Abstract
Stress is an established risk factor for negative health outcomes. Salivary cortisol and testosterone concentrations increase in response to acute psychosocial stress. It’s crucial to reduce stress for health and well-being through evidence-based interventions. Body-mind interventions such as meditation and Tai Chi have shown reduced cortisol levels but mixed results in testosterone concentration after stress. To address this research gap, we conducted a pilot randomized controlled trial to examine the modulating effects of a short-term (seven 20-minute sessions) mindfulness meditation on testosterone and cortisol in response to acute stress. Using one form of mindfulness meditation – Integrative Body-Mind Training (IBMT) and an active control–relaxation training (RT), we assessed salivary cortisol and testosterone concentrations at three stages of stress intervention – rest, stress, and an additional 20-min IBMT or RT practice. We found increased cortisol and testosterone concentrations after acute stress in both groups, but testosterone rise was not associated with cortisol rise. Moreover, an additional practice immediately after stress produced higher testosterone concentrations in the IBMT group than the RT group, whereas cortisol concentration increased in the RT group than in the IBMT group at the same time point. These findings indicate that brief mindfulness intervention modulates a dual-hormone profile of testosterone and cortisol in response to acute stress presumably via the co-regulation of hypothalamus-pituitary-adrenal and hypothalamus-pituitary-testicular axes.
1. Introduction
Research has shown that minor yet frequent daily stressors are often better predictors of important health outcomes than chronic stress such as major life events (Tang, Hölzel, & Posner, Citation2015; Tang et al., Citation2020). It is imperative to understand how the hypothalamus-pituitary-adrenal (HPA) axis responds to acute stress that affects health and well-being. Stress-induced activation of the HPA axis leads to the release of cortisol. Although acute HPA axis responses to stress are adaptive for engaging with novel and uncertain situations, exaggerated or chronic secretion of cortisol can suppress immune function and have negative effects on health and well-being (Almeida et al., Citation2020). Appropriate reactivity to acute stress and timely cessation of the stress response is critical for an organism’s survival (Glaser, Citation2005; Smyth et al., Citation2018).
Many stress management studies have chosen salivary cortisol as a reliable physiological index to evaluate the efficiency of an intervention (Fan et al., Citation2009; Gaab et al., Citation2003; Hammerfald et al., Citation2006; MacLean et al., Citation1997; Oyola & Handa, Citation2017). Individuals who received cognitive-behavioral stress management training showed an attenuated cortisol response to acute stress relative to those in the waitlist control group without any stress management training, who reached their salivary cortisol maximum about 20 minutes after acute stress (Oyola & Handa, Citation2017). Likewise, our previous randomized controlled trials (RCTs) have shown that short-term Integrative Body-Mind Training (IBMT)—a form of open-monitoring mindfulness meditation (see details in 2.2), improves self-regulation ability and reduces stress compared to an active control group given the same amount of relaxation training (RT) (Fan, Tang, & Posner, Citation2014; Pawlow & Jones, Citation2005; Scholey et al., Citation2009; Tang, Citation2017; Tang et al., Citation2007; Citation2009). For example, five 20-minute sessions of IBMT had a significantly lowered cortisol response to mental stress after training than did the RT. Moreover, an additional 20 minutes of practice immediately after acute stress reduced the cortisol concentration in the IBMT group, relative to the RT group. However, 5 sessions of IBMT did not change the basal cortisol levels. Thus, another study using the same RCT design further explored whether increasing amounts of IBMT could decrease basal cortisol. Our results showed that the basal cortisol levels decreased significantly in the IBMT but not in the RT after 2 and 4 weeks of training (5-10 hours in total). An additional IBMT practice immediately after acute stress produced significantly lower cortisol release for the IBMT group in comparison with the RT group at weeks 2 and 4. These results indicate that IBMT produces a change in the basal endocrine system and larger acute effects as the dose of training increases (Pawlow & Jones, Citation2005; Scholey et al., Citation2009; Tang et al., Citation2007).
Gonadal hormones play a key role in the regulation of HPA axis (Glaser, Citation2005; Smyth et al., Citation2018). Testosterone, the end-product of the hypothalamus-pituitary–testicular (HPT) axis, is implicated in male reproductive development and behavior. Though they often exert different effects, the HPT and HPA axes do not operate independently and instead communicate with and co-regulate one another. For instance, testosterone modulates HPA axis men’s reactivity to psychosocial stress (Chichinadze & Chichinadze, Citation2008; Knight et al., Citation2017; Tang, Tang, & Gross, Citation2019) and men with lower basal cortisol concentrations show larger increases in the testosterone response to social stress (Tang, Tang, Posner, & Gross, Citation2022; Viau, Citation2002).
Prior work showed that testosterone levels may either decrease or increase under acute stress (Barel et al., Citation2018; Bedgood, Boggiano, & Turan, Citation2014), a possible reason for this inconsistency may be the social nature of the stressful event (Chichinadze & Chichinadze, Citation2008; Tang et al., Citation2022). It has been suggested that the increase in testosterone levels in males has a preparatory role in stress challenges by inducing competitive and dominant behavior (Lennartsson et al., Citation2012). The increase in testosterone may be part of an adaptive strategy when facing social challenges (Tang et al., Citation2022; Zueger, Annen, & Ehlert, Citation2023). Even though testosterone levels may increase in the initial phase of acute psychosocial stress, the HPT axis may be inhibited during prolonged periods of stress (Bedgood et al., Citation2014). In contrast to the tendency of chronic stress to elevate cortisol levels, chronic stress decreases testosterone levels in males. Lower levels of testosterone in men have been associated with depression and lower life satisfaction (Kutlikova et al., Citation2020).
Body-mind techniques include various practices such as mindfulness meditation, Tai Chi, Yoga, Kung-Fu training, and relaxation training (Pawlow & Jones, Citation2005; Scholey et al., Citation2009). Although these techniques differ, they share several key components such as body relaxation, bodily awareness, breath adjustment, mental imagery, and mindfulness practice, which can help facilitate practitioners to get into meditative states easily (Fan et al., Citation2014; Pawlow & Jones, Citation2005; Scholey et al., Citation2009; Tang, Citation2017; Tang et al., Citation2007; Citation2009). So far, only a few studies have examined the effects of these techniques on both cortisol and testosterone levels. One study reported that after 4 months of Transcendental Meditation practice in young men, basal cortisol concentration was decreased, but basal testosterone concentration was unchanged (Gaab et al., Citation2003). Similarly, compared to the sedentary control, long-term Tai Chi (one form of movement meditation) showed lower basal cortisol concentration but did not affect basal testosterone concentration among middle-aged men (Booth, Shelley, Mazur, Tharp, & Kittok, Citation1989). However, Tai Chi qigong, and/or self-defense Kung-Fu training in aging men were neither associated with testosterone nor cortisol compared to the age-matched controls (Kutlikova et al., Citation2020). Therefore, the pattern of testosterone response to body-mind practices remains unclear.
