Abstract
Background: Despite the rapid increase in reports of exhaustion syndrome (ES) due to daily occupational stress, the mechanisms underlying ES are unknown. In the present study, we investigated whether occupational ES is associated with specific modifications of the subfields of the amygdala and hippocampus resembling those described in other chronic stress conditions. Special focus was paid to possible sex differences.
Methods: As a follow up to our previous studies of occupational ES, we carried out MRI-based subfield segmentation of the hippocampus and amygdala volumes in 58 patients with occupational ES (22 males) and 65 age-matched controls (27 males) (age range 30–46 years).
Results: There was a significant and bilateral enlargement of the lateral, basal and central nucleus of the amygdala in patients with ES (corrected for the total intracranial volume (ICV)). These differences were detected only in females. Higher values in the right central and right basal amygdala remained when the whole amygdala volume was used as reference, instead of the ICV. Notably, in female patients the volumes of these specific nuclei were positively correlated with the degree of perceived stress. No changes in the hippocampus subfields were detected in female or male patients.
Conclusions: The findings underline that ES is a chronic stress condition, suggesting that not only extreme forms of stress, but also the everyday stress is associated with localized differences from controls in the amygdala. The absence of significant alterations among men with ES despite a similar degree of perceived stress supports the notion that women seem more susceptible to stress-related cerebral changes, and may explain the higher prevalence of ES among women.
Introduction
There are increasing reports about damaging effects of chronic occupational stress on cognitive functions (Abe et al., Citation2022; Gavelin et al., Citation2020; Glise et al., Citation2020; Grossi et al., Citation2015; Lee et al., Citation2021; Savic, Citation2015; Savic et al., Citation2018). The affected persons, typically young and healthy, describe stereotypical symptoms with disturbed sleep, memory and attention problems, and profound fatigue. As opposed to the chronic fatigue syndrome (myalgic encephalomyelitis), this fatigue does not worsen with exertion, pain is not the major symptom, and there are no signs of inflammation (Bested & Marshall, Citation2015). The symptoms develop gradually and are attributed to occupational stress, due to high workload, long hours without break, and maladaptive coping behaviors (Grossi et al., Citation2015; Savic et al., Citation2018). The symptoms are frequently misdiagnosed as depression. However, antidepressants have very rarely any effect (Asberg et al., Citation2010). The condition was in 2019 recognized by the WHO, and labeled occupational burnout, or exhaustion syndrome (ES). ES is more common in women and is still not accepted as a medical condition, and is by many regarded as ‘functional’, meaning without cerebral correlates. Based on combined brain imaging and behavioral studies, we queried this notion and suggested that ES indeed is a medical condition, and that it is related to chronic stress, which we search to further elaborate in the present manuscript.
Across several study groups with ES (Abe et al., Citation2022; Blix et al., 2013; Gavelin et al., Citation2020; Glise et al., Citation2020; Lee et al., Citation2021), cerebral differences in relation to controls have been detected, resembling those reported in post-traumatic stress disorder (PTSD), and in early life stress. In specific, among persons with ES there was a selective thinning of the mesial prefrontal cortex (mPFC), and the superior temporal gyrus, along with an enlargement of the amygdala volume and reduction of the caudate volume (Abe et al., Citation2022; Blix et al., Citation2013; Lee et al., Citation2021; Savic, Citation2015; Savic et al., Citation2018). Notably, enlargement of the amygdala volumes was detected only in women. At variance from some other stress conditions in studied in humans, like PTSD and early life stress, which often show a reduction of the hippocampus volume (De Bellis et al., Citation2001; Hanson et al., Citation2015; Henigsberg et al., Citation2019; Kitayama et al., Citation2005), no changes in the hippocampus volumes were detected in our cross sectional or longitudinal studies of ES. The hippocampus is, however, a complex structure, which can be divided into several subfields (Fanselow & Dong, Citation2010). Each subfield presents different histological characteristics and, also, different functions. Chronic stress in rats produces atrophy and debranching of dendrites in pyramidal neurons of the CA3 (Vyas et al., Citation2002), as well as decreased neurogenesis in the dentate gyrus (DG) (Gould et al., Citation1998; McEwen, Citation1999). The hippocampus is also implicated in the regulation of the hypothalamic-pituitary-adrenal (HPA) axis, and there is some evidence that the CA3 and the DG subregions play a crucial role in stress adaptation via the glucocorticoid receptors (McEwen, Citation2007).
The few studies of stress in humans evaluating hippocampus subfields indeed show a reduction of the left CA3, DG and subiculum in maltreated children (Teicher et al., Citation2012), also in the DG (Hayes et al., Citation2017) and CA1–3 in PTSD (Mid-Atlantic MIRECC Workgroup, 2018) with some variation depending of duration of stress exposure in relation to MR measurements. Investigating the specific subfields of hippocampus, may, consequently, reveal regional hippocampal differences from controls that otherwise can be missed.
Another structure highly involved in the physiology of stress is the amygdala, which is the major relay in the brain to detect and appraise stress stimuli. Animal studies have frequently shown alterations, primarily as growth of the dendritic spines, in the amygdala following severe or prolonged stress. Mitra et al (Mitra et al., Citation2005) found that rats subjected to a 10 day chronic stress paradigm led to an increase in spine density in the basolateral amygdala (BLA). Vyas et al. (Citation2004) investigated the long-term effects of chronic immobilization stress in rats, and found that the dendritic remodeling in the BLA was present even after substantial length of recovery time (21 days). Vyas et. al reported in two other studies that chronic immobilization stress led to dendritic arborization in both the basolateral complex (Vyas et al., Citation2002) and the extended amygdala (Vyas et al., Citation2003). The enlargement in amygdala in response to chronic stress could possibly be due to an increase in glucocorticoid-driven expression of brain-derived neurotrophic factor (BDNF) (Bennett & Lagopoulos, Citation2014; Lakshminarasimhan & Chattarji, Citation2012). Stress related increase in BDNF has been reported specifically in the BLA with and neuronal arborization and hypertrophy of spine density (Lakshminarasimhan & Chattarji, Citation2012; Morey et al., Citation2020). Data from humans with respect to amygdala sub-nuclei in relation to stress are still sparse, as the technique to investigate structural subfields has only recently become available. The pattern of structural changes varies between different reports and also on the sex, age at stress exposure, frequency, duration, and the type of biopsychosocial stress (DeCasien et al., Citation2022; Karl et al., Citation2006; Kuo et al., Citation2012; Landré et al., Citation2010; Morey et al., Citation2012; Citation2020; Ousdal et al., Citation2020; Pavlisa et al., Citation2006; Tottenham et al., Citation2010; Veer et al., Citation2015; Woon & Hedges, Citation2009).
