Abstract
Objectives
To investigate the effects of chronic stress on bladder morphology and the impact of food preference (standard or comfort foods) on the bladder of stressed rats.
Methods
In total, 32 Wistar male rats (3 months old) were divided into four groups: control (C), stressed (S), control + comfort food (C + CF), and stressed + comfort food (S + CF). Groups C and C + CF were maintained under normal conditions, while groups S and S + CF were subjected to chronic stress by the restraint method. Groups C and S received standard rat chow, while groups C + CF and S + CF received comfort food (Froot Loops®) and standard chow. The stress stimuli were induced daily for 2 h over 8 weeks. After 8 weeks, all animals were killed, and the bladders were removed and used for histomorphometric analysis.
Results
Body mass was similar among the groups. Stress did not promote differences regarding food intake, but animals receiving comfort food showed higher calories intake (in kcal/Kg) than animals receiving only standard chow. The C + CF and S + CF groups preferred comfort food over the standard chow; this preference was higher in the S + CF than in the C + CF group. The surface density of smooth muscle was reduced in stressed animals, while connective tissue and elastic system fiber content were increased in stressed groups. Further, epithelial height was increased in rats submitted to chronic stress. The surface density of elastic system fibers was decreased by the consumption of comfort food.
Conclusions
Chronic stress induces morphological modifications on the bladder wall and epithelium. These modifications may be related to lower urinary tract symptoms. Additionally, chronic stress caused a higher preference for comfort food intake which did not ameliorate or aggravate the stress-induced bladder alterations.
Introduction
Chronic stress is associated with functional and morphological alterations in different organs and systems (Marchon et al., Citation2018; Ribeiro et al., Citation2018, Citation2019; Tynan et al., Citation2010). Among these organs, our research group has demonstrated that several urogenital organs exhibit deleterious morphological modifications promoted by chronic stress. The kidneys of stressed rats have fewer nephrons than those of non-stressed animals (Benchimol de Souza et al., Citation2011; Marchon et al., Citation2018). In the testicle, chronic stress induces modifications in seminiferous tubules, negatively impacting spermatozoid quality (Ribeiro et al., Citation2018). The penis is also affected by chronic stress, with fibrotic tissue substituting the normal cavernosal smooth muscle content necessary for penile erection (de Souza et al., Citation2012; Ribeiro et al., Citation2019).
Current evidence indicates that chronic stress results in urinary symptoms, such as urinary frequency, urgency, incontinence, and pelvic pain (Chang et al., Citation2009). Several mechanisms have been proposed for stress-associated lower urinary tract dysfunction, including neuronal (both central and peripheric), inflammatory, and local mechanisms (Chang et al., Citation2009; Mann et al., Citation2015). Among the local effects of chronic stress, the impact on bladder morphology has not been extensively investigated. As morphology and function are directly associated, studying the different components of the bladder in stressed individuals can provide morphological evidence of clinically observed functional changes. For this purpose, morphometric methods are quite suitable, as they provide objective and numerical information about the structural components of the bladder.
Besides local morphological alterations, it has been observed that chronic stress also affects normal alimentary behavior (Yau & Potenza, Citation2013). With the increase in glucocorticoid in the bloodstream after stress stimuli, the search for pleasurable activities (such as sugar and fat ingestion or drugs) increases (Dallman et al., Citation2003). Some studies suggest that consuming comfort foods during stress periods may attenuate endocrine imbalance and anxiety-like symptoms (Ortolani et al., Citation2011; Yau & Potenza, Citation2013).
Thus, this study aimed to investigate structural changes in the bladder of chronically stressed rats. Further, we studied the food preference (standard or comfort foods) and its effects on the bladder morphology of chronically stressed rats. The current study hypothesized that (i) chronic stress would alter bladder wall and epithelium morphology; (ii) stressed animals would prefer comfort food over standard food; and (iii) comfort food intake would prevent stress-induced bladder morphological alterations.
Materials and methods
Thirty-two male Wistar rats were used in this study. All animals were bred in the Urogenital Research Unit animal facilities, kept in a room with a controlled temperature (22 °C ± 1 °C) and artificial dark-light cycles (lights on from 7:00 am to 7:00 pm), and had free access to standard rat chow and water. Animals were housed with 3 or 4 animals per cage (>340 cm2 per animal). This study was approved by our local ethics committee (protocol number CEUA-004/2019) and followed national and international regulations on experimental animal use.