To address this issue, the current study aims to examine the modulating effects of short-term IBMT on men’s cortisol and testosterone concentrations. Additionally, it seeks to explore the relationship between changes in cortisol and testosterone concentrations in response to acute psychosocial stress using a reliable stress test among young adults. We hypothesize that short-term IBMT will induce a dual-hormone profile of increased testosterone combined with reduced cortisol than RT in a rigorous RCT design.
2. Methods
2.1. Study design and subjects
We employed a double-blind, randomized controlled design (Davidson, Citation2010; Terburg, Morgan, & van Honk, Citation2009). Subjects were recruited through a notice on the bulletin board of Dalian University of Technology. All responders (Chinese college students) received an in-person screening interview and assessment at the University Psychological Counseling Center and were excluded if having (1) prior or current training experiences such as meditation, relaxation, yoga, or cognitive training; (2) preexisting health conditions or a past or present history of psychiatric, immune, metabolic, or endocrine disease, or use of medication with known immune or endocrine effects.
Based on our prior studies on cortisol concentration changes following IBMT (Glaser, Citation2005; Pawlow & Jones, Citation2005; Tang et al., Citation2007), we conducted a power analysis with a medium to large effect size (f = 0.32) for repeated measures of ANOVA, yielding a total sample size of 18 for two groups to achieve at least 80% power. To account for potential attrition/dropout and potential underpower because of individual fluctuations in cortisol and testosterone concentrations, we doubled the sample size and ended up with a total of 32 people who were eligible to participate and complete the study. Thirty-two healthy male college students (mean age ± SD = 21.33 ± 0.63) without any previous training experiences were randomly assigned to an experimental group (16 subjects, IBMT practice) and 16 control subjects who received an equal period of RT practice. There were no significant differences in gender, age, education, and ethnicity between the two groups. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Dalian University of Technology (DUT200304). Informed consent was obtained from all subjects involved in the study.
2.2. Training Methods
Mindfulness meditation involves paying attention to the present moment without judgment and usually has two forms: focus attention and open monitoring. In literature, IBMT is termed as a form of open-monitoring mindfulness meditation (Terburg et al., Citation2009). As described in our prior work (Fan et al., Citation2014; Pawlow & Jones, Citation2005; Scholey et al., Citation2009; Tang, Citation2017; Tang et al., Citation2007; Citation2009; Tang & Tang, Citation2023; Terburg et al., Citation2009), different from some practices with the effort to control or manipulate the mind, IBMT stresses no effort to control or manipulate thoughts and feelings but emphasizes an awareness of the natural state of body and mind, and accepts whatever arises in one’s awareness at each moment. To achieve this mindfulness state, IBMT strengthens body and mind interaction and often uses stretching postures to first engage in the body (bodifulness) and then facilitate mental states with full awareness (mindfulness), also see review (Fan et al., Citation2014). Guided by an experienced trainer, subjects start the gentle and gradual adjustment and exercise of body postures with full awareness, to achieve bodily presence, balance, and integration. When the body is naturally relaxed, the mind is easily calm but alert effortlessly. Our RCTs showed that this effortless body-mind practice is supported by the interaction and optimization of the central and autonomic nervous systems (Fan et al., Citation2014; Pawlow & Jones, Citation2005; Scholey et al., Citation2009; Tang, Citation2017; Tang et al., Citation2007; Citation2009; Terburg et al., Citation2009), which can promote mindfulness awareness effectively.
Relaxation Training (RT) is a form of progressive muscle relaxation technique very popular in the West. RT involves progressively relaxing different muscle groups over the face, head, shoulders, arms, legs, chest, back, and abdomen, guided by an experienced trainer. Stretching and adjusting body postures are encouraged to facilitate relaxation. With eyes closed and in a sequential pattern, one is instructed to concentrate on the sensation of relaxation, such as the feelings of warmth and heaviness. This progressive training helps the participant achieve physical and mental relaxation and calmness. RT has been used as an active control in our and other RCTs (Lazar et al., Citation2000; Pawlow & Jones, Citation2005; Scholey et al., Citation2009; Tang et al., Citation2007; Taren et al., Citation2015). IBMT and RT share several components of body-mind techniques, but the main difference between IBMT and RT is with or without a mindfulness component, suggesting RT is an adequate control for IBMT.
2.3. Experimental procedure
Before training, the qualified IBMT and RT trainers separately gathered the subjects to introduce the structure of the program and the training method, answer questions, and also set up the exact time, training room, and disciplines for the group practice. Experimental (IBMT) and control (RT) subjects completed a group training (4-5 subjects) on campus for 7 consecutive days with a 20-minute session per day (without any extra sessions such as home practice). Following the IBMT or RT instructions guided by the trainers, the subjects sat in a chair closed their eyes, and practiced IBMT or RT respectively in different rooms. After each training session, every subject filled out an in-house self-report questionnaire and evaluated the practice. The trainers gave brief and immediate responses to questions the subjects may have (Fan et al., Citation2014; Pawlow & Jones, Citation2005; Scholey et al., Citation2009; Tang, Citation2017; Tang et al., Citation2007).
All subjects performed the stress intervention test after 7 sessions of training. The stress intervention test included three stages—rest, stress, and additional 20-minute practice. After a 5-minute rest, subjects performed a 3-minute mental arithmetic task (see 2.4) to induce acute stress. Then, the experimental subjects practiced IBMT for 20 minutes, whereas the controls practiced a 20-minute RT.
