Abstract
Chronic stress exposure during development can have lasting behavioral consequences that differ in males and females. More specifically, increased depressive behaviors in females, but not males, are observed in both humans and rodent models of chronic stress. Despite these known stress-induced outcomes, the molecular consequences of chronic adolescent stress in the adult brain are less clear. The stress hormone corticosterone activates the glucocorticoid receptor, and activity of the receptor is regulated through interactions with co-chaperones—such as the immunophilin FK506 binding proteins 5 (FKBP5). Previously, it has been reported that the adult stress response is modified by a history of chronic stress; therefore, the current study assessed the impact of chronic adolescent stress on the interactions of the glucocorticoid receptor (GR) with its regulatory co-chaperone FKBP5 in response to acute stress in adulthood. Although protein presence for FKBP5 did not differ by group, assessment of GR-FKBP5 interactions demonstrated that adult females with a history of chronic adolescent stress had elevated GR-FKBP5 interactions in the hippocampus following an acute stress challenge which could potentially contribute to a reduced translocation pattern given previous literature describing the impact of FKBP5 on GR activity. Interestingly, the altered co-chaperone interactions of the GR in the stressed female hippocampus were not coupled to an observable difference in transcription of GR-regulated genes. Together, these studies show that chronic adolescent stress causes lasting changes to co-chaperone interactions with the glucocorticoid receptor following stress exposure in adulthood and highlight the potential role that FKBP5 plays in these modifications. Understanding the long-term implications of adolescent stress exposure will provide a mechanistic framework to guide the development of interventions for adult disorders related to early life stress exposures.
Introduction
Women exhibit increased incidence of stress-related disorders, such as depression and anxiety compared to men (Cover et al., Citation2014; Pratt & Brody, Citation2014). This disparity may be a result of developmental mechanisms that differ in males and females. During adolescence, dendritic maturation (Markham et al., Citation2013), changes in brain volume (Giedd, Citation2004), and extensive maturation of the neuroendocrine system (Foilb et al., Citation2011; Romeo et al., Citation2016) occur, and chronic stress experienced during this developmental period can disrupt these processes leading to long-term consequences in the stress response and neural health (Spear, Citation2000). To isolate the mechanisms by which chronic adolescent stress may induce changes in the adult brain, animal models have been developed that mirror the changes observed in humans. A rat model of chronic adolescent stress (CAS) has been used to study long-term consequences of CAS exposure in adult animals (Bourke & Neigh, Citation2011; Bourke et al., Citation2013; Bekhbat et al., Citation2019; Rowson et al., Citation2019; Hyer et al., Citation2021; Hyer et al., Citation2023) and has been shown to induce increases in depressive-like behavior in females that last into adulthood (Bourke & Neigh, Citation2011). More specifically, in females, exposure to CAS reduces sucrose but not water consumption, and reduces struggling and latency to float in the forced swim test at the end of adolescence and in adulthood (Bourke & Neigh, Citation2011). Despite these known behavioral outcomes, the underlying molecular consequences of CAS exposure in adulthood are not fully understood.
The glucocorticoid receptor (GR) is a critical regulator of the impact of glucocorticoids, and GR activity in the hippocampus is involved in negative feedback on the hypothalamic pituitary adrenal (HPA) axis response (Feldman & Weidenfeld, Citation1999). Cellular activity of the GR is modulated locally through chaperone proteins, including the immunophilin FK506 binding proteins 5 (FKBP5) (Wochnik et al., Citation2005). Interaction with FKBP5 inhibits efficient translocation of the GR to the nucleus, reducing its activity (Wochnik et al., Citation2005). In humans, polymorphisms of the Fkbp5 gene have been associated with increased number of depressive episodes, altered response to antidepressant treatment (Binder et al., Citation2004), and increased depression risk (Fan et al., Citation2021). Increased interactions between GR and FKBP5 have been found following laboratory assessment of fear conditioning in mice and in patients with post-traumatic stress disorder (Li et al., Citation2020). However, the consequences of exposure to developmental stressors on adult interactions between the GR and FKBP5 in the brain are not well characterized.
While FKBP5’s impact on the GR complex has previously been investigated in cell lines (Davies et al., Citation2002; Wochnik et al., Citation2005), neuronal cultures (Tatro et al., Citation2009), and whole mouse brain samples (Li et al., Citation2020), the current study assesses the interactions between the GR and its co-chaperone FKBP5 in the rat hippocampus in situ. Specifically, these studies investigate the effect of a history of chronic adolescent stress and a subsequent acute stress challenge in adulthood on interactions between the GR and FKBP5 in the hippocampus—a brain region that plays a considerable role in the stress response and mediates depression and anxiety. In order to understand the extent to which CAS alters adult Fkbp5 expression and interactions between the GR and FKBP5, we used a CAS paradigm that confers sex-dependent behavioral and molecular effects on co-chaperones of the GR (Bourke & Neigh, Citation2011; Bourke et al., Citation2013). Furthermore, we assessed gene expression of its co-chaperones and a subset of GR target genes to understand the lasting impact of CAS on the GR in adulthood.
Methods
Animal husbandry
Rats were housed on a 14:10 reverse light:dark cycle with standard rat chow and water available ad libitum in AAALAC-approved facilities at Emory University. All procedures were approved by the Institutional Animal Care and Use Committee at Emory University. Pups from Wistar dams (Charles River Laboratories) were culled to litters of eight (4 males and 4 females) on postnatal day (PND) 3. On PND 21, litters were weaned into same-sex pairs. Separate cohorts of rats were used for PCR, western blots, FKBP5 immunohistochemistry, and proximity ligation assay due to tissue requirements. All procedures for the FKBP5 immunohistochemistry cohort of rats were conducted at Virginia Commonwealth University and were performed in accordance with the Institutional Animal Care and Use Committee, as well as the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chronic adolescent stress paradigm
On PND 35, male and female rats were divided into chronic adolescent stress (CAS) and non-stress (NS) groups. Male and female rats in the CAS group were individually housed beginning on PND 35 through the end of the study. On PND 38-49, CAS rats were exposed to a mixed-modality CAS paradigm consisting of restraint, social defeat, and isolation as previously described (Bourke & Neigh, Citation2011; Bourke et al., Citation2013; Harrell et al., Citation2013; Harrell et al., Citation2015; Rowson et al., Citation2016; Rowson et al., Citation2018; Bekhbat et al., Citation2019; Rowson et al. 2 019, Bekhbat et al., Citation2021; Hyer et al., Citation2021).
Weight analysis
Regardless of endpoint assignment, all rats were weighed on the first day of isolation housing (PND 35) and Day 1, 5, and 10 during the CAS paradigm. NS rats were weighed on the same days as littermate CAS rats. Terminal weight was measured prior to acute stressor exposure on the day of collection.
Acute stress and collection
In adulthood (at least six weeks following the end of CAS exposure, PND 90-120), rats were exposed to the novel acute stressor—a five-minute forced swim. Rats were removed from their home cage for acute stressor exposure and immediately placed in a clear acrylic cylinder (60 cm tall, 22 cm diameter) with 25 °C water for five minutes. Immediately following the forced swim exposure, rats were dried and replaced in their home cage. Rats were rapidly decapitated 15, 30, or 120 minutes following swim exposure and brain tissue was collected. Brains were frozen on dry ice and stored at −80 °C for future processing. A separate group of rats was collected in adulthood without exposure to the acute novel swim stressor.
Corticosterone ELISA
At the time of euthanasia, trunk blood was collected in EDTA tubes (BD Vacutainer) on ice. Blood was centrifuged at 2500 rpm in a Sorvall RC-5B Refrigerated Superspeed Centrifuge and SM-24 rotor for 20 minutes at 4 °C. Plasma was collected and stored at −80 °C. An Enzo Life Sciences Enzyme-linked Immunosorbant Assay (ELISA) was used to assess plasma corticosterone according to manufacturer’s instructions. Samples were performed in duplicate, and only data from samples that had a CV% <15 were included in analyses.