When the animals reached 10 weeks of age, they were included in the experiment. The experimental design was the same as described by Marchon et al. (Citation2023) Four experimental groups, each consisting of eight rats, were randomly assigned as follows: control group (C); stressed group (S); control + comfort food group (C + CF); and stressed + comfort food group (S + CF).
Groups C and S received only standard rat chow (Nuvilab CR-1, Quimtia, Colombo, Brazil), while groups C + CF and S + CF received Froot Loops (Kellogg Brazil, São Paulo, Brazil) in addition to the standard rat chow. Froot Loops was used as comfort food because it is mainly composed of simple carbohydrates, as well as being very attractive and easy to gnaw. Its nutritional characteristics are listed in supplementary Table 1. For all groups, the standard chow present in each cage was weighed daily and replenished to 50 g per animal. In addition to the standard chow, groups C + CF and S + CF received 30 g of Froot Loops per rat, and its consumption was also weighed daily. Food intake (measured as grams) calories intake (measured as Kilocalories per body mass) and food preference (percentage of consumption in grams; for groups C + CF and S + CF) were calculated and compared among the groups. Capillary blood glucose was measured, using an appropriate glucometer (Accucheck performa, Roche, São Paulo, Brazil), at the beginning and at the end of the study. To do this, the animals underwent an eight-hour fasting and the caudal vein was punctured to collect blood samples.
Animals in groups S and S + CF were submitted to a chronic stress protocol using the restriction of movements method (Benchimol de Souza et al., Citation2011; de Souza et al., Citation2012). Each animal was placed in a rigid opaque plastic tube to restrain its movements, 2 h daily (from 17:00 to 19:00), for 8 weeks. Tubes with different diameters and lengths were adjusted weekly depending on the animal’s size. On the other hand, the control groups (C and C + CF) were kept under normal conditions and not subjected to any stress procedure; nevertheless, food was removed during the same period as the stressed groups (2 h daily) to avoid any bias in food intake measurements.
After eight weeks of experiments, the animals were weighed and euthanized by isoflurane (Isofluorano, BioChimico, Itatiaia, Brazil) inhalation in an induction chamber (Procópio et al., Citation2021). The bladder was dissected, and its middle portion was fixed by immersion in a 4% buffered formaldehyde solution. The bladder fundus and neck portion were avoided since the muscle fibers in these portions have different characteristics and dispositions. Subsequently, samples were routinely processed for paraffin embedding, and 5 μm thick sections were used for histomorphometric evaluations (Ribeiro et al., Citation2014).
The surface density (Sv) of bladder wall components (Smooth muscle, connective tissue, and elastic system fibers) was evaluated using images captured under 600x or 1000x magnification by a digital camera (DP70, Olympus, Tokyo, Japan) coupled to a microscope (BX51, Olympus). Masson’s trichrome stained sections were analyzed for smooth muscle and connective tissue surface densities, while Weigert’s resorcin fuchsin stained sections were used to analyze the surface density of elastic system fibers. The surface density was calculated (for each structure) by the point counting method (Baddeley et al., Citation1986; Felix-Patrício et al., Citation2015). Briefly, a 100-point grid was superimposed over the images using the Image J software (version 1.45s, National Institutes of Health, Bethesda, USA), and each structure “touched” by a point was counted. The result, expressed as a percentage, was calculated after measuring 25 images from different randomly captured fields for each animal, for each Sv analysis (Da Silva et al., Citation2020; Felix-Patrício et al., Citation2015). Results are expressed as a percentage, calculated by the number of points that superimpose each bladder component (Baddeley et al., Citation1986; Ribeiro et al., Citation2014).
The epithelial height was measured at a final magnification of X600 of Masson’s trichrome-stained sections. The “straight line” tool of ImageJ software was used for this purpose after the program was calibrated for absolute values. Epithelial height, expressed in µm, was measured in different locations per field, resulting in 50 measures per animal (Ribeiro et al., Citation2014). The mean of these values was considered the epithelial height of the animal.