2.4. Mental arithmetic task
Mental arithmetic was used as an acute and reliable laboratory stressor shown in research (Glaser, Citation2005; Lai, Liu, Lin, Tsai, & Chien, Citation2017; Pawlow & Jones, Citation2005; Tang et al., Citation2007; Walther, Lacker, & Ehlert, Citation2018). Subjects read the introduction writing on a computer screen and performed serial subtraction of 47 from a four-digit number. During the 3-minute mental arithmetic task, subjects were prompted to respond verbally as fast and accurately as possible. The experimenter checked the correct answers printed on a piece of paper. If the subject did not give the correct answer in 5 seconds, the experimenter would press the mouse button, and then the computer would produce a harsh sound to remind the subject, who was required to restart the task and do it again.
2.5. Sampling methods and biochemical analysis
Saliva samples were collected repeatedly before and immediately after the stress, and immediately after the 20-minute practice. To control for variations of cortisol and testosterone levels over the circadian rhythm, saliva sample collection was performed from 14.00 to 18.00 pm. Subjects were required to not smoke or drink alcohol on the day of the test or eat a large meal or engage in physical activity for four hours prior to the test. They were asked to rinse their mouths before collecting samples. Subjects collected about 1 ml saliva samples using the disposable syringe (BD EmeraldTM). The experimenter then put the saliva into the test tubes, which were labeled and placed into a refrigerator under −20 °C and then thawed 24 h later for analysis. The concentrations of cortisol and testosterone were analyzed by Radioimmunoassay at the third-party clinical service center at the Affiliated Zhongshan Hospital of Dalian University following structured clinical protocols using GammaCoatTM RIA Kit. Intra- and inter-assay coefficients of variation were below 10%. To reduce error variance caused by imprecision of the intra-assay, all samples of each subject were analyzed in the same run and in duplicates.
2.6. Statistical analyses
All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) 17.0 for Windows. Data were analyzed using the ANOVA with group and time as the between-subjects and within-subjects factors, respectively. If a significant effect was detected, post-hoc t-tests were conducted to identify significant differences in the means. Pearson’s correlation analysis was conducted to assess the potential relationship between different measures. There were no missing values and outliers observed in this pilot study.
3. Results
3.1. Salivary cortisol
Salivary cortisol concentrations at the three stages of the stress intervention test are shown in . A 2 (group: IBMT, RT) × 3 (time point: rest, immediately after stress, additional practice) ANOVA revealed a significant main effect for time (F(1,30)=25.130, p<.001, ηp2=.456) and a significant interaction for group × time (F(2,60)=4.669, p<.05, ηp2=.135). Relative to baseline, cortisol concentrations increased after exposure to the stressor in both groups. Moreover, cortisol concentration increased significantly in the RT group after the additional 20-minute practice (t(15) = 3.034, p<.01, Cohen’s d=.758), and was also higher than that of the IBMT group at the same time point (t(30)=-2.185, p<.05, Cohen’s d=-.335).
Figure 1. Salivary cortisol (nmol/L) concentrations at the three stages of the stress intervention test.
Note. # p<.05, integrative body-mind training (IBMT) group versus relaxation training (RT) group.
▲p<.05, ▲▲p<.01, ▲▲▲p<.001, immediately after stress versus rest.
* p<.05, ** p<.01, immediately after an additional practice versus immediately after stress.
Error bars depict Mean ± SE.

3.2. Salivary testosterone
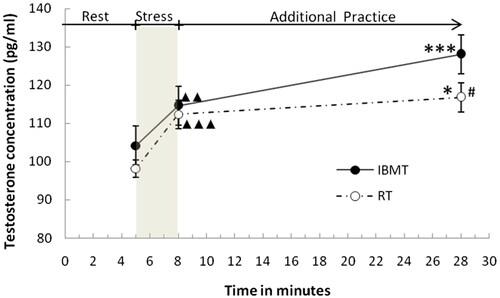
Salivary testosterone concentrations at the three stages of the stress intervention test are shown in . The same ANOVA used for cortisol was applied to testosterone and revealed a significant main effect for time (F(1,30)=76.057, p<.001, ηp2=.717) and a significant interaction for group × time (F(2,60)= 3.454, p<.05, ηp2=.103). Like cortisol, testosterone concentrations immediately after stress were higher relative to baseline in both groups. Testosterone concentrations also increased after the additional 20-minute practice in both the IBMT group (t(15) =5.386, p<.001, Cohen’s d = 1.347) and RT group (t(15) =2.684, p<.05, Cohen’s d=.671). Most importantly, the extent of this increase was greater in the IBMT group relative to the RT group (t(30) =3.019, p<.05, Cohen’s d = 1.067).
Figure 2. Salivary testosterone (pg/ml) concentrations at the three stages of the stress intervention test.
Note. # p<.05, integrative body-mind training (IBMT) group versus relaxation training (RT) group.
▲p<.05, ▲▲p<.01, ▲▲▲p<.001, immediately after stress versus rest.
* p<.05, ** p<.01, *** p<.001, immediately after an additional practice versus immediately after stress.
Error bars depict Mean ± SE.
3.3. Relationship between the changes in cortisol and testosterone concentrations responses to stress
Cortisol and testosterone concentrations increased in response to acute stress in both groups, but the magnitude of the increase in the concentrations of cortisol was not correlated with the testosterone rise (r(32)=.092, p=.616) based on the difference between rest and immediately after stress (stage 2 – stage 1).
4. Discussion
This study aimed to examine the intervention effects of short-term mindfulness meditation on cortisol and testosterone concentrations and their relationships in response to acute psychosocial stress. Consistent with our hypotheses, we found increased cortisol and testosterone levels after acute stress in both groups, but cortisol rise was not associated with testosterone rise. We also found an additional 20-minute practice immediately after stress produced higher testosterone concentrations in the IBMT group than the RT group, whereas cortisol concentration increased in the RT group than in the IBMT group at the same time point. These findings indicate that IBMT outperforms RT in modulating these two hormones in response to acute stress.
Stress leads to the secretion of cortisol. Exaggerated release of cortisol can suppress aspects of immune function and have negative effects on health and wellbeing (Almeida et al., Citation2020). Salivary cortisol is a delayed peripheral response to acute stress (Ring et al., Citation2000). Salivary cortisol level reaches its maximum about 20 minutes after acute stress reaction (Fan et al., Citation2009; Oyola & Handa, Citation2017). Our previous studies examined the dynamic changes in salivary cortisol and salivary secretory immunoglobulin A (sIgA, an index of mucosal immunity) responses to acute stress, and found that the sIgA decrease was significantly correlated with the cortisol increase during the 20 minutes after stress (Glaser, Citation2005). Moreover, we demonstrated that an additional 20-minute IBMT practice immediately after acute stress rendered the participants lower salivary cortisol and higher sIgA in a group that received 5 sessions of IBMT training, in comparison to the same amount of RT (Pawlow & Jones, Citation2005; Tang et al., Citation2007). These studies indicate that IBMT can serve as an effective intervention to modulate endocrine and immune responses to stress. In the present study, patterns of variations in salivary cortisol concentrations across the three stages of the stress intervention test were consistent with our previous studies (Pawlow & Jones, Citation2005; Scholey et al., Citation2009; Tang et al., Citation2007), further showing that a 20-minute session of IBMT practice after acute stress appeared to attenuate the release of cortisol.