Morey et al. found in 149 veterans with PTSD smaller volumes of the left and right lateral and paralaminar nuclei, but with larger left and right central, medial, and cortical nuclei compared to those who were in was but did not develop PTSD (Morey et al., Citation2020). The time between scan and trauma expose was however, not reported. This may indeed be of importance as shown Ousdal et al., (Citation2020) investigated amygdala nuclei volumes of the basolateral and the centro-cortico-medial complex in relation to PTSD symptom severity in 47 young survivors from the 2011 Norwegian terror attack 24–36 months post-trauma. They found that increased PTSD symptom severity 24–36 months post-trauma was associated with volumetric reductions of all basolateral as well as the central and the medial nuclei. However, only the lateral nucleus was associated with longitudinal symptom development and mediated the association post-trauma symptoms after 36 months post trauma (Ousdal et al., Citation2020). The importance of careful clinical investigations was also shown by a study of Kuo et al. (Citation2012). Whereas they detected an overall increase in total amygdala volumes among veterans with PTSD compared to those without PTSD, (no sub-segmentation was carried out), they also found that the presence of early life trauma and the severity of combat exposure were significantly associated with smaller total amygdala volume. In contrast, Morey et al. (Citation2012) reported a smaller amygdala volume among soldiers with PTSD (compared to without those without PTSD). Furthermore, there was no correlation between trauma load or chronicity of PTSD and amygdala volume, which was interpreted to suggest that a smaller amygdala represents a vulnerability to developing PTSD. Considering variability of findings, especially of the analysis did not include sub segmentation, it is not surprising that Woon et al., in a meta-analysis of amygdala volumes in PTSD did not show any differences from controls (Woon & Hedges, Citation2009). Another meta-analysis (Karl et (Karl et al., Citation2006) found smaller left but not right whole amygdala volume investigating reports with 10 years or more between scans and trauma exposure. No sub segmentation data were reported.
In another type of chronic stress in humans, namely the early life traumas, Veer et al reported reduction of the right but not left whole amygdala volume (no sub segmentation was done), (Veer et al., Citation2015). Finally, in a very small sample (n = 7) of women exposed to sexual abuse and 17 controls no difference in amygdala volume was detected (Landré et al., Citation2010). Obviously, the findings are divergent (see also the meta-analysis by Heningsberg et al. (Vyas et al., Citation2002). Several factors can have contributed to these divergent results. First, most of studies with PTSD were carried out only in men or with a considerably higher proportion of men. Sex was used as covariate, and no separate results for men and women were provided. Secondly, only few studies considered amygdala sub volumes. Third, differences to time to stress exposure seems to be of importance with a reduction of the amygdala volume with time.
Both the hippocampus and amygdala are sex dimorphic structures with amygdala volume being larger in men and the hippocampus volume larger in women, even when correcting for the total intracranial volume (ICV) (DeCasien et al., Citation2022; Fish et al., Citation2020). There are also emerging studies, suggesting sex differences in effects of stress on these structures. Wang et al. (Citation2007) found that increase in cerebral blood flow in response to acute stress in the right prefrontal cortex was greater among men than women, whereas in women the amygdala activation was more prolonged. Kogler et al. (Kogler et al., Citation2015) noticed a more pronounced stress activation of the left amygdala and the right superior temporal gyrus in women, and a more robust activation of the left putamen in men.
More recently, the same group also found a sex by cortisol interaction (Kogler et al., Citation2016), congruent with previous hypotheses that stress-induced cortisol release (measured in saliva) is less pronounced in women and varies during the menstrual cycle (lower response when estrogen is high) (Albert et al., Citation2015; Kajantie & Phillips, Citation2006; Kirschbaum et al., Citation1999).
Both hippocampus and amygdala are sex hormone sensitive structures, and it is possible that effects of stress are different in men and women, not only with respect to the total structural volumes, but also the specific subfields and subnuclei of the hippocampus and the amygdala.
We therefore carried out detailed investigations of the amygdala and hippocampus volumes in patients with ES and controls. We opted at a further evaluation of the notion that occupational ES is a stress related condition. We also investigated whether this condition is associated with sex differences in the pattern of stress related structural modification of the amygdala and hippocampus. A newly developed FreeSurfer method, that allows for an accurate and reproducible segmentation of several hippocampal and amygdala subfields, was employed. The study groups were expanded compared to our previous studies, allowing selection of age matched persons with ES and controls.
Based on our previous data and the available literature, we hypothesized:
That persons with ES would have larger basal and lateral amygdala volumes than controls
That the volume of CA1–3 and the DG of the hippocampus would be reduced in persons with ES
That differences in the amygdala volumes would be detected primarily among female patients. Whether this could be attributed to specific subnuclei was an open question.
Materials and methods
Participants
Initially, 75 participants complaining of symptoms consistent with occupational ES (Glise et al. Citation2020; Hanson et al. Citation2015; Henigsberg et al. Citation2019; Fanselow et al. Citation2010) were included, (47 females, age 38 ± 7 and 28 males, age 41 ± 7). 87 right-handed healthy and unstressed participants (44 females, age 32 ± 7 years, 43 males, age 30 ± 7 years) served as controls. Persons with ES had been diagnosed as having a “reaction to severe stress and an adjustment disorder” (ICD-10, F43.8A), and were recruited from the Stress Clinic Foundation in Stockholm, Sweden, where they were patients (they will therefore be labeled as patients here). Stress was defined in accordance with Lazarus and Folkman (Citation1987), as resulting from an “imbalance between demands and resources.” According to the patients own descriptions, they all had typical maladaptive coping behaviors, in the form of underestimating the significance of occupational load, accepting high workload, and/or feeling that the work situation can be controlled by increasing their working hours. They reported working 60–70 h per week continuously over several years prior to the onset of symptoms, which was documented in medical charts. All described a characteristic symptom course of disturbed sleep, memory and attention problems, profound fatigue, and reduced work capacity (confirmed by the employers), attributed to chronic occupational stress.
Table 1. Demographic data.