All data were tested for normal distribution using the Kolmogorov–Smirnov test. A two-way ANOVA was performed to analyze the effect of Stress and Comfort food on the morphological bladder parameters. Tukey`s multiple comparisons test was used for determining between-groups differences. The Student’s t-test was used to analyze food preference. A p < 0.05 was considered statistically significant. Additionally, all results are presented as mean ± standard deviation. All statistical analyses were performed using GraphPad Prism version 8.0 (GraphPad Software, San Diego, USA).
Results
The body mass and capillary blood glucose levels were similar among all groups at the beginning and the end of the study. Two-way ANOVA revealed that there was no statistically significant effect of stress, comfort food or the interaction for these parameters. Chronic stress did not have a statistically significant effect on food intake (p = 0.0515) or on calories intake (p = 0.1819). However, simple main effects analysis showed that comfort food did have a statistically significant effect on food intake calculated in grams (p = 0.0004) or in kcal/kg of body mass (p = 0.0495). Animals with access to comfort food consumed 25.16 g ± 1.91, less than groups without access to comfort food, which consumed 27.79 g ± 0.03. Post-hoc test showed differences between groups S vs. S + CF (p = 0.0009), and C + CF vs. S + CF (p = 0.0324). Meanwhile, the calories intake, calculated in kcal/kg of body mass, was higher in animals with access to comfort foods (0.26 kcal/kg ± 0.02) than those without access (0.24 kcal/kg ± 0.01). Also, there was a statistically significant interaction between the effects of stress and comfort food on food intake, calculated in grams (p = 0.0449).
For both groups that had access to comfort food (C + CF and S + CF), Froot Loops were preferred over standard chow. Animals in group C + CF the food intake was 63.4% of comfort food, and 36.5% of standard chow. However, in group S + CF the preference for Froot Loops was even higher; food intake was 69.1% of comfort food, and 30.9% of standard chow. When comparing these results, it was observed that stressed animals consumed more comfort food than non-stressed animals.
Effects of stress on vesical wall
Regarding the vesical wall morphometry, it was observed that chronic stress induced some modifications. Simple main effects analysis showed that stress did have a statistically significant effect on smooth muscle surface density (p = 0.0005), connective tissue surface density (p = 0.0005), elastic system fiber surface density (p = 0.0013), and epithelial height (p < 0.0001).
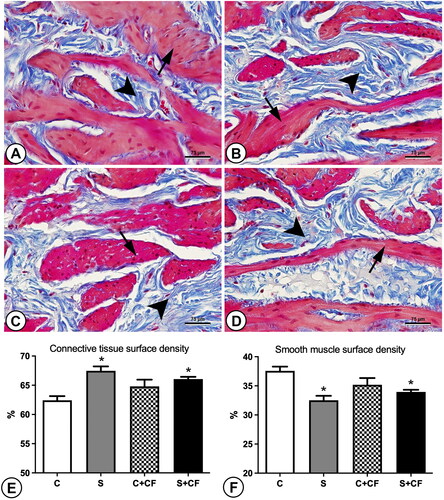
Smooth muscle surface density was reduced from 36.39% ± 1.69 in non-stressed animals to 33.24% ± 1.00 in stressed animals. Post-hoc test showed difference between group C vs. S (p = 0.0007). Meanwhile, connective tissue was increased in stressed groups (66.76% ± 1.00) in comparison to control groups (63.21% ± 1.69 as represented in . Again, post-hoc test showed difference between group C vs. S (p = 0.0007).
Figure 1. Photomicrographs and graphics representing the connective tissue (shown in blue and pointed by arrowheads) and smooth muscle contents (shown in Pink and pointed by arrows) in the bladder wall of different groups. (A) Control group (B) Stressed group (C) Control + comfort food group (D) Stressed + comfort food group. Sections were stained using Masson’s trichrome and captured under 600× magnification. The higher presence of connective tissue (blue stained areas) indicates tissue fibrosis. (E) Connective tissue surface density, (F) smooth muscle surface density. Asterisks represents statistical differences (p = 0.0005) between stressed and control animals. Scale bar represents 75 µm.
The elastic system fiber surface density was also increased, from 2.99% ± 0.70 in control groups to 3.71% ± 0.89 in stressed groups, as shown in . Post-hoc test showed difference between group C vs. S (p = 0.0271). The epithelial height was also increased by chronic stress. Control animals showed a mean of 19.58 µm ± 0.03 while stressed animals had 26.10 µm ± 0.09 of epithelial height. illustrates these findings. Post-hoc test showed differences between groups C vs. S (p = 0.0136), and C + CF vs. S + CF (p = 0.0122). All numerical data are presented in .