Growing evidence suggests that the effects of mindfulness meditation derive from improved self-regulation of attention, emotion, and self-awareness (Terburg et al., Citation2009). For example, mindfulness meditation involves paying attention to the present moment without judgment and encourages subjects to become more aware of their thoughts, feelings, and bodily sensations. This heightened self-awareness helps identify stress triggers and patterns, allowing for proactive stress management through positive reappraisal, emotion regulation, or other strategies, thereby modulating cortisol and testosterone profiles effectively (Goldin et al., Citation2021; González-Palau & Medrano, Citation2022; Hanley et al., Citation2021; Tang, Tang, & Posner, Citation2016; Terburg et al., Citation2009). In addition, stress is often associated with negative emotions. Mindfulness practice helps subjects become aware of these emotions and detect subtle affective changes related to stressors. By observing emotions in a non-judgmental and accepting way, subjects can cultivate a more balanced and non-reactive response to stressors, promoting better emotion regulation and stress reduction (Fan et al., Citation2014; González-Palau & Medrano, Citation2022; Pawlow & Jones, Citation2005; Tang et al., Citation2016). Consistent with these findings, a series of RCT studies have shown that short-term IBMT improves attention control, emotion regulation, and self-awareness, as well as brain plasticity in self-regulation networks, suggesting IBMT reduces stress through strengthening self-control (Pawlow & Jones, Citation2005; Scholey et al., Citation2009; Tang et al., Citation2007; Tang, Citation2017; Fan et al., Citation2014; Tang et al., Citation2009; Terburg et al., Citation2009).
In this study, salivary testosterone concentrations significantly increased immediately after the mental arithmetic task in both groups relative to rest, but the magnitude of change in testosterone concentrations was not associated with the increase in cortisol. The mechanism behind the acute stress-induced increase in testosterone remains unclear. It has been suggested that social status and sympathetic reactivity contributed to acute stress-induced increases in testosterone (Barel et al., Citation2018). The acute psychosocial stress-induced increase of testosterone levels has been suggested to have a preparatory purpose in stress challenges by inducing competitive and dominant behavior (Lennartsson et al., Citation2012), which may be beneficial to overcome the stressor. Research showed that testosterone level temporarily increases in the initial phase of acute psychosocial stress, after 30 minutes of recovery the testosterone level was significantly reduced (Bedgood et al., Citation2014). The HPA axis and HPT axis are competitive systems and during prolonged periods of stress, the HPT axis and the production of sex steroids could be inhibited (Bedgood et al., Citation2014). In contrast to the tendency of chronic stress to elevate cortisol levels, chronic stress decreases testosterone levels in males. Lower levels of testosterone have been associated with depression and lower life satisfaction (Kutlikova et al., Citation2020).
People need to cope with high day-to-day stressful life events. Testosterone and cortisol work together to maintain an appropriate biological and psychological balance (Willemsen, Ring, McKeever, & Carroll, Citation2000). Testosterone and cortisol act in concert with and contribute to adaptive responses to stress (Mehta & Prasad, Citation2015); for example, the dual-hormone profile of increased testosterone and reduced cortisol is related to adaptive decision-making in stressful social settings (Wang et al., Citation2005). Dysregulation of the HPA and HPT axes can result in compromised responses to stressful life events (Smyth et al., Citation2018). Our studies showed that an additional 20-minute IBMT practice after stress could induce a larger release of salivary testosterone and attenuated release of salivary cortisol than RT practice. Therefore, IBMT could provide a convenient approach to modulating endocrine response to stress in time (Pawlow & Jones, Citation2005; Scholey et al., Citation2009; Tang et al., Citation2007). It should be noted that IBMT emphasizes body-mind integration and optimization toward achieving an optimal meditative state characterized by calm and restful alertness of the present moment, it may allow subjects to quickly recover from their previous stressful state and return to a pre-stress brain and body state. Our previous RCTs indicated that brief IBMT could induce several neurophysiological and psychological changes (Scholey et al., Citation2009; Terburg et al., Citation2009). For example, 5-10 sessions of training produced functional and structural changes in brain self-regulation networks including anterior cingulate and adjacent prefrontal cortices and striatum, reduced cortisol and negative moods, enhanced testosterone, immune function, and positive moods, and improved quality of life (Fan et al., Citation2014; Mehta, Mor, Yap, & Prasad, Citation2015; Pawlow & Jones, Citation2005; Scholey et al., Citation2009; Tang, Citation2017; Tang et al., Citation2007; Citation2009; Terburg et al., Citation2009). Moreover, these positive brain, physiological, and behavioral changes were accompanied by the interaction of both central and autonomic nervous systems (Fan et al., Citation2014; Scholey et al., Citation2009; Tang, Citation2017; Tang et al., Citation2007; Citation2009). It is thus possible that IBMT may contribute to improved homeostasis in response to stress via a specific neuroendocrine pathway - co-regulation of the HPT and HPA axes, subsequently influencing both testosterone and cortisol concentrations in response to stress.
Testosterone plays an important role in male physiology and behavior (Mazur & Booth, Citation1998; Wingfield, Hegner, Dufty, & Ball, Citation1990), and most previous research on testosterone in the context of stress has focused primarily on males (Dismukes, Johnson, Vitacco, Iturri, & Shirtcliff, Citation2015; Knight et al., Citation2017; Kutlikova et al., Citation2020). The present study was also conducted in males to build on the previous literature. However, growing evidence suggests that testosterone levels are also related to female stress responses and behavior (Casto & Edwards, Citation2016; Casto, Edwards, Akinola, Davis, & Mehta, Citation2020). Some research has shown similar fluctuations in testosterone levels in both men and women in response to acute stress (Knight & Mehta, Citation2017; Lennartsson et al. Citation2012), whereas other studies found sex differences (Schultheiss et al., Citation2005; Kivlighan, Granger, & Booth, Citation2005). These sex differences in the context of stress might be explained by key differences between the male and female endocrine systems. Whereas men’s testosterone is secreted primarily from the gonads, women’s testosterone levels are secreted primarily by the adrenal glands (Schultheiss et al., Citation2005). Given that fewer studies have examined testosterone levels, stress, and behavior in females compared to males (Casto & Prasad, Citation2017), it remains unclear whether the findings from the current study conducted in males will generalize to females. As such, this remains an important question to investigate in future research.