Quantitative reverse-transcription polymerase chain reaction
Hippocampal regions were dissected, and the left hippocampus was used for quantitative reverse-transcription polymerase chain reaction (qRT-PCR). Total RNA was extracted with Qiagen RNAeasy mini kits (Germantown, MA) according to manufacturer’s instructions in the Yerkes Nonhuman Primate Genomics Core Laboratory. A nanodrop 2000 was used to assess RNA concentration. RNA was standardized to 1 μg in 10 μl nuclease-free water. Applied Biosystems High-Capacity cDNA Reverse Transcription kit was used to create cDNA (Applied Biosystems by Thermo Fisher Scientific, Vilnius, Lithuania). Quant-IT Pico Green dsDNA Assay kit (Invitrogen) was used to standardize cDNA. qRT-PCR was performed with Thermo Scientific Absolute Blue qPCR SYBR Green ROX Mix and primers (Thermo Scientific, Carlsbad, CA) for Nr3c1, Fkbp5, Ppid, Bag1, Fkbp4, Per1, Dusp1, Sgk1, Tsc22d3, and Hprt1 housekeeping gene (Supplemental Table 1). qRT-PCR was performed in triplicate and a CV cutoff of 4% was used. Gene expression was normalized to Hprt1 housekeeping gene, and the 2-ΔΔCt method was used to calculate and graph fold change (Livak & Schmittgen, Citation2001).
Proximity ligation assay
Brains were frozen at −80 °C until processing. Brains were cryosectioned on a Leica cryostat at 7 μm thickness. Coronal sections from ∼-3.3 mm and ∼-5.3 mm Bregma (Paxinos & Watson, Citation1998; Kjonigsen et al., Citation2011) were fixed in 10% formalin and washed with 1X phosphate buffered saline (PBS) for five minutes three times. Antigen retrieval was performed at 96 °C, and tissue was permeabilized in 0.2% Triton-X in 1X PBS. Tissue was washed with 1X PBS and blocked in Duolink blocking buffer (Sigma) for one hour at 37 °C. Tissue was incubated overnight at 4 °C with rabbit anti-glucocorticoid receptor (1:600, Bethyl A303-491A) and goat anti-FKBP5 (1 ug/ml, R&D Systems R&D Systems AF4094) primary antibodies in Duolink antibody diluent. A separate section (Bregma ∼-3.3 mm) was incubated overnight at 4 °C with only anti-FKBP5 primary antibody as a negative technical control. Proximity ligation assay (PLA) with far red detection reagents was performed according to manufacturer’s instructions (Sigma). Briefly, slides were washed 5 times for 5 minutes in Duolink Fluorescence Wash Buffer A then incubated with anti-goat (1:5) and anti-rabbit (1:5) Duolink secondary antibodies in Duolink antibody diluent for one hour at 37 °C. Slides were washed in Wash Buffer A and incubated for 30 minutes at 37 °C with ligation solution, consisting of 1:5 ligation stock (5x) and 1:40 ligase (1 U/μl) in nuclease-free water (Duolink). Slides were then washed in Wash Buffer A and incubated with amplification solution consisting of 1:5 Far Red Amplification Stock (5x, Duolink) and 1:80 polymerase (10 U/μl) in nuclease-free water for 100 minutes at 37 °C, protected from light. Slides were washed with Wash Buffer B 5 times for 5 minutes followed by a single 5-minute wash of 0.01x Wash Buffer B with agitation. Slides were dried overnight and cover slipped with Duolink DAPI mounting media.
Images were acquired on a Leica TCS SP8 confocal microscope. Twenty-one stacks of 0.35 μm thickness were acquired as z-stacks (for a total of 7 μm thickness) with a HC PL APO CS2 40x/1.3 OIL objective. Far red signal was acquired at 653 to 691 nm emission spectra on a HyD detector. DAPI counter stain was acquired at 447 to 481 nm emission spectra on HyD detector. All images were acquired with the same acquisition settings. Three z-stacks centered on the pyramidal or granule cells in the CA1, CA3, and dentate gyrus (DG) were acquired from each stained section.
Duolink ImageTool Software was used to quantify number of PLA signals (PLA counts) in each acquired image with signal threshold set to 644 and a signal size threshold of 3 pixels. The number of PLA signals for the three images within each region (dorsal and ventral CA1, CA3, and DG) were averaged (one CA1 animal used an average of two images due to an imaging error). Average PLA counts in treated samples were divided by the average PLA counts in the single antibody negative technical control (only anti-FKBP5 primary antibody, dorsal section) to control for batch-to-batch variability. Representative images are maximum intensity z projections in Fiji.
FKBP5 immunofluorescence
On PND 119, animals were transcardially perfused with PBS, immediately followed by 4% paraformaldehyde. Brains were extracted and post-fixed for 48 hours in 4% paraformaldehyde then cryoprotected in 30% sucrose. Tissue was sectioned at 40 µm on a cryostat (Leica) before staining. Hippocampal and prefrontal sections were mounted onto glass slides and allowed to dry. Sections were subsequently rinsed in phosphate buffered saline (PBS) immersed in citrate buffer for antigen retrieval, rinsed with PBS, blocked in 5% normal goat serum with 0.4% Triton X for one hour at room temperature, and incubated overnight in goat anti-FKBP5 primary antibody (1:300, R&D Systems, cat. no. AF4094) at 4 °C. Tissue was then rinsed in PBS and incubated in anti-goat Alexafluor 488 (1:500, Invitrogen, ThermoFisher cat. no. A32723) for one hour and then rinsed in PBS. Slides were coverslipped with Vectashield Hardset Anti-Fade Mounting Media with DAPI (Vector, cat. no. H-1500). Image stacks measuring 10 µm with 1 µm intervals were acquired on a Zeiss LSM 700. Eight image stacks per region were acquired for the DG, CA3, and CA1. Image stacks were uploaded to Volocity (Quorum Technologies) for volumetric quantification of immunofluorescent labeling density.
Nuclear and cytosolic protein extraction
Hippocampal hemispheres were dissected on dry ice, and the right hemisphere was used for all protein analyses. Nuclear and cytosolic protein fractions were extracted as previously described (Bourke et al., Citation2013). Briefly, tissue was homogenized in 50 mM Tris, 6 mM MgCl2, 1 mM EDTA, 10% sucrose buffer with 1:1000 protease inhibitor. Homogenized protein was spun at 105,000 x g at 4 °C for 30 minutes, and the supernatant was collected as the cytosolic fraction. The pellet was homogenized in 0.5 ml buffer consisting of 50 mM Tris, 6 mM MgCl2, 1 mM EDTA, 0.5 M NaCl, 10% sucrose and 1:1000 protease inhibitor. The pellet was centrifuged and washed twice, incubated in an ice bath for one hour and then centrifuged at 8000 x g for 10 minutes at 4 °C. The supernatant was collected as the nuclear protein fraction. Protein concentrations were determined using a BCA assay (Pierce, Prod #23227) according to the manufacturer’s instructions.
Western blot
A Pierce BCA was used to standardize 10 μg protein in nuclease-free water with 4x Laemmli Sample Buffer (BioRad) and β-mercaptoethanol and run on a Criterion Precast gel (10-20% Tris HCl, 1.0 mm, BioRad) in a BioRad apparatus for 95 minutes at a constant 150 V. Protein was transferred to a PVDF membrane (midi size, BioRad). A BioRad Transblot turbo apparatus was used to perform a semi-dry transfer (BioRad, preset Mixed molecular weight setting) in TransBlot turbo buffer according to manufacturer’s instructions. The membrane was blocked in 7.5% weight/volume nonfat dry milk in TBS-T for one hour at room temperature. The membrane was cut to visualize each of three proteins: GR, H3, and GAPDH. The membrane was incubated with anti-GR antibody (1:10000, ab109022) overnight at 4 °C, anti-H3 (1:100000, ab1791) overnight at 4 °C or anti-GAPDH (1:500000, ab181602) for 30 minutes at room temperature. Membranes then were washed three times in TBS-T and incubated with secondary goat anti-rabbit HRP conjugated antibody (1:5000, ab97051). Membranes were washed three times in TBS-T, and chemiluminescence was visualized with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific) on a Syngene G:BOX system (Syngene, Frederick, MD). Signal was quantified using Li-Cor Image Studio Lite using the user defined background subtraction method (a single background value for each image). Arbitrary densitometry units were normalized to a standard protein lane on each membrane to normalize signal between membranes, and to a housekeeping protein in each lane to normalize between lanes (H3 for nuclear fractions, GAPDH for cytosolic fractions).