Figure 2. Photomicrographs and graphics representing the elastic system fibers (shown as Fine purple lines and pointed by arrowheads) in the bladder wall of different groups. (A) Control group (B) Stressed group (C) Control + comfort food group (D) Stressed + comfort food group. Sections were stained using Weigert’s resorcin fuchsin and captured under 1000× magnification. (E) Results of elastic system fibers surface density. Asterisks represents statistical differences (p = 0.0013) between stressed and control animals. Hashtag represents statistical differences (p < 0.0001) between comfort food and standard chow fed animals. Scale bar represents 200 µm.
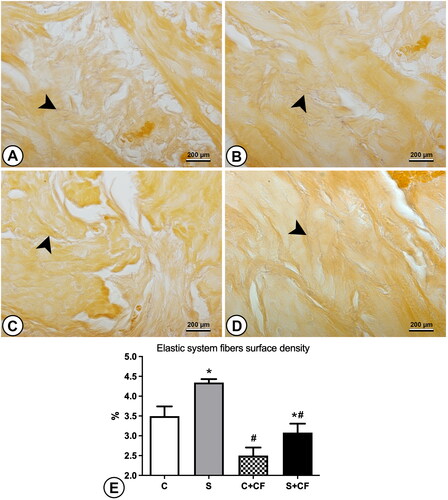
Figure 3. Photomicrographs and graphics representing the epithelial height in the bladder of different groups. The epithelium is marked (in random areas) by double-headed arrows to indicate the differences in epithelial height. (A) Control group (B) Stressed group (C) Control + comfort food group (D) Stressed + comfort food group. Sections were stained using Masson’s trichrome and captured under 600× magnification. (E) Results of epithelial height measurements. Asterisks represents statistical differences (p < 0.0001) between stressed and control animals. Scale bar represents 75 µm.
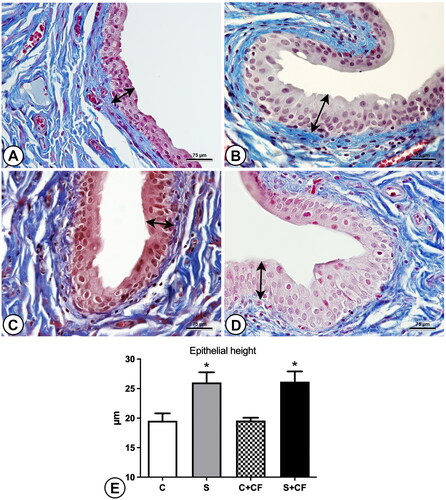
Table 1. Biometric data and bladder morphometric analysis of the experimental groups.
Effects of comfort food on vesical wall
The effects of comfort food on bladder morphology were less drastic. Simple main effects analysis showed that comfort food did have a statistically significant effect on elastic system fiber surface density (p < 0.0001). For this parameter, a decrease from 3.92% ± 0.60 in animals receiving only standard chow to 2.79% ± 0.41 in animals with access to comfort food was observed. Post-hoc test showed differences between groups C vs. C + CF (p = 0.0078), and S vs. S + CF (p = 0006).
Meanwhile, comfort food did not have a statistically significant effect on smooth muscle surface density (p = 0.55), connective tissue surface density (p = 0.55), and epithelial height (p = 0.95).
Interaction effects between comfort food and stress on vesical wall
The two-way ANOVA revealed that there was a statistically significant interaction between the effects of stress and comfort food on smooth muscle surface density (p = 0.024), and connective tissue surface density (p = 0.024).
The interaction effects between the effects of stress and comfort food were not statistically significant for elastic system fiber surface density (p = 0.50), and epithelial height (p = 0.97).
Discussion
The current study showed that chronic stress promotes structural modifications in the bladder, altered the food preference and investigated the effects of this dietary modifications on the bladder morphology of stressed rats. The bladder of stressed animals had an increase of connective tissue, elastic system fibers, and epithelial height, while detrusor musculature was decreased. Although both non-stressed and stressed animals preferred to consume comfort food over standard chow, this preference was more notable in chronically stressed animals. The consumption of comfort food was associated to a reduction in bladder elastic system fibers.