As mentioned before, previous studies have found basal cortisol concentrations decreased after months of transcendental meditation (Gaab et al., Citation2003), or years of Tai Chi (Booth et al., Citation1989), but basal testosterone concentrations were unchanged. Our previous RCTs have shown that basal cortisol levels decreased after just 2-4 weeks of IBMT, suggesting IBMT may be highly effective in regulating acute stress response (Pawlow & Jones, Citation2005; Scholey et al., Citation2009; Tang et al., Citation2007). Future research should examine the effect of longer IBMT on basal testosterone.
This study has some limitations that should be taken into account. First, the short-term training (seven 20-min sessions) did not allow us to examine the longitudinal effects of the training. Future studies should explore the effects of long-term training on the brain and endocrine function in a larger and more diverse sample (Mehta et al., Citation2015). Second, our samples only consisted of young males, which may limit the generalizability of the results. However, this could also be a strength, as dominance and competition in males occur in a more natural setting. Further, recent work indicates that testosterone levels can be measured more accurately in males compared to females due to relatively lower levels in females (Prasad, Lassetter, Welker, & Mehta, Citation2019). Nevertheless, newer techniques such as mass spectrometry hold promise for improving the validity of testosterone measurement, particularly in populations with low levels (e.g. women; Schultheiss, Dlugash, & Mehta, Citation2019; Prasad et al., Citation2019). Thus, future research should adopt these improved measurement techniques to explore whether mindfulness meditation would show similar or different hormone changes in males and females. Third, this study did not use a waitlist control (non-intervention control). The waitlist control has been intensively investigated in the meditation field (Davidson, Citation2010; MacCoon, MacLean, Davidson, Saron, & Lutz, Citation2014; MacCoon et al., Citation2012; Rosenkranz et al., Citation2013; Terburg et al., Citation2009). Early meditation research often used meditation intervention vs. waitlist control and demonstrated the positive training effects. For example, compared to the waitlist control, a widely used meditation program—mindfulness-based stress reduction (MBSR) showed positive effects on stress reduction, mood changes, behavioral symptoms, and pain relief (Terburg et al., Citation2009). However, later rigorous RCT studies using an active control failed to replicate these results. For example, a series of studies used MBSR vs. Health Enhancement Program (HEP) to validate the active control in meditation (Davidson, Citation2010; MacCoon et al., Citation2012; Citation2014; Rosenkranz et al., Citation2013). Both HEP and MBSR were structurally equivalent, having all the matching components both in class and at homework practices. However, results showed both HEP and MBSR were effective in reducing symptoms over time, and MBSR was not superior to HEP. Other longitudinal RCTs also showed no sustained attention differences between MBSR and HEP, MBSR and HEP had comparable post-training stress-evoked cortisol responses, as well as equivalent reductions in self-reported psychological distress and physical symptoms (Davidson, Citation2010; MacCoon et al., Citation2012; Citation2014; Rosenkranz et al., Citation2013). These results indicate that prior studies using waitlist control produced false positive results, and the meditation vs. waitlist design has major flaws because of multiple concerns such as ethical concerns (e.g. delay subjects’ benefits), difficulty in blinding when subjects are aware of the conditions, and high dropout rate due to dissatisfaction with being on the waitlist. In addition, the positive outcomes in the meditation group may derive from social confounders during the whole intervention period such as frequent contact and interaction between trainees and trainers in the meditation group compared to the limited interaction in waitlist control. Therefore, it is imperative to utilize active control conditions that permit a rigorous comparison rather than the waitlist that does not have any active training component (Davidson, Citation2010; Terburg et al., Citation2009). Consequently, for the past 10 years, more and more meditation studies have used longitudinal RCT design with active controls such as relaxation training, health education or enhancement programs, physical exercise, and cognitive tasks (Davidson, Citation2010; MacCoon et al., Citation2012; Citation2014; Rosenkranz et al., Citation2013; Terburg et al., Citation2009). RT has shown positive effects compared to waitlist control and has been used as an active and adequate control in our and other RCTs (Benson & Klipper, Citation2000; Lazar et al., Citation2000; Pawlow & Jones, Citation2005; Scholey et al., Citation2009; Tang et al., Citation2007; Taren et al., Citation2015) as the main difference between mindfulness meditation (e.g. IBMT) and RT is with or without a mindfulness component. Therefore, in contrast to the waitlist design, the rigorous and longitudinal RCT design in the current study could also be seen as a strength (IBMT vs. active control RT) in revealing the training effects and underlying mechanisms.
5. Conclusion
To our knowledge, this RCT demonstrates that even short-term mindfulness meditation can modulate testosterone response to stress and the relationship between the changes in cortisol and testosterone concentrations responses to stress. Our findings suggest IBMT may contribute to improved homeostasis in response to stress via co-regulation of the HPT and HPA axes. Consistent with the dual-hormone hypothesis, IBMT may provide a means to efficiently regulate both cortisol and testosterone to affect dominant and aggressive behavior and improve prosocial behavior (Wang, Zhang, Wu, Qin, & Liu, Citation2022; Wang, Zhang, et al., Citation2022).
Authors’ contributions
Conceptualization, Y.-Y.T.; methodology, Y.-Y.T.; formal analysis, Y.F., Y.C.; investigation, all authors.; resources, Y.-Y.T.; data curation, Y.F., Y.C.; writing—original draft preparation, Y.F.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
The authors declare no competing interests.
Additional information
Funding
Notes on contributors
Yaxin Fan
Yaxin Fan, PhD is a Professor and Associate Director of the Dalian Blood Center, China.Her research focuses on stress hormones and immune function.
Yifen Cui
Yifen Cui, MS is a research staff at the Central lab, Affiliated Zhongshan Hospital of Dalian University, China. Her research focuses on molecular mechanisms of stress response.