Experimental design and statistical analysis
GraphPad Prism was used for statistical analyses with α = 0.05. Two-way ANOVAs (with factors adolescent stress x acute stress) were used for gene expression (PCR), corticosterone ELISA, and PLA analyses separately in males and females because CAS was hypothesized to alter endpoints differently in males and females. A mixed-effects model using the restricted maximum likelihood method was used to assess weight gain across adolescent stress (CAS x day) due to missing values, and an unpaired t-test was used to assess adult terminal weight separately in males and females. A priori t-tests were used to assess the impact of CAS on Fkbp5 gene expression in adulthood without acute stress exposure as well as FKBP5 protein expression in subregions of the hippocampus. Sidak’s multiple comparisons test was used to assess post-hoc comparisons between NS and CAS groups when a significant interaction was observed in weight data. A post-hoc Tukey’s test was used for PLA analyses when a significant interaction was observed. When a main effect of acute stress was found in GR-target gene PCR or corticosterone data, post-acute stress group differences were assessed with a Sidak’s multiple comparisons test. Multiplicity adjusted p-values are reported for multiple comparisons tests. One animal was not included in gene expression analysis due to a technical pipetting error. One male isolation weight was identified as an outlier with Grubbs’ Outlier test and excluded. Two-way ANOVAs (with factors adolescent stress x acute stress) were used for western blot analysis. Sidak’s multiple comparisons test was used to assess post-hoc comparisons between NS and CAS groups when a significant interaction was observed in western blot data. Multiplicity adjusted p-values are reported for multiple comparisons tests. Samples were excluded from western blot analysis due to technical reasons including insufficient protein extraction yield (4 animals), bubbles in protein band (11 samples), or a technical error in mass of protein run (3 animals); exclusions were represented among multiple experimental groups. Sample sizes for each experiment are detailed in Supplemental Table S2.
Results
Weight gain
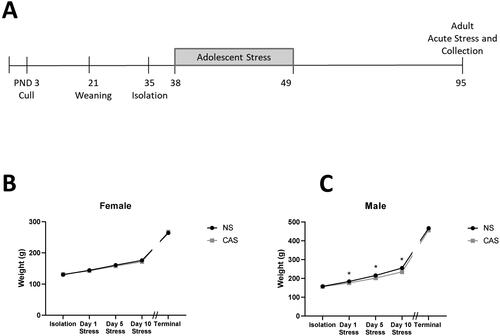
Though both male and female adolescent rats gained weight throughout adolescence during the stress paradigm (Male: F(3, 409) = 3038, p < 0.0001; Female: F(3, 393) = 707.7, p < 0.0001), male rats exposed to CAS gained less weight than NS male controls (F(1, 140) = 16.70, p < 0.0001, interaction: F(3, 409) = 32.84, p < 0.0001, ). On day 1, 5, and 10 of adolescent stress, CAS males exhibited reduced body weight compared to NS male controls (p = 0.038, p < 0.0001, p < 0.0001, respectively). The impact of CAS on weight in males normalized by adulthood at the terminal timepoint (t140=1.556, p > 0.05, ). CAS did not significantly impact weight gain across adolescence in females (F(1, 137) = 1.08, p > 0.05) but there was a significant interaction between CAS and day (F(3, 393) = 2.87, p = 0.036) that was not attributable to a significant difference on one specific day. CAS did not impact adult terminal weight in females (t137=0.929, p > 0.05, ).
Figure 1. Male and female rats were exposed to a mixed-modality CAS paradigm according to the timeline (A). Female (B) and male (C) rats were weighed on isolation day (PND 35), the first (PND 38), fifth (PND 42), and tenth (PND 47) day of the stress paradigm. Both males and females gained weight through adolescence, but males exposed to CAS gained less weight than non-stress (NS) male controls (ANOVA, p < 0.05). CAS does not alter terminal weight in female or male adult rats exposed to CAS using a t-test (p > 0.05). Weight was measured for all animals in the morning on the day of collection. Data are presented as mean ± SEM. * indicates significance in Sidak’s multiple comparisons test comparing CAS to NS groups. α = 0.05. Data are presented as mean ± SEM.
CAS does not impair the resolution of the corticosterone response
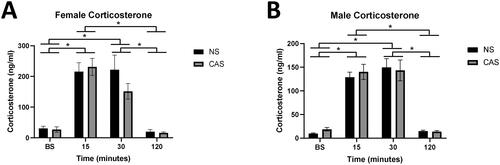
Plasma corticosterone was altered with time following novel acute stressor challenge in both females (F(3, 112) = 43.13, p < 0.0001) and males (F(3, 119) = 74.15, p < 0.0001, ). Specifically, corticosterone was elevated at the 15-minute and 30-minute timepoint compared to baseline (p < 0.0001) and compared to the 120-minute timepoint (p < 0.0001) in both males and females (Sidak’s multiple comparisons test). There was no significant effect of CAS on the corticosterone response in females (F(1, 112) = 0.94, p > 0.05) or males (F(1, 119) = 0.14, p > 0.05).
Figure 2. Trunk blood was collected immediately following rapid decapitation in female (A) and male (B) adult rats at baseline (BS; no acute stressor exposure) or 15, 30, or 120-minutes following a five-minute novel acute forced swim stressor challenge. Both males and females exhibited a main effect of time following acute stressor (p < 0.05), but CAS did not elicit a significant impact on plasma corticosterone. A Sidak’s multiple comparisons test showed that corticosterone at BS and 120 minutes following acute stressor exposure differed from 15 and 30 minutes, pooled across NS and CAS groups in both females (A) and males (B; p < 0.05, indicated by *). Data are presented as mean ± SEM.
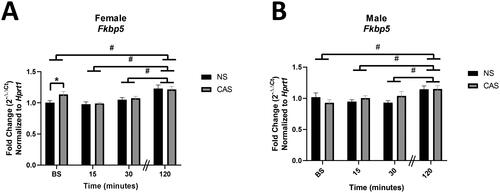
Chronic adolescent stress increases basal Fkbp5 gene expression in females and acute adult stress increases Fkbp5 gene expression for both sexes regardless of adolescent stress history
Gene expression of Fkbp5 increased over time following novel acute forced swim stressor exposure in both males (F(3,64)=5.42, p = 0.0022) and females (F(3, 67) = 13.20, p < 0.0001). Males and females had higher Fkbp5 gene expression 120 minutes following acute stressor exposure compared to baseline, 15, and 30 minutes (p < 0.05, Sidak’s multiple comparisons test) regardless of their adolescent stress history. CAS females also exhibited elevated Fkbp5 gene expression at baseline (t16=2.27, p = 0.038), as compared to the NS female group ().
Figure 3. Male and female rats were exposed to CAS or non-stress (NS) control conditions and collected at baseline or 15, 30, or 120 minutes following exposure to an acute novel stressor in adulthood. Females exposed to CAS exhibited elevated Fkbp5 gene expression at baseline. There was no significant effect of CAS in males. Both females (A) and males (B) exhibited elevated expression of Fkbp5 with time following acute novel forced swim stressor challenge. Data are expressed as mean fold change (2-ΔΔCt) ± SEM normalized to same-sex NS baseline group. * indicates a significant difference in CAS to NS comparison with a priori t-test, p < 0.05. # indicates that gene expression at 120 minutes differs from baseline, 15, and 30 minutes, data pooled across NS and CAS groups with Sidak’s multiple comparisons test (p < 0.05).