It is well known that chronic stress leads to functional and morphological alterations of the urogenital organs (Marchon et al., Citation2018; Ribeiro et al., Citation2018, Citation2019). There is robust evidence showing that psychological stress negatively influences bladder function (Chess-Williams et al., Citation2021). However, the relationship between chronic stress and bladder morphology has not been fully studied. Chang et al. (Citation2009) demonstrated that the bladders of stressed mice suffer a remodeling process with increased organ proportional mass. Further, Mann et al. (Citation2015) confirmed this bladder mass increase in stressed mice, also showing that stress promoted the thickening of the bladder wall. The present study is the first to examine the proportional contents of the bladder wall after an animal model of chronic stress.
One of the most noticeable findings in the current study was the increase of connective tissue and decrease of smooth muscle fibers in stressed animals, what indicates bladder fibrosis as a consequence of stress stimuli. Bladder fibrosis is a feature of bladder wall morphology that accompanies many benign lower urinary tract conditions (Fry et al., Citation2018). The release of inflammatory factors is considered one of the initial disruptors that initiate increased collagen production. In this regard, there is evidence of increased inflammatory responses to stress systemically (Cheng et al., Citation2015) and locally on the bladder (Merrill et al., Citation2013). This increased release of inflammatory mediators due to stress has also been linked to changes in bladder function, with increased micturition frequency (Merrill et al., Citation2013; Pierce et al., Citation2016).
Another interesting finding of the present study was the elevated proportional content of elastic system fibers in stressed animals. Elastic fibers are the major component of the extracellular matrix involved in bladder elasticity (Nagatomi et al., Citation2004; Ribeiro et al., Citation2014). The increase in extracellular matrix components, including elastic system fibers, in the bladder wall may lead to increased resistance for detrusor muscle relaxation, thereby diminishing compliance. Thus, the significant increase in elastic system fibers observed in this study may explain the increased urinary frequency and urgency observed in stressed animals and patients (Chess-Williams et al., Citation2021; Merrill et al., Citation2013).
The increase in connective tissue (including collagen and elastic system fibers) deposited in the bladder wall may lead to a stiffer bladder wall resulting in reduced filling compliance (Fry et al., Citation2018). As in other conditions (Metcalfe et al., Citation2010), it is possible to believe that bladder fibrosis begins with an inflammatory process after stress stimuli and ends in voiding symptoms.
The increased thickness of bladder epithelium may, at least partially, explain the previous findings of increased bladder wall thickness in stressed animals (Mann et al., Citation2015). Proliferative factors were reported to be over-expressed in the bladder of stressed animals, and this could locally induce epithelial growth in response to stress stimuli (de Rijk et al., Citation2022; Merrill et al., Citation2013). These results confirm the hypothesis that chronic stress would alter bladder wall and epithelium morphology.
As expected, animals with access to comfort foods preferred it over standard chow. Nonetheless, it was interesting that stressed animals showed a higher preference to consume comfort foods. As previously mentioned, high palatability and high caloric food intake increase during stressful situations (Dallman et al., Citation2003). This study’s results corroborate these arguments and confirm the hypothesis that stressed animals would prefer comfort food intake rather than standard chow.
Another hypothesis raised was that comfort food intake would ameliorate stress-induced bladder morphological alterations. This hypothesis was based on previous studies which had shown that the elevated serum corticosterone after induced stress is attenuated when rats had access to comfort food (Ortolani et al., Citation2011). Corticosterone could be implicated in physiological and morphological modifications associated with stress; hence, it was reasonable to raise the mentioned hypothesis. In this study, we found that access to comfort food only prevented stress-induced modification in the proportion of elastic system fibers. Specifically, this parameter was found to be lower in group C + CF compared to group C. This suggests that comfort food intake may specifically reduce the proportion of elastic system fibers regardless of stress stimuli. For smooth muscle and connective tissue surface densities, the results of groups C + CF and S + CF were similar, denoting that when using comfort foods, there were no stress-induced bladder alterations. However, for these parameters, the results of groups S and S + CF were also statistically similar, clearly indicating that no protective effect was achieved by comfort food intake. Overall, these results show that access to comfort food does not ameliorate (or worsen) stress-induced bladder morphological alterations.