To be included in the study, ES patients were also required to have had a symptom duration of at least 1 year (range 1.5–3.5 years, irrespective of gender), to have been on sick leave (≥50%) for stress-related symptoms for a minimum of 3 months before entering the study, and to have an average stress-burnout score of ≥3.0 on the Maslach Stress- Burnout Inventory-General Survey (MBI-GS) as described previously (Savic, Citation2015; Savic et al., Citation2018). Their cortisol levels were within normal ranges, as reported earlier (Savic et al., Citation2018). The controls were healthy, unmedicated, reported no psycho-social stress, scoring <2.5 (mean ± SD of the general population) on the MBI-GS questionnaire.
Participants were excluded if they had a previous history of psychosis, personality disorder, major or bipolar depression, alcohol or substance abuse, chronic fatigue, chronic pain, fibromyalgia, neurological or endocrine disease. Those who experienced prominent stress factors in their private life or a major traumatic event at any time in their life, were also excluded. This was done on the basis of selected items of the Holms and Raphe scale (Holmes & Rahe, Citation1967), (items related to work were not relevant to our population and were therefore omitted). Furthermore, because this scale lacks some important aspects about impactful stress exposure such as early life trauma, incest, rape, life threatening situations as cancer, we added these specific items to the clinical interview. This procedure was used only to determine which participants should be included in the study. Daily medication was allowed during the 2 months prior to the study, except contraceptives (16 of the finally included female patients and 16 of the finally included female controls were using contraceptives at the time of the study). According to a review of their pharmacological treatment histories, none of them had taken drugs that are known to affect brain structure (e.g., psychopharmaca). None of the participants was a smoker. The patient and control groups had similar sex distributions.
Participants were investigated with the Swedish version of the Mini-International Neuropsychiatric Interview, and the Montgomery-Åsberg Depression Rating Scale (MADRS), as described previously (Savic, Citation2015; Savic et al., Citation2018). Patients also received a medical screening (physical examination, test of thyroid function). All female participants had regular menstruations and were investigated during the first week of their menstrual cycles. Only age matched participant groups were selected for statistical comparisons, which was done by removing outliers (using 2 SD of the mean age) (in the entire more extensive study populations patients were slightly elder than controls). The groups were homogenous with respect to type of profession and education. The final data for analysis and group comparisons consisted of 22 male patients and 27 male controls. Among female participants, we selected 36 patients and 38 controls.
The study was approved by the Ethics Committee at Karolinska Institute, and written informed consent was received from each patient.
MR data acquisition
Magnetic resonance imaging was carried out using a 3D T1-weighted spoiled gradient echo pulse sequence (SPGR) acquired with a 3 Tesla MRI medical scanner (Discovery 3 T GE-MR750, General Electric, Milwaukee, Wisconsin). The data was recorded with 1mm3 isotropic voxel size and with an 8-channel head coil using the following specifications: TE = 3.1 ms, TR = 7.9 ms, TI = 450 ms, FoV = 24 cm, 176 axial slices, flip angle of 12°.
Data processing
After visual inspection and radiological assessment of the acquired data, the T1-weighted scans were processed using FreeSurfer 7.0 with the standard cross-sectional recon-all pipeline (Dale et al., Citation1999; Fischl et al., Citation1999).These steps included Talairach registration, correction for bias field artifacts and skull stripping. In a next step, white and gray matter were segmented leading to calculations of the white and the pial surfaces. The 7.0 version of the FreeSurfer was used in order to allow comparisons with our previous calculations of the amygdala and hippocampus volumes.
Segmentation of hippocampal subfields and the amygdala nuclei
Hippocampal subfields and the nuclei of the amygdala were calculated with FreeSurfer 7.1.1 using the cross-sectional (Iglesias et al., Citation2015; Saygin et al., Citation2017) as well as the longitudinal processing stream (the latter was used for future follow up analyses). The different sub-compartments were segmented via a Bayesian inference approach, by using intensities and prior knowledge from a probabilistic atlas, based on ultra-high resolution ex-vivo MRI data (Chiappiniello et al., Citation2021). The final segmentations were statistically checked for outliers, followed by a complete visual inspection by two different raters to ensure high segmentation quality and to exclude subjects with putative segmentation errors. For the sake of consistency, we also used the 7.1.1 version for calculation of the whole hippocampus volume, whole amygdala volume and the ICV. The calculation of ICV with the 7.1.1 version is used in the result report.
The hippocampus was segmented into 19 subfields: parasubiculum, HATA, fimbria, hippocampal fissure, hippocampal tail, presubiculum head, presubiculum body, subiculum head, subiculum body, CA1 head, CA1 body, CA3 head, CA3 body, CA4 head, CA4 body, GC-ML-DG head, GC-ML-DG body, molecular layer head and molecular layer body. Furthermore, volumes of the whole hippocampus on the right and left side were calculated.
The FreeSurfer subfield tool was also used for amygdala sub-segmentation. In addition to the whole amygdala volumes the FreeSurfer tool was used to segment 7 subnuclei of the amygdala: lateral nucleus, basal nucleus, central nucleus, medial nucleus, cortical nucleus, accessory basal nucleus, and the paralaminar nucleus, the cortico-amygdaloid transition area, and the anterior amygdaloid area (AAA).
The FreeSurfer pipeline delivers robust results with reliable segmentations of most of the hippocampal and amygdala subfields (Brown et al., Citation2020; Chiappiniello et al., Citation2021), () with exception of certain smaller structures. This applies particularly to the hippocampal fissure, parasubiculum and fimbria, which show lower measurement-consistency (Brown et al., Citation2020; Chiappiniello et al., Citation2021). Taking these methodological aspects into consideration, and the fact that just certain subfield regions provided by the FreeSurfer software are relevant for studies of psychosocial stress, only selected subfield measures were used as input. For the hippocampus these subfields were the right and left CA1, CA3, CA4, DG, the presubiculum, and subiculum. We used the mean values of the head, body and tail of the hippocampus in the final group comparison in order to reduce the number of comparisons, and that there was no specific hypothesis that a specific part (head, body, or tail) or subfield of the hippocampus would be affected. With respect to the amygdala, we included the right and left anterior amygdala nucleus, the lateral, medial, basal, and central nucleus.
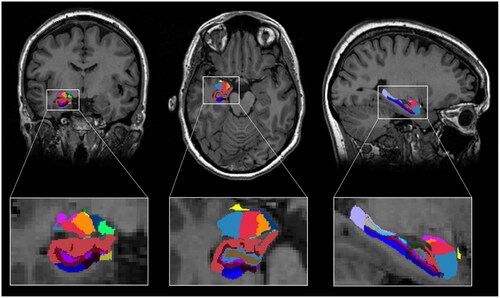
Figure 1. Segmentation of hippocampal subfields and amygdala nuclei. T1-weighted scan with segmentations from a representative subject using the longitudinal stream. Results are displayed in coronal-, axial- and sagittal view and radiological convention (right = left).