In addition, gene expression of other known GR co-chaperones was assessed in males and females following CAS and acute stressor exposure (Supplemental Figure S1). Females with a history of CAS exposure exhibited reduced Bag1 gene expression (Main effect CAS: F(1, 67) = 4.26, p = 0.043) that was not impacted by exposure to the acute stressor (F(3,67)=0.451, p > 0.05). CAS did not impact Bag1 gene expression in males (F(1, 64) = 3.12, p = 0.082) regardless of acute stressor exposure in adulthood (F(3.64)=1.48, p > 0.05).Neither adolescent (Female: F(1, 67) = 0.253, p > 0.05; Male: F(1, 64) = 0.897, p > 0.05) nor acute stress (Female: F(3, 67) = 0.572, p > 0.05; Male: F(3, 64) = 1.558, p > 0.05) impacted Fkbp4 gene expression in males or females. Chronic adolescent (Female: F(1, 67) = 1.26, p > 0.05; Male: F(1, 64)= 0.149, p > 0.05) and acute (Female: F(3, 67) = 1.223, p > 0.05; Male: F(3, 64) = 0.584, p > 0.05) stress also did not impact Ppid gene expression. Nr3c1 gene expression was not significantly altered by chronic adolescent (Female: F(1, 67) = 1.251, p > 0.05; Male: F(1, 64) = 3.137, p = 0.081) or acute stress (Female: F(3, 67) = 1.576, p > 0.05; Male: F(3, 64) = 2.48, p = 0.069) in males or females.
Chronic adolescent stress does not alter FKBP5 protein levels in the hippocampus
There was no effect of CAS on FKBP5 staining in the female DG (t10=0.043, p = 0.97), CA1 (t10=0.36, p = 0.73), or CA3 (t10=0.95, p = 0.36) regions of the hippocampus. Similarly, males did not show an effect of CAS on FKBP5 in either the DG (t10=0.06, p = 0.95), CA1 (t10=1.13, p = 0.29), or CA3 (t10=1.39, p = 0.20) regions of the hippocampus (Representative images in Supplemental Figure S2).
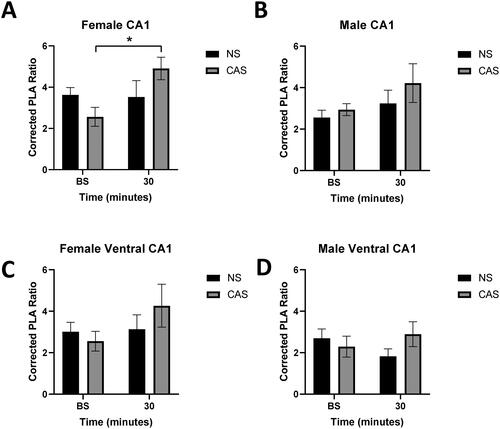
Females exposed to CAS exhibit increased GR-FKBP5 interactions following acute stressor exposure
A proximity ligation assay (PLA) was used to assess protein-protein interactions between GR and FKBP5 in situ in the adult rat hippocampus. Acute stress exposure did not significantly increase GR-FKBP5 interactions (measured by the corrected PLA ratio) in the dorsal CA1 in adult female rats (F(1, 20) = 4.01, p = 0.0589), but females exhibited a significant acute stress by CAS interaction (F(1, 20) = 4.77, p = 0.041) (, Supplemental Figure S3). Post-hoc analysis demonstrated that females with a history of CAS exposure exhibited increased GR-FKBP5 interactions in the dorsal CA1 following exposure to a novel acute stressor (p = 0.036, Tukey’s multiple comparisons test), but NS females did not (p > 0.05). Males did not exhibit an impact of acute (F(1, 20) = 2.57, p = 0.125) or adolescent (F(1, 20) = 1.25, p > 0.05) stress on GR-FKBP5 interactions in the dorsal CA1 (). Representative images are shown in Supplemental Figure S2. Acute stress (Female: F(1, 20) = 1.70, p > 0.05; Male: F(1, 20) = 0.08, p > 0.05) or CAS (Female: F(1, 20) = 0.23, p > 0.05; Male: F(1, 20) = 0.47, p > 0.05) did not significantly impact GR-FKBP5 interactions in the ventral CA1 in males or females (). There were also no significant interactions in the ventral CA1 of males or females (Female: F(1,20) = 1.28, p > 0.05; Male: F(1,20) = 2.30, p > 0.05).
Figure 4. Female and male rats were exposed to CAS. In adulthood, tissue was collected at baseline (no acute stressor exposure) or 30 minutes following an acute stressor challenge (acute). A proximity ligation assay (PLA) was used to assess interactions between GR and FKBP5 protein. The corrected PLA ratio was calculated by dividing PLA counts in treated images by PLA counts in a negative technical control (single antibody, dorsal section). PLA signals in the dorsal CA1 in females (A) exhibit a significant interaction between adolescent stress and time after acute stress (p < 0.05). Adolescent or acute stress did not significantly impact PLA signal in the male dorsal CA1 (B), female ventral CA1 (C), or male ventral CA1 (D) (p > 0.05). * denotes significant effect in a post-hoc Tukey’s test. n = 6 per group. Data are presented as mean ± SEM.
GR-FKBP5 interactions were also assessed in the dorsal and ventral CA3 and dentate gyrus (DG). Acute stress increased the corrected PLA ratio in females in the DG (F(1, 20) = 6.386, p = 0.02). A history of CAS or acute stressor exposure did not significantly impact GR-FKBP5 interactions in the dorsal or ventral CA3 or DG in males or in the dorsal or ventral CA3 or ventral DG in females (p > 0.05) (Supplemental Figure S4).
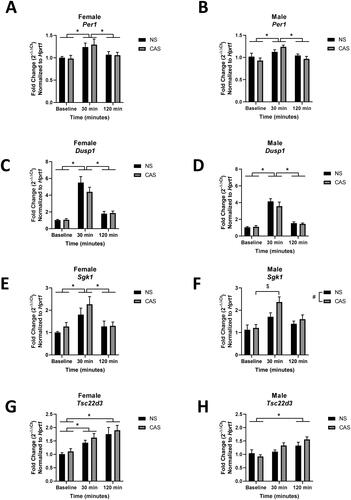
Gene expression of GR-target genes
As expected, following exposure to an acute novel stressor, gene expression of GR target genes Per1 (Female: F(2, 49) = 5.909, p = 0.005; Male: F(2, 48) = 8.349, p = 0.0008), Dusp1 (Female: F(2,49) = 53.99, p < 0.0001; Male: F(2,48) = 67.13, p < 0.0001), Sgk1 (Female: F(2,49) = 8.126, p = 0.0009; Male: F(2,48) = 11.34, p < 0.0001), and Tsc22d3 (Female: F(2,49) = 11.57, p < 0.0001; Male: F(2,48) = 11.05, p = 0.0001) were increased (). Males exposed to CAS showed increased gene expression of Sgk1 (F(1, 48) = 4.513, p = 0.039), but CAS did not impact Sgk1 gene expression in females (F(1, 49) = 1.739, p > 0.05). A post hoc test revealed that males exposed to CAS increased gene expression of Sgk1 30 minutes following acute stressor exposure compared to baseline (p = 0.0004). CAS did not significantly impact gene expression of Per1, Dusp1, and Tsc22d3 in either sex (p > 0.05).
Figure 5. Male and female rats were exposed to CAS or non-stress (NS) control conditions and collected at baseline, 30, or 120 minutes following exposure to an acute novel stressor in adulthood. Data are expressed as mean fold change (2-ΔΔCt) ± SEM normalized to same-sex NS baseline group. # indicates a main effect of CAS. * indicates a significant difference between post-acute stress time points with Sidak’s multiple comparisons test. $ indicates a significant effect of a Sidak’s multiple comparisons test between groups.
CAS impact on nuclear localization of protein
Protein expression of GR in nuclear and cytosolic cell fractions were assessed using an anti-GR antibody (Abcam ab109022). However, following completion of the study, Abcam ab109022 was discontinued due to new evidence that the antibody was nonspecific to the glucocorticoid receptor as determined by using NR3C1-knockout A549 whole cell lysate by the manufacturer (https://www.abcam.com/glucocorticoid-receptor-antibody-epr4595-ab109022.html). Because this antibody labeled protein in a knockout sample, it is not clear if the observed effects of CAS on the western blot protein data () are due to impact of CAS on GR translocation or due to an impact on other nuclear receptors or proteins. We report the collected data here as this antibody appears in the literature (Boncompagni et al., Citation2015; Herring et al., Citation2016; Wei et al., Citation2016; Engel et al., Citation2018; Hadamitzky et al., Citation2018; Sugimoto et al., Citation2019; Edvinsson et al., Citation2020; Wang et al., Citation2020; Shi et al., Citation2021; Lin et al., Citation2022) and identification of the labeled protein may be reported at a future timepoint.