There is increasing evidence that chronic stress can result in the development of lower urinary tract symptoms such as urinary frequency, urgency, incontinence, and pelvic pain. Chess-Williams et al. (Citation2021) raised important questions regarding the treatment of urinary symptoms induced by chronic stress. Anxiolytics and other psychiatric drugs reduce stress-induced behavioral changes with minimal effects on voiding symptoms (Chess-Williams et al., Citation2021). On the other hand, traditional drugs used for detrusor overactivity (oxybutynin and mirabegron) ameliorated urinary symptoms without affecting behavior. As chronic stress induces morphological alterations of the bladder wall, the treatment goal should be restoring normal bladder wall morphology, which may not be achieved using any of the drugs mentioned. Studying other urogenital organs (penis, testes, and kidneys) has shown that stress stimuli removal promotes either the recovery of the stress-induced morphological modifications or the interruption of disease progression (Marchon et al., Citation2018; Ribeiro et al., Citation2018, Citation2019). These results, in some way, corroborate the positive results obtained with mindfulness, hypnosis, and yoga for treating stress-related urinary symptoms (Chess-Williams et al., Citation2021). Future studies investigating the effects of stress-stimuli removal on bladder morphological modifications are warranted to elucidate this topic further.
This study has some limitations. Froot loops was chosen as a comfort food because it is mainly composed of simple carbohydrates, but other types of comfort foods (high fat and/or high sucrose foods) could be used as well. This does not isolate the individual effect of simple carbohydrates or lipids but reflects the usual comfort food consumed. As an experimental study, the results obtained reflect only the tested conditions and may not be directly transposed to other conditions (types of stressors, species, diets, etc.) that are different from the experiments conducted. Additionally, animals from control groups were not handled daily (as animals from stressed groups were), thus the effects observed could be not only due to the tube restraint stress but also due to the daily handling of the animals which was necessary to perform the study. One other aspect that should be considered is that we used only male rats in this study. The influences of chronic stress on the female bladder may be different than what was observed in males, and the differences in how comfort foods impact female food behaviors should also be considered. Nevertheless, the results of the present study help to understand some aspects of the biology of stress, its effects on bladder morphology, and its relation to lower urinary tract symptoms.
Conclusion
Chronic stress resulted in morphological alterations in the bladder wall and epithelium, which may be related to lower urinary tract symptoms. Stressed animals showed an increased preference for consuming comfort foods; however, this feeding pattern did not ameliorate or worsen the bladder alterations caused by chronic stress.
Supplemental Material
Download MS Word (13.4 KB)Acknowledgments
This study was supported by grants from the Foundation for Research Support of Rio de Janeiro (FAPERJ), the Coordination for the Improvement of Higher Education Personnel (CAPES), and the National Council for Scientific and Technological Development (CNPq). These foundations were not involved in the study design, data collection, analysis, and interpretation, drafting of the manuscript, and the decision to submit for publication.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
Notes on contributors
Roger G. Marchon
Roger G. Marchon is a recently graduated PhD in the Urogenital Research Unit, State University of Rio de Janeiro. This manuscript is part of his thesis.
Bianca M. Gregório
Bianca M. Gregório is an associate Professor in the Urogenital Research Unit, State University of Rio de Janeiro. Her work mainly focuses on nutritional aspects related to the urogenital organs.
Marco A. Pereira-Sampaio
Marco A. Pereira-Sampaio is an associate Professor at the Department of Morphology, Federal Fluminense University and a collaborator at the Urogenital Research Unit, State University of Rio de Janeiro. His work focuses on morphological aspects of animal models.
Waldemar S. Costa
Waldemar S. Costa is an emeritus Professor in the Urogenital Research Unit, State University of Rio de Janeiro. His work mainly focuses on histological and morphometrical evaluation of the urogenital organs.
Francisco J. Sampaio
Francisco J. Sampaio is a full Professor, and head of the Urogenital Research Unit, State University of Rio de Janeiro. His work investigates the morphological aspects of the urogenital system at normal and altered conditions in humans and animal models.
Diogo B. De Souza
Diogo B. De Souza is an associate Professor in the Urogenital Research Unit, State University of Rio de Janeiro. He has been dedicated to study the urogenital organs under different conditions in animal models. Among his research lines, one is to study the impact of chronic stress on the kidney, testis, penis, and bladder.