Statistical analyses
Because of the known sex differences in the hippocampus and amygdala volumes even when correcting for differences in the ICV (DeCasien et al., Citation2022) and the reported sex differences among ES patients (Blix et al., Citation2013; Landré et al., Citation2010; Savic et al., Citation2018), all the comparisons were carried out between female ES patients and female controls, male ES patients and male controls separately. Possible group differences in education and the ICV were tested using one way ANOVA (p<.05). Group comparisons in total amygdala volume, total hippocampus volume, as well as subfield- and substructural- volumes were first based on region/ICV ratios using multivariate general linear model multivariate multiple regression (that is, multiple dependent variables for different regions). When the omnibus test was significant (Wilk’s lamda p<.05), we examined group differences for the individual subfields and subnuclei on both left and right side (p < 0.05) without having to control for multiple post hoc tests, according to Fisher’s Least significant difference procedure.
Group differences were tested, in addition, with respect to subfield and subnucleus volumes in relation to the ipsilateral whole hippocampus and whole amygdala volume. The rationale was that the specific sub-volumes are not necessarily linearly correlated with the ICV. Another rationale was that we searched to evaluate whether and which specific volume differences remained when correcting for possible diffuse/overall group difference in the hippocampus and/or amygdala.
Results
Demography
The demographic data are presented in . There were no significant group differences in years of education (patients 17.1 years ± 3.3, controls 16.4 years ± 2.9, p=.36), age, or ICV. Men had, as expected, larger ICV than women. Both MADRAS and MBI-GS mean scores were significantly higher in ES patients, independently of sex (F > 50, p<.001 for all the comparisons. Difference between the two male groups, as well as the two female groups was detected with respect to stress scores (p<.001), MADRAS scores (p<.001), but not ICV nor age.
Volume differences between ES patients and controls
No significant group difference were detected between female ES patients and female controls in the whole hippocampus volume, nor in any of the hippocampal subfields on the right or left side, (Wilk’s lambda = 0.653, F(27,47)=1.055; p=.624) (). Neither did the corresponding comparisons between males with ES and male controls reveal an overall group difference (Wilk’s lambda = 0.519, F(18,39)=1.147, p=.378).
Table 2a. Volumes of the hippocampus subfields.
Table 2b. Volumes of the amygdala subfields.
Statistical comparisons with respect to amygdala sub-fields showed a different pattern. There was an overall group difference (Wilk’s lambda = 0.662, F(10,56)=2.850, p=.006). Furthermore, this difference was detected only between the two groups of females. It was constituted by an enlargement of the left and right basal amygdala (p=.009 and .021, respectively), left and right lateral amygdala (p=.025 and p=.012), left and right central amygdala (p=.028 and <.001). There was also an enlargement of the left and right whole amygdala volume (p<.001), all calculated as ratios with ICV among female ES patients. The described group differences in females remained for the right central amygdala (p=.02), and right basal amygdala (p=.017) when using the ipsilateral whole amygdala volume as reference, instead of ICV ().
In contrast to the female groups, no difference in the whole amygdala volumes or in amygdala subfield volumes (in relation to ICV) was detected between the male ES patients and male controls (Wilk’s Lambda = 0.657, F(10,38)=1.983, p=.063). This was also true when comparisons were based on amygdala subfield volumes in relation to the whole amygdala volume (Wilks lambda =0.764, F(8,80)=1.542, p=.174). No difference was detected between the two male groups. There were no group differences in the hippocampus subfields normalized with the whole hippocampus volume (Wilk’s lambda =0.648, F(10,38)=2.062, p=.053 when comparing male ES patients with male controls, and, Wilks lambda =0.832, F(8,65)=1.649, p=.128, when comparing the two groups of females).
Post hoc analysis
In a post hoc analysis, we also directly compared the volume of the hippocampal subfields and the amygdala sub volumes corrected for ICV between males and females with ES. There was an overall difference (Wilk’s Lambda = 0.005, p<.001), with a significantly larger right lateral amygdala (p=.038), right basal amygdala (p=.033) and right central amygdala (p=.005) among female patients with ES. Again, no significant differences were observed for the hippocampus subfields (Wilk’s Lamda 0.821, F(8,58) p=.171).
Correlation analysis with respect to amygdala subfields, stress perception and depression scores
Among female participants with ES there was a significant correlation between mean MBI-GS scores and the whole amygdala volumes relative to ICV (right amygdala/ICV, p=.005, r = 0.380; left amygdala/ICV, p=.032, r = 0.276; Spearman’s correlation p<.05). No such correlation was detected with the hippocampus volumes relative to ICV. Stress scores were, among female patients with ES, also positively correlated with the volume of the central amygdala/ICV (p=.009, r = 0.35). The corresponding value for left central nucleus/ICV was p=.048, r = 0.248. The right and left lateral amygdala volume/ICV showed no significant correlations with the MBI-GS scores. The right lateral nucleus/right whole amygdala volume showed, however, a significant correlation with MBI-GS scores (p=.021; r = 0.310), as did the right central amygdala nucleus/right whole amygdala volume (p=.009, r = 0.348). Significant p-values were also detected for the left and right basal nuclei, but the correlation values were below 0.3 and were regarded weak. No correlations were detected among male participants. Notably, MBI-GS scores were significantly elevated among both female and male ES patients, without any sex difference (F(1,46)=0.265, p=.609, (3.9 ± 0.5) for the respective group). In contrast to the stress scores, MADRAS scores showed no correlation with any of the measured volumes.
Discussion
The present study investigates whether chronic occupational stress is associated with changes in specific subfields of the amygdala and hippocampus, if such changes accord with those described in more “conventional” stress conditions, and whether the pattern of tentative changes differs between men and women. The generated data add to the existing literature by showing that chronic occupational stress is associated with a localized, bilateral, enlargement of the basal and central amygdala. These specific nuclei are known to be particularly involved in chronic stress conditions (Bennett & Lagopoulos, Citation2014; Hanson et al., Citation2015; Henigsberg et al., Citation2019; Lakshminarasimhan & Chattarji, Citation2012; McEwen, Citation2007; Morey et al., 2012; Citation2020; Ousdal et al., Citation2020; Tottenham et al., Citation2010; Veer et al., Citation2015; Vyas et al., Citation2002). The described pattern of changes was detected only among female patients with ES, independently of whether the ipsilateral whole amygdala volume or the ICV was used as reference. Interestingly, the degree of perceived stress, indexed by MBI-GS scores, was among female ES patients positively correlated to the volume of the right amygdala and the right central amygdala (normalized with the ipsilateral whole amygdala volume), whereas there was no such correlation in male patients. This was even though the MBI-GS scores did not differ between female and male ES patients; they were significantly elevated among both groups.