Figure 6. Female and male rats were exposed to CAS or non-stress (NS) control conditions. In adulthood, hippocampal tissue was collected at baseline (BS, no acute stressor exposure) and 15, 30, or 120-minutes following exposure to an acute novel stressor. Nuclear (A,B) and cytosolic (C,D) GR protein was assessed with western blot. Changes in GR antibody-tagged protein were impacted by CAS. There was a significant interaction between time following acute stressor exposure and history of CAS on nuclear GR antibody-tagged protein expression (A). Arbitrary densitometry units were normalized to H3 nuclear housekeeping protein or GAPDH cytosolic protein and a standard protein sample and expressed as mean ± SEM. * indicates significant effect in CAS to NS comparison with Sidak’s multiple comparisons test, p < 0.05. Data are presented as mean ± SEM. Note that following completion of this study, the antibody was discontinued by the company which limits interpretation of these data.
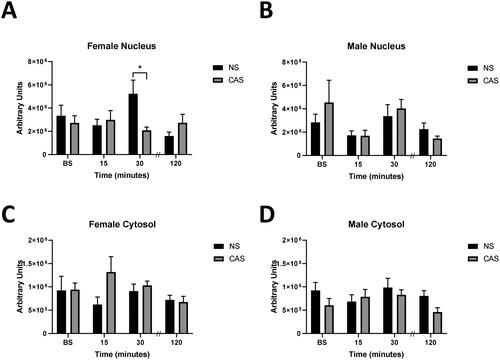
Using ab109022, females exhibited a significant interaction between time following acute stressor exposure and history of CAS on nuclear antibody tagged protein expression (F(3, 65) = 3.516, p = 0.02, ), such that females exposed to CAS exhibited reduced antibody-tagged nuclear protein 30 minutes following the acute stressor compared to NS controls (Sidak’s multiple comparisons test, p = 0.013). There were no main effects of time following acute stressor exposure (F(3, 65) = 1.535, p > 0.05) or history of CAS (F(1, 65) = 1.187, p > 0.05) on antibody-tagged nuclear protein content in females. CAS did not impact antibody-tagged nuclear protein content in males (F(1, 62) = 0.314, p > 0.05; ) but there was a non-significant trend in a main effect of time following acute stressor exposure on nuclear antibody-tagged protein (F(3, 62) = 2.682, p = 0.0545) suggesting an increase in nuclear expression following the acute stressor. Cytosolic antibody-tagged protein expression was not impacted by exposure to the acute stressor (Female: F(3, 66) = 0.93, p > 0.05; Male: F(3, 60) = 1.127, p > 0.05) or CAS (Female: F(1, 66) = 2.075, p > 0.05, Male: F(1, 60) = 3.025, p = 0.087) in females or males ().
Discussion
Exposure to CAS can cause lasting disruptions to stress-sensitive behaviors in female rats (Bourke & Neigh, Citation2011; Hyer et al., Citation2021). Here, we investigated whether a history of CAS alters adult co-chaperone regulation of the GR in the hippocampus to examine a potential underlying mechanism for risk in females. We demonstrate that although FKBP5 protein expression in the hippocampus is similar in females with and without a history of adolescent stress, expression of Fkbp5 is elevated in the hippocampus of adult females with a history of CAS and interactions between GR and FKBP5 are augmented following an acute stressor in females with a history of adolescent stress. Collectively, the data reported here suggest that there is a lasting impact of CAS on co-chaperone regulation of the GR.
The impact of CAS on behavior initiates a complex behavioral profile that differs between males and females. Work from our group that assessed the adolescent and adult impact of CAS on affective-like behaviors showed that CAS increased depressive-like behaviors in adult female rats (Bourke & Neigh, Citation2011). We have also observed that rats exposed to CAS exhibit impacted learning and memory behaviors. Following an LPS challenge, males demonstrated deficits in initial learning while females exhibited impacted reversal learning in the attention set shift task (Hyer et al., Citation2023). Further, in the Barnes Maze task, while CAS females have an initial reduction in latency to locate a goal box compared to non-stressed controls, CAS females but not males, showed impairments in cognitive flexibility with reversal learning (Hyer et al., Citation2021). While females show altered depressive-like behavior and learning and memory performance following CAS, CAS increases social avoidance in adult male rats but not females (Bekhbat et al., Citation2021). These data show a complex impact of CAS on adult behaviors that differs considerably between males and females. These differences in behavior suggest that the physiological mechanisms underlying these behaviors are also sex-specifically altered by CAS.
In order to assess a potential mechanism underlying the behavioral impact of CAS and directly assess the impact of CAS on co-chaperone regulation of the GR, we assessed protein interactions between GR and FKBP5 in situ. Interactions between these proteins have been previously reported in vitro (Davies et al., Citation2002; Wochnik et al., Citation2005) or with co-immunoprecipitation in the whole mouse brain (Li et al., Citation2020). The current studies show that CAS has lasting effects on stress-induced GR-FKBP5 interactions in the hippocampus in adulthood such that GR-FKBP5 interactions in the dorsal CA1 increase following acute stressor exposure in adult female rats with a history of CAS. These data also indicate that CAS shifts regulation of these proteins differently in males and females as CAS exposure does not impact GR-FKBP5 interactions in males. Increased interactions between GR and FKBP5 have been observed following fear conditioning in mice and in patients with PTSD, and reducing interactions between GR-FKBP5 reduces the behavioral impact of fear conditioning in mice (Li et al., Citation2020), further implicating interactions between GR and FKBP5 in stress-related behaviors. Following acute stress exposure, GR-FKBP5 interactions also increased in the dorsal DG in females, indicating that increases in GR association with FKBP5 following acute stressor exposure occurs rapidly, within 30 minutes, though there was no additional impact of CAS. Although a basal increase in Fkbp5 gene expression was observed for females with a history of CAS, we did not document an increase in FKPB5 protein suggesting that protein availability of FKBP5 does not solely account for the increase in GR-FKBP5 interactions. However, it is important to note that immunohistochemistry was used to measure FKBP5 protein and this technique is not robustly quantitative limiting interpretation of this specific result. We did not detect a significant impact of acute stress or CAS on GR-FKBP5 interactions in the dorsal or ventral CA3 in males or females, or in the dorsal or ventral DG in males (Supplemental Figure S4). Other groups have reported that dexamethasone treatment increases Fkbp5 gene expression in the mouse CA1 and DG, but not in the CA3 (Scharf et al., Citation2011), suggesting that there may be hippocampal region-specific control of Fkbp5 expression or modulation of FKBP5 activity, such that the CA1 is more responsive to alterations in FKBP5 regulation with CAS, in line with the current results. Though there was not a significant impact of CAS on GR-FKBP5 interactions in the dorsal or ventral CA3 or DG, it is possible that there are meaningful changes in GR-FKBP5 interactions only within specific cell types. Because we only assessed GR-FKBP5 interactions regionally, we do not have data on potentially impactful changes within specific cell types. In addition to neurons, microglia (Wohleb et al., Citation2011) and astrocytes (Carter et al., Citation2012; Tertil et al., Citation2018) express both GR and FKBP5. Differences in distribution of microglia density in the mouse hippocampus have been found across dorsal and ventral hippocampal regions (Jinno et al., Citation2007), and it is therefore possible that differential contribution of different cell types to PLA interactions could dilute an effect in a single cell type. Future work should consider the additional potential for cell type-specific contributions of GR-FKBP5 interactions.
Given the increased interactions of GR with its co-chaperone FKBP5, which is known to impair GR-nuclear translocation (Wochnik et al., Citation2005), we hypothesized that GR-nuclear translocation following acute stressor exposure would be attenuated in females previously exposed to CAS. We assessed this question using Western Blot to determine GR expression in nuclear and cytosolic fractions. However, the anti-GR antibody used in these experiments (Abcam ab109022) was discontinued after the completion of these experiments due to new evidence from the manufacturer that the antibody was not specific to GR protein using a GR- knockout cell model. It is therefore not possible to conclude whether the impact of CAS on GR-antibody tagged nuclear protein expression we observed in adult females is due to the GR or other proteins contributing to the observed signal (). Future experiments will need to assess the impact of CAS exposure on adult translocation and activity of the GR in adulthood.