Rongxiang Tang
Rongxiang Tang, PhD is a postdoctoral scholar in the Department of Psychiatry at the University of California San Diego, USA. Her research broadly focuses on health neuroscience, including understanding the mechanisms of stress.
Amar Sarkar
Amar Sarkar is a PhD student in the Department of Evolutionary Biology at Harvard University. He is broadly interested in human health, development, and evolution.
Pranjal Mehta
Pranjal Mehta, PhD, is an Associate Professor of Experimental Psychology at University College London. He studies the neuroendocrine systems involved in social hierarchy, stress, and decision-making.
Yi-Yuan Tang
Yi-Yuan Tang, PhD, is a Professor and Director of the Health Neuroscience Collaboratory at Arizona State University, USA. He studies the neuroscience of whole person health and behavior change over the lifespan. He developed the integrative body-mind training (IBMT).
References
- Almeida, D. M., Charles, S. T., Mogle, J., Drewelies, J., Aldwin, C. M., Spiro, A., & Gerstorf, D. (2020). Charting adult development through (historically changing) daily stress processes. The American Psychologist, 75(4), 1–9. https://doi.org/10.1037/amp0000597
- Barel, E., Abu-Shkara, R., Colodner, R., Masalha, R., Mahagna, L., Zemel, O. C., & Cohen, A. (2018). Gonadal hormones modulate the HPA-axis and the SNS in response to psychosocial stress. Journal of Neuroscience Research, 96(8), 1388–1397. https://doi.org/10.1002/jnr.24259
- Bedgood, D., Boggiano, M. M., & Turan, B. (2014). Testosterone and social evaluative stress: The moderating role of basal cortisol. Psychoneuroendocrinology, 47, 107–115. https://doi.org/10.1016/j.psyneuen.2014.05.007
- Benson, H., & Klipper, M. Z. (2000). The relaxation response. William Morrow Paperbacks.
- Booth, A., Shelley, G., Mazur, A., Tharp, G., & Kittok, R. (1989). Testosterone, and winning and losing in human competition. Hormones and Behavior, 23(4), 556–571. https://doi.org/10.1016/0018-506x(89)90042-1
- Casto, K.V., & Prasad, S. (2017). Recommendations for the study of women in hormones and competition research. Hormones and Behavior, 92, 190–194.
- Casto, K. V., & Edwards, D. A. (2016). Before, during, and after: How phases of competition differentially affect testosterone, cortisol, and estradiol levels in women athletes. Adaptive Human Behavior and Physiology, 2(1), 11–25. https://doi.org/10.1007/s40750-015-0028-2
- Casto, K. V., Edwards, D. A., Akinola, M., Davis, C., & Mehta, P. H. (2020). Testosterone reactivity to competition and competitive endurance in men and women. Hormones and Behavior, 123, 104665. https://doi.org/10.1016/j.yhbeh.2019.104665
- Chichinadze, K., & Chichinadze, N. (2008). Stress-induced increase of testosterone: Contributions of social status and sympathetic reactivity. Physiology & Behavior, 94(4), 595–603. https://doi.org/10.1016/j.physbeh.2008.03.020
- Davidson, R. J. (2010). Empirical explorations of mindfulness: Conceptual and methodological conundrums. Emotion (Washington, D.C.), 10(1), 8–11. https://doi.org/10.1037/a0018480
- Dismukes, A. R., Johnson, M. M., Vitacco, M. J., Iturri, F., & Shirtcliff, E. A. (2015). Coupling of the HPA and HPG axes in the context of early life adversity in incarcerated male adolescents. Developmental Psychobiology, 57(6), 705–718. https://doi.org/10.1002/dev.21231
- Fan, Y. X., Tang, Y. Y., & Posner, M. I. (2014). Cortisol level modulated by integrative meditation in a dose-dependent fashion. Stress and Health: Journal of the International Society for the Investigation of Stress, 30(1), 65–70. https://doi.org/10.1002/smi.2497
- Fan, Y., Tang, Y., Lu, Q., Feng, S., Yu, Q., Sui, D., Zhao, Q., Ma, Y., & Li, S. (2009). Dynamic changes in salivary cortisol and secretory immunoglobulin A response to acute stress. Stress and Health, 25(2), 189–194. https://doi.org/10.1002/smi.1239
- Gaab, J., Blättler, N., Menzi, T., Pabst, B., Stoyer, S., & Ehlert, U. (2003). Randomized controlled evaluation of the effects of cogni-tive-behavioral stress management on cortisol responses to acute stress in healthy subjects. Psychoneuroendocrinology, 28(6), 767–779. https://doi.org/10.1016/s0306-4530(02)00069-0
- Glaser, R. (2005). Stress-associated immune dysregulation and its importance for human health: A personal history of psychoneuroimmunology. Brain, Behavior, and Immunity, 19(1), 3–11. https://doi.org/10.1016/j.bbi.2004.06.003
- Goldin, P. R., Thurston, M., Allende, S., Moodie, C., Dixon, M. L., Heimberg, R. G., & Gross, J. J. (2021). Evaluation of cognitive behavioral therapy versus mindfulness meditation related brain changes during reappraisal and acceptance for social anxiety disorder: A randomized clinical trial. JAMA Psychiatry, 78(10), 1134–1142. https://doi.org/10.1001/jamapsychiatry.2021.1862
- González-Palau, F., & Medrano, L. A. (2022). A Mini-Review of work stress and mindfulness: A neuropsychological point of view. Frontiers in Psychology, 13, 854204. https://doi.org/10.3389/fpsyg.2022.854204
- Hammerfald, K., Eberle, C., Grau, M., Kinsperger, A., Zimmermann, A., Ehlert, U., & Gaab, J. (2006). Persistent effects of cognitive-behavioral stress management on cortisol responses to acute stress in healthy subjects–a randomized controlled trial. Psychoneuroendocrinology, 31(3), 333–339. https://doi.org/10.1016/j.psyneuen.2005.08.007
- Hanley, A. W., de Vibe, M., Solhaug, I., Farb, N., Goldin, P. R., Gross, J. J., & Garland, E. L. (2021). Modeling the Mindfulness-to-Meaning theory’s mindful reappraisal hypothesis: Replication with longitudinal data from a randomized controlled study. Stress and Health, 37(4), 778–789. https://doi.org/10.1002/smi.3035
- Kivlighan, K. T., Granger, D. A., & Booth, A. (2005). Gender differences in testosterone and cortisol response to competition. Psychoneuroendocrinology, 30(1), 58–71. https://doi.org/10.1016/j.psyneuen.2004.05.009