References
- Baddeley, A. J., Gundersen, H. J., & Cruz-Orive, L. M. (1986). Estimation of surface area from vertical sections. Journal of Microscopy, 142(Pt 3), 1–9. https://doi.org/10.1111/j.1365-2818.1986.tb04282.x
- Benchimol de Souza, D., Silva, D., Marinho Costa Silva, C., Barcellos Sampaio, F. J., Silva Costa, W., & Martins Cortez, C. (2011). Effects of immobilization stress on kidneys of Wistar male rats: A morphometrical and stereological analysis. Kidney & Blood Pressure Research, 34(6), 424–429. https://doi.org/10.1159/000328331
- Chang, A., Butler, S., Sliwoski, J., Valentino, R., Canning, D., & Zderic, S. (2009, October). Social stress in mice induces voiding dysfunction and bladder wall remodeling. American Journal of Physiology. Renal Physiology, 297(4), F1101–F1108. https://doi.org/10.1152/ajprenal.90749.2008
- Cheng, Y., Jope, R. S., & Beurel, E. (2015, May 7). A pre-conditioning stress accelerates increases in mouse plasma inflammatory cytokines induced by stress. BMC Neuroscience, 16(1), 31. https://doi.org/10.1186/s12868-015-0169-z
- Chess-Williams, R., McDermott, C., Sellers, D. J., West, E. G., & Mills, K. A. (2021, October). Chronic psychological stress and lower urinary tract symptoms. Lower Urinary Tract Symptoms, 13(4), 414–424. https://doi.org/10.1111/luts.12395
- Da Silva, M. H. A., Medeiros, J. L., Jr, Costa, W. S., Sampaio, F. J. B., & De Souza, D. B. (2020, December). Effects of the dutasteride and sildenafil association in the penis of a benign prostatic hyperplasia animal model. The Aging Male, 23(5), 1009–1015. https://doi.org/10.1080/13685538.2019.1653839
- Dallman, M. F., Pecoraro, N., Akana, S. F., La Fleur, S. E., Gomez, F., Houshyar, H., Bell, M. E., Bhatnagar, S., Laugero, K. D., & Manalo, S. (2003). Chronic stress and obesity: A new view of “comfort food.” Proceedings of the National Academy of Sciences of the United States of America, 100(20), 11696–11701. https://doi.org/10.1073/pnas.1934666100
- de Rijk, M. M., Wolf-Johnston, A., Kullmann, A. F., Maringer, K., Sims-Lucas, S., van Koeveringe, G. A., Rodríguez, L. V., & Birder, L. A. (2022, December). Stress-induced changes in trophic factor expression in the rodent urinary bladder: Possible links with angiogenesis. International Neurourology Journal, 26(4), 299–307. https://doi.org/10.5213/inj.2244118.059
- de Souza, D. B., Silva, D., Cortez, C. M., Costa, W. S., & Sampaio, F. J. (2012, July-August). Effects of chronic stress on penile corpus cavernosum of rats. Journal of Andrology, 33(4), 735–739. https://doi.org/10.2164/jandrol.111.014225
- Felix-Patrício, B., De Souza, D. B., Gregório, B. M., Costa, W. S., & Sampaio, F. J. (2015). How to quantify penile corpus cavernosum structures with histomorphometry: Comparison of two methods. BioMed Research International, 2015, 832156–832156. https://doi.org/10.1155/2015/832156
- Felix-Patrício, B., Medeiros, J. L., Jr, De Souza, D. B., Costa, W. S., & Sampaio, F. J. (2015). Penile histomorphometrical evaluation in hypertensive rats treated with sildenafil or enalapril alone or in combination: A comparison with normotensive and untreated hypertensive rats. The Journal of Sexual Medicine, 12(1), 39–47. https://doi.org/10.1111/jsm.12750
- Fry, C. H., Kitney, D. G., Paniker, J., Drake, M. J., Kanai, A., & Andersson, K. E. (2018, June). Fibrosis and the bladder, implications for function ICI-RS 2017. Neurourology and Urodynamics, 37(S4), S7–S12. https://doi.org/10.1002/nau.23725
- Mann, E. A., Alam, Z., Hufgard, J. R., Mogle, M., Williams, M. T., Vorhees, C. V., & Reddy, P. (2015, October 15). Chronic social defeat, but not restraint stress, alters bladder function in mice. Physiology & Behavior, 150, 83–92. https://doi.org/10.1016/j.physbeh.2015.02.021