Following acute stressor exposure, the GR rapidly interacts with glucocorticoid response elements (GREs) in target genes in the hippocampus to regulate gene expression (Mifsud & Reul, Citation2016). As expected, upon exposure to an acute stressor, we observed a robust increase in gene expression of GR-target genes in both males and females. Although CAS interacted with acute stress to increase interactions between GR and FKBP5 in adult females, potentially disrupting activity of the GR, we did not observe an effect of CAS on gene expression in the subset of GR-target genes assessed in females, suggesting that CAS does not cause a less transcriptionally active GR at these specific target genes. Activity of the GR is regulated at multiple levels- through interaction with multiple co-chaperones, post-translational modifications, and interactions with co-activators, among other mechanisms (Bekhbat et al., Citation2017). Differential interaction of the GR with co-activators or preferential interaction of the GR with specific GR-response elements (specifically within the genes assessed in this study) could allow for unchanged GR-mediated transcription at specific target genes even with altered GR co-chaperone activity.
Interestingly, adult males exposed to CAS showed increased expression of the GR-target gene Sgk1. Serum and glucocorticoid inducible kinase 1 (SGK1) is involved in GR activation and has been implicated in the effects of cortisol on hippocampal neurogenesis and GR translocation and is increased in patients with depression (Anacker et al., Citation2013), suggesting that though we did not observe any difference in GR-FKBP5 interactions in adult males, the system is affected by CAS exposure. Our group has previously reported impacts of CAS on adult immune reactivity with exaggerated impact in the peripheral immune system of males as well as alterations in male social behavior following CAS (Bekhbat et al., Citation2019; Bekhbat et al., Citation2021). Given the role of SGK1 in immune-related processes (Wu et al., Citation2013; Inoue et al., Citation2016; Dattilo et al., Citation2020), the impact of CAS on SGK1 in male rats will be of particular importance. The impact of CAS on gene transcription in the hippocampus is complex and differs in males and females (Rowson et al., Citation2019; Bekhbat et al., Citation2021). Specific targeting of the transcriptional changes due to CAS may be informative to further elucidate the mechanisms contributing to the impact of CAS on behavior in adulthood.
Adult male and female rats exhibited a robust corticosterone response following acute stressor exposure, consistent with the observed increased expression of the GR target genes. Interestingly, the resolution of the corticosterone response to acute stress was not impacted by CAS. Our group has previously reported a prolonged corticosterone response to an acute stressor in females exposed to CAS at the end of adolescence (Bourke et al., Citation2013). The difference in this study is likely a reflection of the age of assessment as it is well established that the corticosterone response to acute stress varies with age (Foilb et al., Citation2011; Lui et al., Citation2012; Girard-Joyal et al., Citation2015), and this may further interact with the length of time between the final chronic stressor and the acute stress challenge (Rich & Romero, Citation2005; Ostrander et al., Citation2006; Lin et al., Citation2008).
Together, these results suggest that the transcriptional activity of GR may be unchanged despite increased interactions between GR and FKBP5 in the hippocampus of females with a history of chronic adolescent stress. Altered transactivator and transrepressor activity of the GR (Bekhbat et al., Citation2017) or epigenetic changes at target genes could underlie these effects, as epigenetic modifications of GR-target genes have been previously observed in specific GR-target genes (Lebow et al., Citation2019). Increased GR sensitivity or a shifted balance of GR activity to different subsets of genes could be disruptive; for example, both high and low activity of the GR have been implicated in disrupting neurogenesis (Anacker et al., Citation2011; Saaltink & Vreugdenhil, Citation2014). This disruption could be driven in part through changes in interactions with FKBP5, as FKBP5 inhibition increases neurite outgrowth in hippocampal neurons (Gaali et al., Citation2015). Increased GR association with FKBP5 alone has been implicated in stress-related disorders (Li et al., Citation2020), and interactions with FKBP5 could be involved in shifting the balance of GR activity. These shifted GR-FKBP5 profiles could underlie the behavioral impact of CAS in adulthood (Bourke & Neigh, Citation2011; Hyer et al., Citation2021). Compensations in the system to counteract increased FKBP5 interactions following acute stressor exposure may be sufficient to prevent transcriptional impact of CAS earlier in adulthood, but with aging or if faced with additional health challenges, these mechanisms may no longer be sufficient and could cause further dysregulation.
Supplemental Material
Download Zip (980.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Sydney Rowson
Sydney Rowson, Phd is a Associate Laboratory Project Set-Up Manager with expertise in both translational animal models and clinical research
Mandakh Bekhbat
Mandakh Bekhbat, Phd is a postdoctoral fellow with expertise in the study of immune and endocrine mechanisms underlying psychiatric illnesses.
Sean Kelly
Sean Kelly, is a Senior Research Specialist with expertise in animal models of developmental stress and neuroimmune methods.
Molly M. Hyer
Molly M. Hyer, Phd is Director of Research Development and Innovation for the VCU Institute of Women’s Health and an experienced researcher in psychoneuroendocrinology.
Samya Dyer
Samya Dyer, MPH professional experience performing laboratory research involving continuous data collection, analysis, and interpretation in team settings.
David Weinshenker
David Weinshenker, Phd is a Professor of Human Genetics at Emory University and Scientific Director of the Emory School of Medicine Rodent Behavioral Core.
Gretchen Neigh
Gretchen Neigh, PhD, MBA is a Professor of Anatomy & Neurobiology. Dr. Neigh is director of Translational Research for the VCU Institute of Women’s Health, co-director of research for BIRCWH, and co-director for the Clinical and Translational Sciences PhD Program at Virginia Commonwealth University.
References
- Anacker, C., Cattaneo, A., Musaelyan, K., Zunszain, P. A., Horowitz, M., Molteni, R., Luoni, A., Calabrese, F., Tansey, K., Gennarelli, M., Thuret, S., Price, J., Uher, R., Riva, M. A., & Pariante, C. M. (2013). Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proceedings of the National Academy of Sciences of the United States of America, 110(21), 1–14. https://doi.org/10.1073/pnas.1300886110
- Anacker, C., Zunszain, P. A., Carvalho, L. A., & Pariante, C. M. (2011). The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology, 36(3), 415–425. https://doi.org/10.1016/j.psyneuen.2010.03.007
- Bekhbat, M., Howell, P. A., Rowson, S. A., Kelly, S. D., Tansey, M. G., & Neigh, G. N. (2019). Chronic adolescent stress sex-specifically alters central and peripheral neuro-immune reactivity in rats. Brain, Behavior, and Immunity, 76, 248–257. https://doi.org/10.1016/j.bbi.2018.12.005
- Bekhbat, M., Mukhara, D., Dozmorov, M. G., Stansfield, J. C., Benusa, S. D., Hyer, M. M., Rowson, S. A., Kelly, S. D., Qin, Z., Dupree, J. L., Tharp, G. K., Tansey, M. G., & Neigh, G. N. (2021). Adolescent stress sensitizes the adult neuroimmune transcriptome and leads to sex-specific microglial and behavioral phenotypes. Neuropsychopharmacology: official Publication of the American College of Neuropsychopharmacology, 46(5), 949–958. https://doi.org/10.1038/s41386-021-00970-2
- Bekhbat, M., Rowson, S. A., & Neigh, G. N. (2017). Checks and balances: The glucocorticoid receptor and NFĸB in good times and bad. Frontiers in Neuroendocrinology, 46, 15–31. https://doi.org/10.1016/j.yfrne.2017.05.001
