenia, S., Polman, A., Mijalkovic, J., Lachize, S., Datson, N., de Kloet, E. R., & Meijer, O. C. (2010). Disrupted corticosterone pulsatile patterns attenuate responsiveness to glucocorticoid signaling in rat brain. Endocrinology, 151(3), 1177–1186. https://doi.org/10.1210/en.2009-1119
- Scarpa, J. R., Fatma, M., Loh, Y. H. E., Traore, S. R., Stefan, T., Chen, T. H., Nestler, E. J., & Labonté, B. (2020). Shared transcriptional signatures in major depressive disorder and mouse chronic stress models. Biological Psychiatry, 88(2), 159–168. https://doi.org/10.1016/j.biopsych.2019.12.029
- Schmidt, M. V., Levine, S., Alam, S., Harbich, D., Sterlemann, V., Ganea, K., de Kloet, E. R., Holsboer, F., & Müller, M. B. (2006). Metabolic signals modulate hypothalamic-pituitary-adrenal axis activation during maternal separation of the neonatal mouse. Journal of Neuroendocrinology, 18(11), 865–874. https://doi.org/10.1111/j.1365-2826.2006.01482.x
- Schwabe, L., Hermans, E. J., Joëls, M., & Roozendaal, B. (2022). Mechanisms of memory under stress. Neuron, 110(9), 1450–1467. https://doi.org/10.1016/j.neuron.2022.02.020
- Schwabe, L., & Wolf, O. T. (2013). Stress and multiple memory systems: from “thinking” to “doing”. Trends in Cognitive Sciences, 17(2), 60–68. https://doi.org/10.1016/j.tics.2012.12.001
- Seckl, J. R., & Fink, G. (1992). Antidepressants increase glucocorticoid and mineralocorticoid receptor mRNA expression in rat hippocampus in vivo. Neuroendocrinology, 55(6), 621–626. https://doi.org/http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1321353 https://doi.org/10.1159/000126180
- Selye, H. (1946). The general adaptation syndrome and the diseases of adaptation. The Journal of Clinical Endocrinology & Metabolism, 6(2), 117–230. https://doi.org/10.1016/j.ajog.2010.07.025
- Selye, H. (1950). STRESS – The physiology and pathology of exposure to stress. In Acta Inc Montreal, 203, 1025. https://doi.org/10.1016/S0016-5085(51)80143-4
- Shorter, E., & Fink, M. (2010). Endocrine psychiatry : solving the riddle of melancholia. (p. 193). Oxford University Press.
- Ribeiro, S. (2021). The Oracle of Night: the history and science of dreams. Bantam Press.
- Solomon, M. B., Wulsin, A. C., Rice, T., Wick, D., Myers, B., McKlveen, J., Flak, J. N., Ulrich-Lai, Y., & Herman, J. P. (2014). The selective glucocorticoid receptor antagonist CORT 108297 decreases neuroendocrine stress responses and immobility in the forced swim test. Hormones and Behavior, 65(4), 363–371. https://doi.org/10.1016/j.yhbeh.2014.02.002
- Sousa, N. (2016). The dynamics of the stress neuromatrix. Molecular Psychiatry, 21(3), 302–312. https://doi.org/10.1038/mp.2015.196
- Stranahan, A. M., Arumugam, T. V., Cutler, R. G., Lee, K., Egan, J. M., & Mattson, M. P. (2008). Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nature Neuroscience, 11(3), 309–317. https://doi.org/10.1038/NN2055
- Taubenfeld, S. M., Riceberg, J. S., New, A. S., & Alberini, C. M. (2009). Preclinical assessment for selectively disrupting a traumatic memory via postretrieval inhibition of glucocorticoid receptors. Biological Psychiatry, 65(3), 249–257. https://doi.org/10.1016/J.BIOPSYCH.2008.07.005
- Taylor, S. E., Klein, L. C., Lewis, B. P., Gruenewald, T. L., Gurung, R. A., & Updegraff, J. A. (2000). Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review, 107(3), 411–429. https://doi.org/10.1037/0033-295X.107.3.411