To the best of our knowledge this is the first report from a study about amygdala and hippocampus subfield volumes in occupational stress. Occupational stress is highly debated, and there is still skepticism as to whether occupational ES is a stress related medical condition, despite a stress typical array of clinical symptoms. The present finding of selective amygdala changes resembling those described in known stress conditions, therefore, deserve attention.
Tentative underlying mechanisms
Data from animal studies of stress point rather unanimously to the basolateral amygdala as the primary relay to stress stimuli. The basolateral amygdala, or basolateral complex, consists of the lateral, basal and accessory-basal nuclei. The lateral nuclei receive the sensory information directly from the temporal lobe structures, including the hippocampus and primary auditory cortex. The basolateral amygdala also receives dense neuro modulatory inputs from ventral tegmental area, locus coeruleus, and basal forebrain. The information is then processed by the basolateral complex and sent to the central nucleus of the amygdala, which is also found to be affected in conditions with chronic stress.
Animal data suggest that stress has differentiated effects on the amygdala and hippocampus, although the exact molecular mechanisms are not yet fully understood. A prominent role seems to be played by stress related glucocorticoid driven expression of BDNF (Bennett & Lagopoulos, Citation2014; Lakshminarasimhan & Chattarji, Citation2012) together with glutamate (McEwen, Citation1999; Citation2007; Savic, Citation2020). Whereas chronic stress causes shortening of dendrites in the CA3 region of rats, the basolateral amygdala responds by an expansion of dendrites (Vyas et al., Citation2002) Furthermore, whereas in CA3 of the hippocampus, there is a downregulation in BDNF, this growth factor is increased in the basolateral amygdala (Bonne et al., Citation2001; Lakshminarasimhan & Chattarji, Citation2012).
The lateral amygdala is densely populated by glutamatergic and GABAergic neurons, which express estrogen receptors (Gan et al., Citation2014). During initial periods of chronic stress exposure, basolateral amygdala sends strong excitatory glutamatergic connections to the mPFC, which responds with inhibitory feedback to the amygdala, thereby modulating stress perception (McEwen, Citation1999; Citation2007). One observation in our previous brain imaging studies of occupational stress is that glutamate concentration was increased in the mPFC (Savic, Citation2020), a region also showing a selective cortical thinning (Savic, Citation2015; Savic et al., Citation2018). One possibility is that during the course of chronic exposure to stress, the thinning of mPFC leads to impaired inhibitory feed back to the lateral amygdala rendering this particular region more exposed to the internal glutamatergic excitotoxicity subsequently leading to dendritic shrinkage in the glutamate receptor abundant lateral nucleus. Such a mechanism, although yet hypothetical, is supported by some human studies (see further).
Data from segmentation analyses of the hippocampus and amygdala subfields in chronic stress conditions in humans are sparse and has been focused on PTSD and early life stress, both representing an extreme form of stress. The degree and direction of stress-associated difference in humans (increase vs. reduction of the basolateral amygdala volume) seems to depend on age, type of stress, duration, and frequency of exposure to stressful events. These variables can explain some seemingly inconsistent reports about exact direction of changes in the basolateral amygdala. In addition, recent studies of PTSD show that basolateral and central amygdala may be differentially affected in PTSD (Morey et al., Citation2020), tentatively depending on the stage of the condition.
Several publications show that trauma during vulnerable periods early in life can result in hypertrophy of the basolateral amygdala (Tottenham et al., Citation2010) which is consistent with enhanced spinogenesis and dendritic arborization of this region (Gan et al., Citation2014; Lakshminarasimhan & Chattarji, Citation2012; Tottenham et al., Citation2010). However, like in PTSD, the data are somewhat conflicting depending on the developmental timing of stress exposure, as well as the duration and intensity of the stress (Ousdal et al., Citation2020; Veer et al., Citation2015). The patients in the present study were investigated upon receiving the diagnosis of ES, although stress exposure lasted for typically several months up to a year before coming to the stress clinic. That the right basolateral and central amygdala volumes were increased could indicate that MRI investigations were carried out relatively early during stress exposure, a hypothesis than needs specific testing in future longitudinal studies of occupational ES. The major finding, that the changes in patients with everyday stress, have similar selective location as more established stress conditions is, nevertheless, of note.
It may seem contradictory that group difference in amygdala in our ES population were found only in women, although the degree of perceived stress, indexed in MBI-GS scores was similar (). Our previous studies with partly different populations (only 50% of the patients in the present study participated in our previously published study of amygdala and hippocampus volumes) (Savic et al., Citation2018), also showed increased amygdala volumes only among the female group of ES patients. Whilst the present study group was large enough (68 ES patients and 75 controls) the total number of males was lower by about 30%, as ES is widely more common in women. This could theoretically have contributed to a type 1 error lower power in men making it more difficult to detect significant differences in men. However, when carrying out the comparisons among female patients and controls reducing the study group to 22 patients and 27 controls to match the size of study group with males, the significant changes on the basal, lateral, and central amygdala remained only in women (Wilks lambda = 0.614, F(10,39) = 2.451, p=.022). The majority of stress related conditions are more common in women. One tentative explanation is that the neurons of the basal and lateral nuclei have a dense expression of estrogen receptors, and estrogen is known to facilitate glutamatergic activity (Landré et al., Citation2010; Veer et al., Citation2015) rendering female patients more vulnerable to stress related perturbations. It is also possible, as suggested earlier (Savic et al., Citation2018) that male and female patients have different vulnerability patterns involving different cerebral regions. Future studies with larger and more sex balanced groups and including structural and functional connectivity between cortical and subcortical regions are needed to further elucidate this interesting issue.