- Binder, E. B., Salyakina, D., Lichtner, P., Wochnik, G. M., Ising, M., Pütz, B., Papiol, S., Seaman, S., Lucae, S., Kohli, M. A., Nickel, T., Künzel, H. E., Fuchs, B., Majer, M., Pfennig, A., Kern, N., Brunner, J., Modell, S., Baghai, T., … Muller-Myhsok, B. (2004). Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nature Genetics, 36(12), 1319–1325. https://doi.org/10.1038/ng1479
- Boncompagni, S., Arthurton, L., Akujuru, E., Pearson, T., Steverding, D., Protasi, F., & Mutungi, G. (2015). Membrane glucocorticoid receptors are localised in the extracellular matrix and signal through the MAPK pathway in mammalian skeletal muscle fibres. The Journal of Physiology, 593(12), 2679–2692. https://doi.org/10.1113/JP270502
- Bourke, C. H., & Neigh, G. N. (2011). Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Hormones and Behavior, 60(1), 112–120. https://doi.org/10.1016/j.yhbeh.2011.03.011
- Bourke, C. H., Raees, M. Q., Malviya, S., Bradburn, C. A., Binder, E. B., & Neigh, G. N. (2013). Glucocorticoid sensitizers Bag1 and Ppid are regulated by adolescent stress in a sex-dependent manner. Psychoneuroendocrinology, 38(1), 84–93. https://doi.org/10.1016/j.psyneuen.2012.05.001
- Carter, B. S., Meng, F., & Thompson, R. C. (2012). Glucocorticoid treatment of astrocytes results in temporally dynamic transcriptome regulation and astrocyte-enriched mRNA changes in vitro. Physiological Genomics, 44(24), 1188–1200. https://doi.org/10.1152/physiolgenomics.00097.2012
- Cover, K. K., Maeng, L. Y., Lebrón-Milad, K., & Milad, M. R. (2014). Mechanisms of estradiol in fear circuitry: implications for sex differences in psychopathology. Translational Psychiatry, 4(8), e422–e422. https://doi.org/10.1038/tp.2014.67
- Dattilo, V., Amato, R., Perrotti, N., & Gennarelli, M. (2020). The emerging role of SGK1 (Serum- and Glucocorticoid-Regulated Kinase 1) in major depressive disorder: Hypothesis and mechanisms. Frontiers in Genetics, 11, 826. https://doi.org/10.3389/fgene.2020.00826
- Davies, T. H., Ning, Y.-M., & Sánchez, E. R. (2002). A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. The Journal of Biological Chemistry, 277(7), 4597–4600. https://doi.org/10.1074/jbc.C100531200
- Edvinsson, Å., Hoyer, A., Hansson, M., Kallak, T. K., Sundström-Poromaa, I., Skalkidou, A., & Lager, S. (2020). Placental glucocorticoid receptors are not affected by maternal depression or SSRI treatment. Upsala Journal of Medical Sciences, 125(1), 30–36. https://doi.org/10.1080/03009734.2019.1702126
- Engel, M., Eggert, C., Kaplick, P. M., Eder, M., Röh, S., Tietze, L., Namendorf, C., Arloth, J., Weber, P., Rex-Haffner, M., Geula, S., Jakovcevski, M., Hanna, J. H., Leshkowitz, D., Uhr, M., Wotjak, C. T., Schmidt, M. V., Deussing, J. M., Binder, E. B., & Chen, A. (2018). The role of m(6)A/m-RNA methylation in stress response regulation. Neuron, 99(2), 389–403.e9. e389. https://doi.org/10.1016/j.neuron.2018.07.009
- Fan, B., Ma, J., Zhang, H., Liao, Y., Wang, W., Zhang, S., Lu, C., & Guo, L. (2021). Association of FKBP5 gene variants with depression susceptibility: A comprehensive meta-analysis. Asia-Pacific Psychiatry: official Journal of the Pacific Rim College of Psychiatrists, 13(2), e12464. https://doi.org/10.1111/appy.12464
- Feldman, S., & Weidenfeld, J. (1999). Glucocorticoid receptor antagonists in the hippocampus modify the negative feedback following neural stimuli. Brain Research, 821(1), 33–37. https://doi.org/10.1016/s0006-8993(99)01054-9
- Foilb, A. R., Lui, P., & Romeo, R. D. (2011). The transformation of hormonal stress responses throughout puberty and adolescence. The Journal of Endocrinology, 210(3), 391–398. https://doi.org/10.1530/JOE-11-0206
- Gaali, S., Kirschner, A., Cuboni, S., Hartmann, J., Kozany, C., Balsevich, G., Namendorf, C., Fernandez-Vizarra, P., Sippel, C., Zannas, A. S., Draenert, R., Binder, E. B., Almeida, O. F., Rühter, G., Uhr, M., Schmidt, M. V., Touma, C., Bracher, A., & Hausch, F. (2015). Selective inhibitors of the FK506-binding protein 51 by induced fit. Nature Chemical Biology, 11(1), 33–37. https://doi.org/10.1038/nchembio.1699
- Giedd, J. N. (2004). Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences, 1021(1), 77–85. https://doi.org/10.1196/annals.1308.009
- Girard-Joyal, O., Faragher, A., Bradley, K., Kane, L., Hrycyk, L., & Ismail, N. (2015). Age and sex differences in c-Fos expression and serum corticosterone concentration following LPS treatment. Neuroscience, 305, 293–301. https://doi.org/10.1016/j.neuroscience.2015.06.035
- Hadamitzky, M., Herring, A., Kirchhof, J., Bendix, I., Haight, M. J., Keyvani, K., Lückemann, L., Unteroberdörster, M., & Schedlowski, M. (2018). Repeated Systemic Treatment with Rapamycin Affects Behavior and Amygdala Protein Expression in Rats. The International Journal of Neuropsychopharmacology, 21(6), 592–602. https://doi.org/10.1093/ijnp/pyy017
- Harrell, C. S., Burgado, J., Kelly, S. D., Johnson, Z. P., & Neigh, G. N. (2015). High-fructose diet during periadolescent development increases depressive-like behavior and remodels the hypothalamic transcriptome in male rats. Psychoneuroendocrinology, 62, 252–264. https://doi.org/10.1016/j.psyneuen.2015.08.025
- Harrell, C. S., Hardy, E., Boss-Williams, K., Weiss, J. M., & Neigh, G. N. (2013). Sex and lineage interact to predict behavioral effects of chronic adolescent stress in rats. Behavioural Brain Research, 248, 57–61. https://doi.org/10.1016/j.bbr.2013.04.003
- Herring, A., Münster, Y., Akkaya, T., Moghaddam, S., Deinsberger, K., Meyer, J., Zahel, J., Sanchez-Mendoza, E., Wang, Y., Hermann, D. M., Arzberger, T., Teuber-Hanselmann, S., & Keyvani, K. (2016). Kallikrein-8 inhibition attenuates Alzheimer’s disease pathology in mice. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 12(12), 1273–1287. https://doi.org/10.1016/j.jalz.2016.05.006
- Hyer, M. M., Shaw, G. A., Goswamee, P., Dyer, S. K., Burns, C. M., Soriano, E., Sanchez, C. S., Rowson, S. A., McQuiston, A. R., & Neigh, G. N. (2021). Chronic adolescent stress causes sustained impairment of cognitive flexibility and hippocampal synaptic strength in female rats. Neurobiology of Stress, 14, 100303. https://doi.org/10.1016/j.ynstr.2021.100303
- Hyer, M. M., Wegener, A. J., Targett, I., Dyer, S. K., & Neigh, G. N. (2023). Chronic stress beginning in adolescence decreases spatial memory following an acute inflammatory challenge in adulthood. Behavioural Brain Research, 442, 114323. https://doi.org/10.1016/j.bbr.2023.114323
- Inoue, K., Sakuma, E., Morimoto, H., Asai, H., Koide, Y., Leng, T., Wada, I., Xiong, Z. G., & Ueki, T. (2016). Serum- and glucocorticoid-inducible kinases in microglia. Biochemical and Biophysical Research Communications, 478(1), 53–59. https://doi.org/10.1016/j.bbrc.2016.07.094
- Jinno, S., Fleischer, F., Eckel, S., Schmidt, V., & Kosaka, T. (2007). Spatial arrangement of microglia in the mouse hippocampus: a stereological study in comparison with astrocytes. Glia, 55(13), 1334–1347. https://doi.org/10.1002/glia.20552
- Kjonigsen, L. J., Leergaard, T. B., Witter, M. P., & Bjaalie, J. G. (2011). Digital atlas of anatomical subdivisions and boundaries of the rat hippocampal region. Frontiers in Neuroinformatics, 5, 2. https://doi.org/10.3389/fninf.2011.00002
- Lebow, M. A., Schroeder, M., Tsoory, M., Holzman-Karniel, D., Mehta, D., Ben-Dor, S., Gil, S., Bradley, B., Smith, A. K., Jovanovic, T., Ressler, K. J., Binder, E. B., & Chen, A. (2019). Glucocorticoid-induced leucine zipper "quantifies" stressors and increases male susceptibility to PTSD. Translational Psychiatry, 9(1), 178. https://doi.org/10.1038/s41398-019-0509-3
- Li, H., Su, P., Lai, T. K., Jiang, A., Liu, J., Zhai, D., Campbell, C. T., Lee, F. H., Yong, W., Pasricha, S., Li, S., Wong, A. H., Ressler, K. J., & Liu, F. (2020). The glucocorticoid receptor-FKBP51 complex contributes to fear conditioning and posttraumatic stress disorder. The Journal of Clinical Investigation, 130(2), 877–889. https://doi.org/10.1172/JCI130363
- Lin, C. C., Cheng, P. Y., Hsiao, M., & Liu, Y. P. (2022). Effects of RU486 in treatment of traumatic stress-induced glucocorticoid dysregulation and fear-related abnormalities: Early versus late intervention. International Journal of Molecular Sciences, 23(10), 1–17.