- Knight, E. L., & Mehta, P. H. (2017). Hierarchy stability moderates the effect of status on stress and performance in humans. Proceedings of the National Academy of Sciences, 114(1), 78–83. https://doi.org/10.1073/pnas.1609811114
- Knight, E. L., Christian, C. B., Morales, P. J., Harbaugh, W. T., Mayr, U., & Mehta, P. H. (2017). Exogenous testosterone enhances cortisol and affective responses to social-evaluative stress in dominant men. Psychoneuroendocrinology, 85, 151–157. https://doi.org/10.1016/j.psyneuen.2017.08.014
- Kutlikova, H. H., Durdiaková, J. B., Wagner, B., Vlček, M., Eisenegger, C., Lamm, C., & Riečanský, I. (2020). The effects of testosterone on the physiological response to social and somatic stressors. Psychoneuroendocrinology, 117, 104693. https://doi.org/10.1016/j.psyneuen.2020.104693
- Lai, H. M., Liu, M. S. Y., Lin, T. J., Tsai, Y. L., & Chien, E. J. (2017). Higher DHEAS Levels Associated with Long-Term Practicing of Tai Chi. The Chinese Journal of Physiology, 60(2), 124–130. https://doi.org/10.4077/CJP.2017.BAF454
- Lazar, S. W., Bush, G., Gollub, R. L., Fricchione, G. L., Khalsa, G., & Benson, H. (2000). Functional brain mapping of the relaxation response and meditation. Neuroreport, 11(7), 1581–1585. https://doi.org/10.1097/00001756-200005150-00042
- Lennartsson, A. K., Kushnir, M. M., Bergquist, J., Billig, H., & Jonsdottir, I. H. (2012). Sex steroid levels temporarily increase in response to acute psychosocial stress in healthy men and women. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 84(3), 246–253. https://doi.org/10.1016/j.ijpsycho.2012.03.001
- Lennartsson, A. K., Kushnir, M. M., Bergquist, J., Billig, H., & Jonsdottir, I. H. (2012). Sex steroid levels temporarily increase in response to acute psychosocial stress in healthy men and women. International Journal of Psychophysiology, 84(3), 246–253. https://doi.org/10.1016/j.ijpsycho.2012.03.001
- MacCoon, D. G., Imel, Z. E., Rosenkranz, M. A., Sheftel, J. G., Weng, H. Y., Sullivan, J. C., Bonus, K. A., Stoney, C. M., Salomons, T. V., Davidson, R. J., & Lutz, A. (2012). The validation of an active control intervention for Mindfulness Based Stress Reduction (MBSR). Behaviour Research and Therapy, 50(1), 3–12. https://doi.org/10.1016/j.brat.2011.10.011
- MacCoon, D. G., MacLean, K. A., Davidson, R. J., Saron, C. D., & Lutz, A. (2014). No sustained attention differences in a longitudinal randomized trial comparing mindfulness-based stress reduction versus active control. PLoS ONE., 9(6), e97551. https://doi.org/10.1371/journal.pone.0097551
- MacLean, C. R., Walton, K. G., Wenneberg, S. R., Levitsky, D. K., Mandarino, J. P., Waziri, R., Hillis, S. L., & Schneider, R. H. (1997). Effects of the transcendental meditation program on adaptive mechanisms: Changes in hormone levels and responses to stress after 4 months of practice. Psychoneuroendocrinology, 22(4), 277–295. https://doi.org/10.1016/s0306-4530(97)00003-6
- Mazur, A., & Booth, A. (1998). Testosterone and dominance in men. The Behavioral and Brain Sciences, 21(3), 353–363. https://doi.org/10.1017/S0140525X98001228
- Mehta, P. H., & Prasad, S. (2015). The dual-hormone hypothesis: A brief review and future research agenda. Current Opinion in Behavioral Sciences, 3, 163–168. https://doi.org/10.1016/j.cobeha.2015.04.008
- Mehta, P. H., Mor, S., Yap, A. J., & Prasad, S. (2015). Dual-hormone changes are related to bargaining performance. Psychological Science, 26(6), 866–876. https://doi.org/10.1177/0956797615572905
- Oyola, M. G., & Handa, R. J. (2017). Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: Sex differences in regulation of stress responsivity. Stress (Amsterdam, Netherlands), 20(5), 476–494. https://doi.org/10.1080/10253890.2017.1369523
- Pawlow, L. A., & Jones, G. E. (2005). The impact of abbreviated progressive muscle relaxation on salivary cortisol and salivary immu-noglobulin A (sIgA). Applied Psychophysiology and Biofeedback, 30(4), 375–387. https://doi.org/10.1007/s10484-005-8423-2
- Prasad, S., Lassetter, B., Welker, K. M., & Mehta, P. H. (2019). Unstable correspondence between salivary testosterone measured with enzyme immunoassays and tandem mass spectrometry. Psychoneuroendocrinology, 109, 104373. https://doi.org/10.1016/j.psyneuen.2019.104373
- Ring, C., Harrison, L. K., Winzer, A., Carroll, D., Drayson, M., & Kendall, M. (2000). Secretory immunoglobulin A and cardiovascular reactions to mental arithmetic, cold pressor, and exercise: Effects of alpha-adrenergic blockade. Psychophysiology, 37(5), 634–643. https://doi.org/10.1111/1469-8986.3750634
- Rosenkranz, M. A., Davidson, R. J., Maccoon, D. G., Sheridan, J. F., Kalin, N. H., & Lutz, A. (2013). A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain, Behavior, and Immunity, 27(1), 174–184. https://doi.org/10.1016/j.bbi.2012.10.013
- Scholey, A., Haskell, C., Robertson, B., Kennedy, D., Milne, A., & Wetherell, M. (2009). Chewing gum alleviates negative mood and reduces cortisol during acute laboratory psychological stress. Physiology & Behavior, 97(3-4), 304–312. https://doi.org/10.1016/j.physbeh.2009.02.028
- Schultheiss, O. C., Wirth, M. M., Torges, C. M., Pang, J. S., Villacorta, M. A., & Welsh, K. M. (2005). Effects of implicit power motivation on men’s and women’s implicit learning and testosterone changes after social victory or defeat. Journal of Personality and Social Psychology, 88(1), 174–188. https://doi.org/10.1037/0022-3514.88.1.174
- Schultheiss, O. C., Dlugash, G., & Mehta, P. H. (2019). Hormone measurement in social neuroendocrinology: A comparison of immunoassay and mass spectrometry methods. In Routledge international handbook of social neuroendocrinology (pp. 26–40). Routledge.