- Tempel, D. L., McEwen, B. S., & Leibowitz, S. F. (1993). Adrenal steroid receptors in the PVN: studies with steroid antagonists in relation to macronutrient intake. Neuroendocrinology, 57(6), 1106–1113. https://doi.org/10.1159/000126477
- ter Horst, J. P., van der Mark, M., Kentrop, J., Arp, M., van der Veen, R., de Kloet, E. R., & Oitzl, M. S. (2014). Deletion of the forebrain mineralocorticoid receptor impairs social discrimination and decision-making in male, but not in female mice. Frontiers in Behavioral Neuroscience, 8, 26. https://doi.org/10.3389/fnbeh.2014.00026
- Turecki, G., & Meaney, M. J. (2016). Effects of the social environment and stress on glucocorticoid receptor gene methylation: A systematic review. Biological Psychiatry, 79(2), 87–96. https://doi.org/10.1016/j.biopsych.2014.11.022
- Tzanoulinou, S., Gantelet, E., Sandi, C., & Márquez, C. (2020). Programming effects of peripubertal stress on spatial learning. Neurobiology of Stress, 13, 100282. https://doi.org/10.1016/j.ynstr.2020.100282
- Ulrich-Lai, Y. M., Christiansen, A. M., Ostrander, M. M., Jones, A. A., Jones, K. R., Choi, D. C., Krause, E. G., Evanson, N. K., Furay, A. R., Davis, J. F., Solomon, M. B., De Kloet, A. D., Tamashiro, K. L., Sakai, R. R., Seeley, R. J., Woods, S. C., & Herman, J. P. (2010). Pleasurable behaviors reduce stress via brain reward pathways. Proceedings of the National Academy of Sciences of the United States of America, 107(47), 20529–20534. https://doi.org/10.1073/PNAS.1007740107
- Ulrich-Lai, Y. M., & Herman, J. P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience, 10(6), 397–409. https://doi.org/10.1038/NRN2647
- van Haarst, A. D., Oitzl, M. S., & de Kloet, E. R. (1997). Facilitation of feedback inhibition through blockade of glucocorticoid receptors in the hippocampus. Neurochemical Research, 22(11), 1323–1328. https://doi.org/10.1023/A:1022010904600
- Van Haarst, A. D., Oitzl, M. S., Workel, J. O., & De Kloet, E. R. (1996). Chronic brain glucocorticoid receptor blockade enhances the rise in circadian and stress-induced pituitary-adrenal activity. Endocrinology, 137(11), 4935–4943. https://doi.org/10.1210/ENDO.137.11.8895366
- van Oers, H. J. J., de Kloet, E. R., Whelan, T., & Levine, S. (1998). Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. The Journal of Neuroscience, 18(23), 10171–10179. https://doi.org/10.1523/JNEUROSCI.18-23-10171.1998
- van Weert, L. T. C. M., Buurstede, J. C., Sips, H. C. M., Mol, I. M., Puri, T., Damsteegt, R., Roozendaal, B., Sarabdjitsingh, R. A., & Meijer, O. C. (2019). Mechanistic insights in NeuroD potentiation of mineralocorticoid receptor signaling. International Journal of Molecular Sciences, 20(7), 1575. https://doi.org/10.3390/ijms20071575
- Viho, E. M. G., Kroon, J., Feelders, R. A., Houtman, R., van den Dungen, E. S. R., Pereira, A. M., Hunt, H. J., Hofland, L. J., & Meijer, O. C. (2022). Peripheral glucocorticoid receptor antagonism by relacorilant with modest HPA axis disinhibition. Journal of Endocrinology, 256(2), e220263. https://doi.org/10.1530/JOE-22-0263
- Warris, L. T., Van Den Heuvel-Eibrink, M. M., Aarsen, F. K., Pluijm, S. M. F., Bierings, M. B., Van Bos, C., Den, Zwaan, C. M., Thygesen, H. H., Tissing, W. J. E., Veening, M. A., Pieters, R., & Van Den Akker, E. L. T. (2016). Hydrocortisone as an intervention for dexamethasone-induced adverse effects in pediatric patients with acute lymphoblastic leukemia: Results of a double-blind, randomized controlled trial. Journal of Clinical Oncology, 34(19), 2287–2293. https://doi.org/10.1200/JCO.2015.66.0761