- Lin, Y., Westenbroek, C., Bakker, P., Termeer, J., Liu, A., Li, X., & Ter Horst, G. J. (2008). Effects of long-term stress and recovery on the prefrontal cortex and dentate gyrus in male and female rats. Cerebral Cortex, 18(12), 2762–2774. https://doi.org/10.1093/cercor/bhn035
- Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.), 25(4), 402–408. https://doi.org/10.1006/meth.2001.1262
- Lui, P., Padow, V. A., Franco, D., Hall, B. S., Park, B., Klein, Z. A., & Romeo, R. D. (2012). Divergent stress-induced neuroendocrine and behavioral responses prior to puberty. Physiology & Behavior, 107(1), 104–111. https://doi.org/10.1016/j.physbeh.2012.06.011
- Markham, J. A., Mullins, S. E., & Koenig, J. I. (2013). Periadolescent maturation of the prefrontal cortex is sex-specific and is disrupted by prenatal stress. The Journal of Comparative Neurology, 521(8), 1828–1843. https://doi.org/10.1002/cne.23262
- Mifsud, K. R., & Reul, J. M. (2016). Acute stress enhances heterodimerization and binding of corticosteroid receptors at glucocorticoid target genes in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 113(40), 11336–11341. https://doi.org/10.1073/pnas.1605246113
- Ostrander, M. M., Ulrich-Lai, Y. M., Choi, D. C., Richtand, N. M., & Herman, J. P. (2006). Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology, 147(4), 2008–2017. https://doi.org/10.1210/en.2005-1041
- Paxinos, G., & Watson, C. (1998). The Rat Brain in Stereotaxic Coordinates. Academic Press.
- Pratt, L. A., & Brody, D. J. (2014). Depression in the U.S. household population, 2009–2012. NCHS Data Brief., no 172. Hyattsville, MD, National Center for Health Statistics.
- Rich, E. L., & Romero, L. M. (2005). Exposure to chronic stress downregulates corticosterone responses to acute stressors. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 288(6), R1628–1636. https://doi.org/10.1152/ajpregu.00484.2004
- Romeo, R. D., Patel, R., Pham, L., & So, V. M. (2016). Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neuroscience and Biobehavioral Reviews, 70, 206–216. https://doi.org/10.1016/j.neubiorev.2016.05.020
- Rowson, S. A., Bekhbat, M., Kelly, S. D., Binder, E. B., Hyer, M. M., Shaw, G., Bent, M. A., Hodes, G., Tharp, G., Weinshenker, D., Qin, Z., & Neigh, G. N. (2019). Chronic adolescent stress sex-specifically alters the hippocampal transcriptome in adulthood. Neuropsychopharmacology: official Publication of the American College of Neuropsychopharmacology, 44(7), 1207–1215. https://doi.org/10.1038/s41386-019-0321-z
- Rowson, S. A., Foster, S. L., Weinshenker, D., & Neigh, G. N. (2018). Locomotor sensitization to cocaine in adolescent and adult female Wistar rats. Behavioural Brain Research, 349, 158–162. https://doi.org/10.1016/j.bbr.2018.04.035
- Rowson, S. A., Harrell, C. S., Bekhbat, M., Gangavelli, A., Wu, M. J., Kelly, S. D., Reddy, R., & Neigh, G. N. (2016). Neuroinflammation and Behavior in HIV-1 Transgenic Rats Exposed to Chronic Adolescent Stress. Frontiers in Psychiatry, 7, 102. https://doi.org/10.3389/fpsyt.2016.00102
- Saaltink, D. J., & Vreugdenhil, E. (2014). Stress, glucocorticoid receptors, and adult neurogenesis: a balance between excitation and inhibition? Cellular and Molecular Life Sciences: CMLS, 71(13), 2499–2515. https://doi.org/10.1007/s00018-014-1568-5
- Scharf, S. H., Liebl, C., Binder, E. B., Schmidt, M. V., & Müller, M. B. (2011). Expression and regulation of the Fkbp5 gene in the adult mouse brain. PloS One, 6(2), e16883. https://doi.org/10.1371/journal.pone.0016883
- Shi, X., Huang, Z., Zhou, G., & Li, C. (2021). Dietary Protein From Different Sources Exerted a Great Impact on Lipid Metabolism and Mitochondrial Oxidative Phosphorylation in Rat Liver. Frontiers in Nutrition, 8, 719144. https://doi.org/10.3389/fnut.2021.719144
- Spear, L. P. (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews, 24(4), 417–463. https://doi.org/10.1016/s0149-7634(00)00014-2
- Sugimoto, N., Ishibashi, H., Ueda, Y., Nakamura, H., Yachie, A., & Ohno-Shosaku, T. (2019). Corticosterone inhibits the expression of cannabinoid receptor-1 and cannabinoid receptor agonist-induced decrease in cell viability in glioblastoma cells. Oncology Letters, 18(2), 1557–1563. https://doi.org/10.3892/ol.2019.10456
- Tatro, E. T., Everall, I. P., Kaul, M., & Achim, C. L. (2009). Modulation of glucocorticoid receptor nuclear translocation in neurons by immunophilins FKBP51 and FKBP52: implications for major depressive disorder. Brain Research, 1286, 1–12. https://doi.org/10.1016/j.brainres.2009.06.036
- Tertil, M., Skupio, U., Barut, J., Dubovyk, V., Wawrzczak-Bargiela, A., Soltys, Z., Golda, S., Kudla, L., Wiktorowska, L., Szklarczyk, K., Korostynski, M., Przewlocki, R., & Slezak, M. (2018). Glucocorticoid receptor signaling in astrocytes is required for aversive memory formation. Translational Psychiatry, 8(1), 255. https://doi.org/10.1038/s41398-018-0300-x
- Wang, Z., Zheng, G., Li, G., Wang, M., Ma, Z., Li, H., Wang, X. Y., & Yi, H. (2020). Methylprednisolone alleviates multiple sclerosis by expanding myeloid-derived suppressor cells via glucocorticoid receptor β and S100A8/9 up-regulation. Journal of Cellular and Molecular Medicine, 24(23), 13703–13714. https://doi.org/10.1111/jcmm.15928
- Wei, K., Xu, Y., Zhao, Z., Wu, X., Du, Y., Sun, J., Yi, T., Dong, J., & Liu, B. (2016). Icariin alters the expression of glucocorticoid receptor, FKBP5 and SGK1 in rat brains following exposure to chronic mild stress. International Journal of Molecular Medicine, 38(1), 337–344. https://doi.org/10.3892/ijmm.2016.2591
- Wochnik, G. M., Rüegg, J., Abel, G. A., Schmidt, U., Holsboer, F., & Rein, T. (2005). FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. The Journal of Biological Chemistry, 280(6), 4609–4616. https://doi.org/10.1074/jbc.M407498200
- Wohleb, E. S., Hanke, M. L., Corona, A. W., Powell, N. D., Stiner, L. M., Bailey, M. T., Nelson, R. J., Godbout, J. P., & Sheridan, J. F. (2011). beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(17), 6277–6288. https://doi.org/10.1523/JNEUROSCI.0450-11.2011
- Wu, C., Yosef, N., Thalhamer, T., Zhu, C., Xiao, S., Kishi, Y., Regev, A., & Kuchroo, V. K. (2013). Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature, 496(7446), 513–517. https://doi.org/10.1038/nature11984