The absence of hippocampal changes needs a comment. According to data from animal experiments hippocampal CA3, CA4 regions as well as the DG are clearly vulnerable to stress exposure. Nevertheless, several studies of chronic stress in humans failed to detect any hippocampal changes (Bonne et al., Citation2001; De Bellis et al., Citation2001), see also introduction. Certain animal data suggest a sex difference with respect to stress linked affection of the CA1–3, with males having an elevated vulnerability (Galea et al., Citation1997; Hillerer et al., Citation2019). This aspect was not confirmed in the present study but may be of interest to investigate in future extended studies.
Causality
That only the basolateral and central nucleus of the amygdala was altered in our patients and that only these specific volumes showed a positive correlation with stress scores could suggest a causal link. The study design does, however, not allow distinction between cause and effect. Our data from 1.5 year follow up including measurements of the whole amygdala volumes show a significant reduction over time among patients, however with a remaining amygdala enlargement compared with controls (Savic et al., Citation2018). Whether this suggests that volume differences from controls in ES could reflect a vulnerability, facilitating further changes along with stress exposure is an open question, and will be further elucidated in an ongoing 5-year follow up study.
Some methodological issues need a further comment. The ICV was smaller in patients compared with controls although the populations were age and sex matched. This concurs with our previous observations in more limited study groups (Savic, Citation2015; Savic et al., Citation2018), and could also be related to stress. Given this significant group difference we chose to use region/ICV ratios as input variables rather than ICV as covariate which could be a more conservative approach.
One could argue that testing group differences normalized for the whole amygdala volume and whole hippocampus volume, respectively, could be superfluous. Although the here reported results from the ICV ratios are more conventional, as explained in the method section, it was important to evaluate which specific subregions could be linked to stress even when correction for differences in the entire amygdala and hippocampus volumes especially as the ICV was reduced in patients. Indeed, as shown by the correlation analyses with MBI-GS and MADRAS scores, the right lateral and right central amygdala consistently seemed to be particularly linked to stress in the current group of patients with occupational ES. One may argue that co-linearity between the investigated subfields and sub volumes may pose a general problem in studies using structural sub segmentation. The present results of selective differences between female control and female patients with ES, as well as between female and male ES patients in regions expected to be affected by psychosocial stress, offer, however, support for such studies.
Conclusions
The mechanisms behind occupational exhaustion in otherwise healthy young and middle-aged persons is debated. The present findings of selective and separated group differences in subregions of the amygdala strongly suggest that we are dealing with a chronic stress condition requiring a professional intervention. The present observations also elucidate the complexity of hippocampus-amygdala alterations associated with stress and call for further studies of a suggested interaction between sex and effects of chronic stress exposure on human brain. The observed sex differences corroborate with previous studies of stress, further underlining that occupational ES is a stress condition.
Acknowledgements
We acknowledge the stress clinic, Sabbatsberg in Stockholm for referral of ES patients. We are especially indebted to Dr Vassiliki Perris for recruiting several of the patients, and Christos Saripanidis for recruiting control subjects.
Declaration of interest
None of the authors has any conflict of interest to disclose.
Additional information
Funding
References
- Abe, K., Tei, S., Takahashi, H., & Fujino, J. (2022). Structural brain correlates of burnout severity in medical professionals: A voxel-based morphometric study. Neuroscience Letters, 772, 1. https://doi.org/10.1016/j.neulet.2022.136484
- Albert, K., Pruessner, J., & Newhouse, P. (2015). Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology, 59, 14–10. https://doi.org/10.1016/j.psyneuen.2015.04.022
- Asberg, M., Grape, T., Krakau, I., Nygren, A., Rohde, M., Wahlberg, A., et al. (2010). [Stress as the cause of mental illness]. Lakartidningen, 107(19–20), 1307–1310.
- Bennett, M. R., & Lagopoulos, J. (2014). Stress and trauma: BDNF control of dendritic-spine formation and regression. Progress in Neurobiology. 112, 80–99. https://doi.org/10.1016/j.pneurobio.2013.10.005
- Bested, A. C., & Marshall, L. M. (2015). Review of myalgic encephalomyelitis/chronic fatigue syndrome: An evidence-based approach to diagnosis and management by clinicians. Rev Environ Health, 30(4), 223–249.
- Blix, E., Perski, A., Berglund, H., & Savic, I. (2013). Long-term occupational stress is associated with regional reductions in brain tissue volumes. PLOS One, 8(6), e64065. https://doi.org/10.1371/journal.pone.0064065
- Bonne, O., Brandes, D., Gilboa, A., Gomori, J. M., Shenton, M. E., Pitman, R. K., & Shalev, A. Y. (2001). Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. American Journal of Psychiatry, 158(8), 1248–1251. https://doi.org/10.1176/appi.ajp.158.8.1248
- Brown, E. M., Pierce, M. E., Clark, D. C., Fischl, B. R., Iglesias, J. E., Milberg, W. P., McGlinchey, R. E., & Salat, D. H. (2020). Test-retest reliability of FreeSurfer automated hippocampal subfield segmentation within and across scanners. NeuroImage, 210, 116563. https://doi.org/10.1016/j.neuroimage.2020.116563
- Chen, L. W., Sun, D., Davis, S. L., Haswell, C. C., Dennis, E. L., Swanson, C. A., Whelan, C. D., Gutman, B., Jahanshad, N., Iglesias, J. E., Thompson, P., Wagner, H. R., Saemann, P., LaBar, K. S., & Morey, R. A,. (2018). Smaller hippocampal CA1 subfield volume in posttraumatic stress disorder. Depression and Anxiety, 35(11), 1018–1029. https://doi.org/10.1002/da.22833