- Marchon, R. G., Gregório, B. M., Costa, W. S., Pereira-Sampaio, M. A., Sampaio, F. J., & De Souza, D. B. (2023). Effects of comfort food diet on the penile morphology of stressed rats. Heliyon, 9(6), e17013. https://doi.org/10.1016/j.heliyon.2023.e17013
- Marchon, R. G., Ribeiro, C. T., Costa, W. S., Sampaio, F. J. B., Pereira-Sampaio, M. A., & de Souza, D. B. (2018). Immediate and late effects of stress on kidneys of prepubertal and adult rats. Kidney & Blood Pressure Research, 43(6), 1919–1926. https://doi.org/10.1159/000496004
- Merrill, L., Malley, S., & Vizzard, M. A. (2013, July 15). Repeated variate stress in male rats induces increased voiding frequency, somatic sensitivity, and urinary bladder nerve growth factor expression. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 305(2), R147–R156. https://doi.org/10.1152/ajpregu.00089.2013
- Metcalfe, P. D., Wang, J., Jiao, H., Huang, Y., Hori, K., Moore, R. B., & Tredget, E. E. (2010, December). Bladder outlet obstruction: progression from inflammation to fibrosis. BJU International, 106(11), 1686–1694. https://doi.org/10.1111/j.1464-410X.2010.09445.x
- Nagatomi, J., Gloeckner, D. C., Chancellor, M. B., DeGroat, W. C., & Sacks, M. S. (2004). Changes in the biaxial viscoelastic response of the urinary bladder following spinal cord injury. Annals of Biomedical Engineering, 32(10), 1409–1419. https://doi.org/10.1114/b:abme.0000042228.89106.48
- Ortolani, D., Oyama, L. M., Ferrari, E. M., Melo, L. L., & Spadari-Bratfisch, R. C. (2011). Effects of comfort food on food intake, anxiety-like behavior and the stress response in rats. Physiology & Behavior, 103(5), 487–492. https://doi.org/10.1016/j.physbeh.2011.03.028
- Pierce, A. N., Di Silvestro, E. R., Eller, O. C., Wang, R., Ryals, J. M., & Christianson, J. A. (2016, May 15). Urinary bladder hypersensitivity and dysfunction in female mice following early life and adult stress. Brain Research, 1639, 58–73. https://doi.org/10.1016/j.brainres.2016.02.039
- Procópio, I. M., Pereira-Sampaio, M. A., Costa, W. S., Sampaio, F. J. B., & Souza, D. B. (2021). December 8 Histomorphometric comparison of the corpus cavernosum of rats submitted to euthanasia with ketamine and xylazine or isoflurane. Acta Cirurgica Brasileira, 36(11), e361103. https://doi.org/10.1590/ACB361103
- Ribeiro, C. T., Costa, W. S., Sampaio, F. J. B., Pereira Sampaio, M. A., & de Souza, D. B. (2019). Evaluation of the effects of chronic stress applied from the prepubertal to the adult stages or only during adulthood on penile morphology in rats. Stress (Amsterdam, Netherlands), 22(2), 248–255. https://doi.org/10.1080/10253890.2018.1553946
- Ribeiro, C. T., De Souza, D. B., Costa, W. S., Sampaio, F. J. B., & Pereira-Sampaio, M. A. (2018). Immediate and late effects of chronic stress in the testes of prepubertal and adult rats. Asian Journal of Andrology, 20(4), 385–390. https://doi.org/10.4103/aja.aja_68_17
- Ribeiro, G. S., Souza, D. B., Cortez, C. M., Silva, D., Costa, W. S., & Sampaio, F. J. (2014). Effects of prepubertal corticosterone treatment on urinary bladder. Acta Cirurgica Brasileira, 29(Suppl 3), 55–59. https://doi.org/10.1590/s0102-86502014001700011
- Tynan, R. J., Naicker, S., Hinwood, M., Nalivaiko, E., Buller, K. M., Pow, D. V., Day, T. A., & Walker, F. R. (2010). Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain, Behavior, and Immunity, 24(7), 1058–1068. https://doi.org/10.1016/j.bbi.2010.02.001
- Yau, Y. H., & Potenza, M. N. (2013). Stress and eating behaviors. Minerva Endocrinol, 38(3), 255–267.