- Smyth, J. M., Sliwinski, M. J., Zawadzki, M. J., Scott, S. B., Conroy, D. E., Lanza, S. T., Marcusson-Clavertz, D., Kim, J., Stawski, R. S., Stoney, C. M., Buxton, O. M., Sciamanna, C. N., Green, P. M., & Almeida, D. M. (2018). Everyday stress response targets in the science of behavior change. Behaviour Research and Therapy, 101, 20–29. https://doi.org/10.1016/j.brat.2017.09.009
- Tang, Y. Y. (2017). The neuroscience of mindfulness meditation: How the body and mind work together to change our behavior? Springer Nature.
- Tang, Y. Y., & Tang, R. (2023). Fundamentals of health neuroscience. Academic Press: Elsevier.
- Tang, Y. Y., Hölzel, B. K., & Posner, M. I. (2015). The neuroscience of mindfulness meditation. Nature Reviews. Neuroscience, 16(4), 213–225. https://doi.org/10.1038/nrn3916
- Tang, Y. Y., Tang, R., & Gross, J. J. (2019). Promoting psychological well-being through an evidence-based mindfulness training program. Frontiers in Human Neuroscience, 13, 237. https://doi.org/10.3389/fnhum.2019.00237
- Tang, Y. Y., Tang, R., & Posner, M. I. (2016). Mindfulness meditation improves emotion regulation and reduces drug abuse. Drug and Alcohol Dependence. 163, S13–S18. https://doi.org/10.1016/j.drugalcdep.2015.11.041
- Tang, Y. Y., Tang, R., Posner, M. I., & Gross, J. J. (2022). Effortless training of attention and self-control: Mechanisms and applications. Trends in Cognitive Sciences, 26(7), 567–577. https://doi.org/10.1016/j.tics.2022.04.006
- Tang, Y.-Y., Fan, Y., Lu, Q., Tan, L.-H., Tang, R., Kaplan, R. M., Pinho, M. C., Thomas, B. P., Chen, K., Friston, K. J., & Reiman, E. M. (2020). Long-term physical exercise and mindfulness practice in an aging population. Frontiers in Psychology, 11, 358. https://doi.org/10.3389/fpsyg.2020.00358
- Tang, Y.-Y., Ma, Y., Fan, Y., Feng, H., Wang, J., Feng, S., Lu, Q., Hu, B., Lin, Y., Li, J., Zhang, Y., Wang, Y., Zhou, L., & Fan, M. (2009). Central and autonomic nervous system interaction is altered by short-term meditation. Proceedings of the National Academy of Sciences of the United States of America, 106(22), 8865–8870. https://doi.org/10.1073/pnas.0904031106
- Tang, Y.-Y., Ma, Y., Wang, J., Fan, Y., Feng, S., Lu, Q., Yu, Q., Sui, D., Rothbart, M. K., Fan, M., & Posner, M. I. (2007). Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences of the United States of America, 104(43), 17152–17156. https://doi.org/10.1073/pnas.0707678104
- Taren, A. A., Gianaros, P. J., Greco, C. M., Lindsay, E. K., Fairgrieve, A., Brown, K. W., Rosen, R. K., Ferris, J. L., Julson, E., Marsland, A. L., Bursley, J. K., Ramsburg, J., & Creswell, J. D. (2015). Mindfulness meditation training alters stress-related amygdala resting state functional connectivity: A randomized controlled trial. Social Cognitive and Affective Neuroscience, 10(12), 1758–1768. https://doi.org/10.1093/scan/nsv066
- Terburg, D., Morgan, B., & van Honk, J. (2009). The testosterone-cortisol ratio: A hormonal marker for proneness to social aggression. International Journal of Law and Psychiatry, 32(4), 216–223. https://doi.org/10.1016/j.ijlp.2009.04.008
- Viau, V. (2002). Functional cross‐talk between the hypothalamic‐pituitary‐gonadal and‐adrenal axes. Journal of Neuroendocrinology, 14(6), 506–513. https://doi.org/10.1046/j.1365-2826.2002.00798.x
- Walther, A., Lacker, T. J., & Ehlert, U. (2018). Everybody was Kung-Fu fighting-The beneficial effects of Tai Chi Qigong and self-defense Kung-Fu training on psychological and endocrine health in middle aged and older men. Complementary Therapies in Medicine, 36, 68–72. https://doi.org/10.1016/j.ctim.2017.11.021
- Wang, H., Zhang, S., Wu, S., Qin, S., & Liu, C. (2022). Cortisol awakening response and testosterone jointly affect adolescents’ theory of mind. Hormones and Behavior, 146, 105258. https://doi.org/10.1016/j.yhbeh.2022.105258
- Wang, H., Zhen, Z., Zhu, R., Yu, B., Qin, S., & Liu, C. (2022). Help or punishment: Acute stress moderates basal testosterone’s association with prosocial behavior. Stress (Amsterdam, Netherlands), 25(1), 179–188. https://doi.org/10.1080/10253890.2022.2054696
- Wang, J., Rao, H., Wetmore, G. S., Furlan, P. M., Korczykowski, M., Dinges, D. F., & Detre, J. A. (2005). Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences of the United States of America, 102(49), 17804–17809. https://doi.org/10.1073/pnas.0503082102
- Willemsen, G., Ring, C., McKeever, S., & Carroll, D. (2000). Secretory immunoglobulin A and cardiovascular activity during mental arithmetic: Effects of task difficulty and task order. Biological Psychology, 52(2), 127–141. https://doi.org/10.1016/s0301-0511(99)00028-9
- Wingfield, J. C., Hegner, R. E., Dufty, A. M., Jr, & Ball, G. F. (1990). The "challenge hypothesis": Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. The American Naturalist, 136(6), 829–846. https://doi.org/10.1086/285134
- Zueger, R., Annen, H., & Ehlert, U. (2023). Testosterone and cortisol responses to acute and prolonged stress during officer training school. Stress (Amsterdam, Netherlands), 26(1), 2199886. https://doi.org/10.1080/10253890.2023.2199886