- Watson, S., Gallagher, P., Porter, R. J., Smith, M. S., Herron, L. J., Bulmer, S., Young, A. H., & Ferrier, I. N. (2012). A randomized trial to examine the effect of mifepristone on neuropsychological performance and mood in patients with bipolar depression. Biological Psychiatry, 72(11), 943–949. https://doi.org/10.1016/J.BIOPSYCH.2012.05.029
- Watts, A. G. (2005). Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: A complexity beyond negative feedback. Frontiers in Neuroendocrinology, 26(3–4), 109–130. https://doi.org/10.1016/j.yfrne.2005.09.001
- Weger, M., Alpern, D., Cherix, A., Ghosal, S., Grosse, J., Russeil, J., Gruetter, R., de Kloet, E. R., Deplancke, B., & Sandi, C. (2020). Mitochondrial gene signature in the prefrontal cortex for differential susceptibility to chronic stress. Scientific Reports, 10(1), 18308. https://doi.org/10.1038/s41598-020-75326-9
- Wirz, L., Bogdanov, M., & Schwabe, L. (2018). Habits under stress: mechanistic insights across different types of learning. Current Opinion in Behavioral Sciences, 20, 9–16. https://doi.org/10.1016/j.cobeha.2017.08.009
- Wood, M., Adil, O., Wallace, T., Fourman, S., Wilson, S. P., Herman, J. P., & Myers, B. (2019). Infralimbic prefrontal cortex structural and functional connectivity with the limbic forebrain: a combined viral genetic and optogenetic analysis. Brain Structure & Function, 224(1), 73–97. https://doi.org/10.1007/S00429-018-1762-6
- Wood, N. E., Rosasco, M. L., Suris, A. M., Spring, J. D., Marin, M. F., Lasko, N. B., Goetz, J. M., Fischer, A. M., Orr, S. P., & Pitman, R. K. (2015). Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: Three negative psychophysiological studies. Psychiatry Research, 225(1-2), 31–39. https://doi.org/10.1016/J.PSYCHRES.2014.09.005
- Wulsin, A. C., Herman, J. P., & Solomon, M. B. (2010). Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology, 35(7), 1100–1112. https://doi.org/10.1016/j.psyneuen.2010.01.011
- Yang-Yen, H. F., Chambard, J. C., Sun, Y. L., Smeal, T., Schmidt, T. J., Drouin, J., & Karin, M. (1990). Transcriptional interference between c-Jun and the glucocorticoid receptor: Mutual inhibition of DNA binding due to direct protein-protein interaction. Cell, 62(6), 1205–1215. https://doi.org/10.1016/0092-8674(90)90396-v
- Yates, F. E., Leeman, S. E., Glenister, D. W., & Dallman, M. F. (1961). Interaction between plasma corticosterone concentration and adrenocorticotropinreleasing stimuli in the rat: Evidence for the reset of an endocrine feedback control. Endocrinology, 69(1), 67–80. https://doi.org/10.1210/ENDO-69-1-67
- Yongue, B. G., & Roy, E. J. (1987). Endogenous aldosterone and corticosterone in brain cell nuclei of adrenal-intact rats: regional distribution and effects of physiological variations in serum steroids. Brain Research, 436(1), 49–61. https://doi.org/10.1016/0006-8993(87)91555-1
- Zalachoras, I., Verhoeve, S. L., Toonen, L. J., van Weert, L. T. C. M., van Vlodrop, A. M., Mol, I. M., Meelis, W., de Kloet, E. R., & Meijer, O. C. (2016). Isoform switching of steroid receptor co-activator-1 attenuates glucocorticoid-induced anxiogenic amygdala CRH expression. Molecular Psychiatry, 21(12), 1733–1739. https://doi.org/10.1038/mp.2016.16