- Chiappiniello, A., Tarducci, R., Muscio, C., Bruzzone, M. G., Bozzali, M., Tiraboschi, P., Nigri, A., Ambrosi, C., Chipi, E., Ferraro, S., Festari, C., Gasparotti, R., Gianeri, R., Giulietti, G., Mascaro, L., Montanucci, C., Nicolosi, V., Rosazza, C., Serra, L., … Jovicich, J. (2021). Automatic multispectral MRI segmentation of human hippocampal subfields: An evaluation of multicentric test-retest reproducibility. Brain Structure and Function, 226(1), 137–150. https://doi.org/10.1007/s00429-020-02172-w
- Dale, A. M., Fischl, B., & Sereno, M. I. (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194. https://doi.org/10.1006/nimg.1998.0395
- De Bellis, M. D., Hall, J., Boring, A. M., Frustaci, K., & Moritz, G. (2001). A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biological Psychiatry, 50(4), 305–309. https://doi.org/10.1016/s0006-3223(01)01105-2
- DeCasien, A. R., Guma, E., Liu, S., & Raznahan, A. (2022). Sex differences in the human brain: a roadmap for more careful analysis and interpretation of a biological reality. Biology of Sex Differences, 13(1), 43. England: © 2022. The Author(s) p. https://doi.org/10.1186/s13293-022-00448-w
- Fanselow, M. S., & Dong, H. W. (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron, 65(1), 7–19. https://doi.org/10.1016/j.neuron.2009.11.031
- Fischl, B., Sereno, M. I., & Dale, A. M. (1999). Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage, 9(2), 195–207. https://doi.org/10.1006/nimg.1998.0396
- Fish, A. M., Nadig, A., Seidlitz, J., Reardon, P. K., Mankiw, C., McDermott, C. L., Blumenthal, J. D., Clasen, L. S., Lalonde, F., Lerch, J. P., Chakravarty, M. M., Shinohara, R. T., & Raznahan, A. (2020). Sex-biased trajectories of amygdalo-hippocampal morphology change over human development. NeuroImage, 204, 116122. https://doi.org/10.1016/j.neuroimage.2019.116122
- Galea, L. A., McEwen, B. S., Tanapat, P., Deak, T., Spencer, R. L., & Dhabhar, F. S. (1997). Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience, 81(3), 689–697. https://doi.org/10.1016/S0306-4522(97)00233-9
- Gan, J. O., Bowline, E., Lourenco, F. S., & Pickel, V. M. (2014). Adolescent social isolation enhances the plasmalemmal density of NMDA NR1 subunits in dendritic spines of principal neurons in the basolateral amygdala of adult mice. Neuroscience, 258, 174–183. https://doi.org/10.1016/j.neuroscience.2013.11.003
- Gavelin, H. M., Neely, A. S., Dunås, T., Eskilsson, T., Järvholm, L. S., & Boraxbekk, C. J. (2020). Mental fatigue in stress-related exhaustion disorder: Structural brain correlates, clinical characteristics and relations with cognitive functioning. NeuroImage. Clinical, 27, 102337. https://doi.org/10.1016/j.nicl.2020.102337
- Glise, K., Wiegner, L., & Jonsdottir, I. H. (2020). Long-term follow-up of residual symptoms in patients treated for stress-related exhaustion. BMC Psychology, 8(1), 26. https://doi.org/10.1186/s40359-020-0395-8
- Gould, E., Tanapat, P., McEwen, B. S., Flügge, G., & Fuchs, E. (1998). Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proceedings of the National Academy of Sciences of the United States of America, 95(6), 3168–3171. https://doi.org/10.1073/pnas.95.6.3168
- Grossi, G., Perski, A., Osika, W., & Savic, I. (2015). Stress-related exhaustion disorder–clinical manifestation of burnout? A review of assessment methods, sleep impairments, cognitive disturbances, and neuro-biological and physiological changes in clinical burnout. Scandinavian Journal of Psychology, 56(6), 626–636. https://doi.org/10.1111/sjop.12251
- Hanson, J. L., Nacewicz, B. M., Sutterer, M. J., Cayo, A. A., Schaefer, S. M., Rudolph, K. D., Shirtcliff, E. A., Pollak, S. D., & Davidson, R. J. (2015). Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry, 77(4), 314–323. https://doi.org/10.1016/j.biopsych.2014.04.020
- Hayes, J. P., Hayes, S., Miller, D. R., Lafleche, G., Logue, M. W., & Verfaellie, M. (2017). Automated measurement of hippocampal subfields in PTSD: Evidence for smaller dentate gyrus volume. Journal of Psychiatric Research, 95, 247–252. https://doi.org/10.1016/j.jpsychires.2017.09.007
- Henigsberg, N., Kalember, P., Petrović, Z. K., & Šečić, A. (2019). Neuroimaging research in posttraumatic stress disorder - Focus on amygdala, hippocampus and prefrontal cortex. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 90, 37–42. https://doi.org/10.1016/j.pnpbp.2018.11.003
- Hillerer, K. M., Slattery, D. A., & Pletzer, B. (2019). Neurobiological mechanisms underlying sex-related differences in strress related disorders. Efects. of Neuroactive Steroids on the Hippocampus.Front Neuroendocrin, 55, 1–55.
- Holmes, T. H., & Rahe, R. H. (1967). The Social readjustment rating scale. Journal of Psychosomatic Research, 11(2), 213–218. https://doi.org/10.1016/0022-3999(67)90010-4
- Iglesias, J. E., Augustinack, J. C., Nguyen, K., Player, C. M., Player, A., Wright, M., Roy, N., Frosch, M. P., McKee, A. C., Wald, L. L., Fischl, B., & Van Leemput, K. (2015). A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. NeuroImage, 115, 117–137. https://doi.org/10.1016/j.neuroimage.2015.04.042
- Kajantie, E., & Phillips, D. I. (2006). The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology, 31(2), 151–178. https://doi.org/10.1016/j.psyneuen.2005.07.002
- Karl, A., Schaefer, M., Malta, L. S., Dörfel, D., Rohleder, N., & Werner, A. (2006). A meta-analysis of structural brain abnormalities in PTSD. Neuroscience & Biobehavioral Reviews. 30(7), 1004–1031. https://doi.org/10.1016/j.neubiorev.2006.03.004
- Kirschbaum, C., Kudielka, B. M., Gaab, J., Schommer, N. C., & Hellhammer, D. H. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine, 61(2), 154–162. https://doi.org/10.1097/00006842-199903000-00006
- Kitayama, N., Vaccarino, V., Kutner, M., Weiss, P., & Bremner, J. D. (2005). Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: A meta-analysis. Journal of Affective Disorders, 88(1), 79–86. https://doi.org/10.1016/j.jad.2005.05.014
- Kogler, L., Gur, R. C., & Derntl, B. (2015). Sex differences in cognitive regulation of psychosocial achievement stress: brain and behavior. Human Brain Mapping, 36(3), 1028–1042. https://doi.org/10.1002/hbm.22683
- Kogler, L., Müller, V. I., Seidel, E.-M., Boubela, R., Kalcher, K., Moser, E., Habel, U., Gur, R. C., Eickhoff, S. B., & Derntl, B. (2016). Sex differences in the functional connectivity of the amygdalae in association with cortisol. NeuroImage, 134, 410–423. https://doi.org/10.1016/j.neuroimage.2016.03.064
- Kuo, J. R., Kaloupek, D. G., & Woodward, S. H. (2012). Amygdala volume in combat-exposed veterans with and without posttraumatic stress disorder: a cross-sectional study. Archives of General Psychiatry, 69(10), 1080–1086. https://doi.org/10.1001/archgenpsychiatry.2012.73
- Lakshminarasimhan, H., & Chattarji, S. (2012). Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLOS One. 7(1), e30481. https://doi.org/10.1371/journal.pone.0030481
- Landré, L., Destrieux, C., Baudry, M., Barantin, L., Cottier, J.-P., Martineau, J., Hommet, C., Isingrini, M., Belzung, C., Gaillard, P., Camus, V., & El Hage, W. (2010). Preserved subcortical volumes and cortical thickness in women with sexual abuse-related PTSD. Psychiatry Research: Neuroimaging, 183(3), 181–186. https://doi.org/10.1016/j.pscychresns.2010.01.015
- Lazarus, R. S., & Folkman, S. (1987). Transactional theory and research on emotions and coping. European Journal of Personality, 1(3), 141–169. https://doi.org/10.1002/per.2410010304
- Lee, D., Kim, W., Lee, J. E., Lee, J., Lee, S.-K., Chang, S.-J., Jeung, D. Y., Hyun, D.-S., Ryu, H.-Y., Kim, C., & Jung, Y.-C. (2021). Regional gray matter volume related to high occupational stress in firefighters. Journal of Korean Medical Science, 36(50), e335. https://doi.org/10.3346/jkms.2021.36.e335
- McEwen, B. S. (1999). Stress and hippocampal plasticity. Annual Review of Neuroscience, 22, 105–122. https://doi.org/10.1146/annurev.neuro.22.1.105
- McEwen, B. S. (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Reviews, 87(3), 873–904. https://doi.org/10.1152/physrev.00041.2006
- Mitra, R., Jadhav, S., McEwen, B. S., Vyas, A., & Chattarji, S. (2005). Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America, 102(26), 9371–9376. https://doi.org/10.1073/pnas.0504011102
- Morey, R. A., Clarke, E. K., Haswell, C. C., Phillips, R. D., Clausen, A. N., Mufford, M. S., et al. (2020). Amygdala nuclei volume and shape in military veterans with posttraumatic stress disorder. Biol Psychiatry Cogn Neurosci Neuroimaging, 5(3), 281–290.
- Morey, R. A., Gold, A. L., LaBar, K. S., Beall, S. K., Brown, V. M., Haswell, C. C., Nasser, J. D., Wagner, H. R., McCarthy, G., & Mid-Atlantic Mirecc Workgroup, f t (2012). Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Archives of General Psychiatry, 69(11), 1169–1178. https://doi.org/10.1001/archgenpsychiatry.2012.50
- Ousdal, O. T., Milde, A. M., Hafstad, G. S., Hodneland, E., Dyb, G., Craven, A. R., Melinder, A., Endestad, T., & Hugdahl, K. (2020). The association of PTSD symptom severity with amygdala nuclei volumes in traumatized youths. Translational Psychiatry, 10(1), 288. https://doi.org/10.1038/s41398-020-00974-4
- Pavlisa, G., Papa, J., & Pavić, L. (2006). Bilateral MR volumetry of the amygdala in chronic PTSD patients. Coll Antropol, 30(3), 565–568.
- Savic, I. (2015). Structural changes of the brain in relation to occupational stress. Cerebral Cortex, 25(6), 1554–1564. https://doi.org/10.1093/cercor/bht348
- Savic, I. (2020). MRS shows regionally increased glutamate levels among patients with exhaustion syndrome due to occupational stress. Cerebral Cortex, 30(6), 3759–3770. https://doi.org/10.1093/cercor/bhz340
- Savic, I., Perski, A., & Osika, W. (2018). MRI shows that exhaustion syndrome due to chronic occupational stress is associated with partially reversible cerebral changes. Cerebral Cortex , 28(3), 894–906. https://doi.org/10.1093/cercor/bhw413
- Saygin, Z. M., Kliemann, D., Iglesias, J. E., van der Kouwe, A. J. W., Boyd, E., Reuter, M., Stevens, A., Van Leemput, K., McKee, A., Frosch, M. P., Fischl, B., & Augustinack, J. C. (2017). High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: Manual segmentation to automatic atlas. NeuroImage, 155, 370–382. https://doi.org/10.1016/j.neuroimage.2017.04.046
- Teicher, M. H., Anderson, C. M., & Polcari, A. (2012). Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci U S A, 109(9), E563–72.
- Tottenham, N., Hare, T. A., Quinn, B. T., McCarry, T. W., Nurse, M., Gilhooly, T., Millner, A., Galvan, A., Davidson, M. C., Eigsti, I.-M., Thomas, K. M., Freed, P. J., Booma, E. S., Gunnar, M. R., Altemus, M., Aronson, J., & Casey, B. J. (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science, 13(1), 46–61. https://doi.org/10.1111/j.1467-7687.2009.00852.x
- Veer, I. M., Oei, N. Y., van Buchem, M. A., Spinhoven, P., Elzinga, B. M., & Rombouts, S. A. (2015). Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatry Research: Neuroimaging, 233(3), 436–442. https://doi.org/10.1016/j.pscychresns.2015.07.016
- Vyas, A., Bernal, S., & Chattarji, S. (2003). Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Research. 965(1-2), 290–294. https://doi.org/10.1016/S0006-8993(02)04162-8
- Vyas, A., Mitra, R., Shankaranarayana Rao, B. S., & Chattarji, S. (2002). Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 22(15), 6810–6818. https://doi.org/10.1523/JNEUROSCI.22-15-06810.2002
- Vyas, A., Pillai, A. G., & Chattarji, S. (2004). Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience, 128(4), 667–673. https://doi.org/10.1016/j.neuroscience.2004.07.013
- Wang, J., Korczykowski, M., Rao, H., Fan, Y., Pluta, J., Gur, R. C., McEwen, B. S., & Detre, J. A. (2007). Gender difference in neural response to psychological stress. Social Cognitive and Affective Neuroscience, 2(3), 227–239. https://doi.org/10.1093/scan/nsm018
- Woon, F. L., & Hedges, D. W. (2009). Amygdala volume in adults with posttraumatic stress disorder: A meta-analysis. The Journal of Neuropsychiatry and Clinical Neurosciences, 21(1), 5–12. https://doi.org/10.1176/jnp.2009.21.1.5

