Abstract
Objectives
The purpose of this study was to determine the relationship between fetal exposure to maternal prenatal stressors and infant parasympathetic (PNS) and sympathetic (SNS) nervous function at 3 timepoints across the first year of life.
Background
Autonomic nervous system impairments may mediate associations between gestational exposure to stressors and later infant health problems. Heart rate variability (HRV) provides a sensitive index of PNS and SNS function. However, no studies have assessed longitudinal associations between prenatal stressors and infant HRV measures of both PNS and SNS over the first year of life.
Methods
During the third trimester of pregnancy, 233 women completed measures of life stressors and depression. At 1, 6 and 12 months of age, a stressor protocol was administered while infant electrocardiographic (ECG) data were collected from a baseline through a post-stressor period. HRV measures of PNS and SNS activity (HF, LF, LF/HF ratio) were generated from ECG data. We used multilevel regression to examine the aims, adjusting for maternal depression and neonatal morbidity.
Results
There were no associations between prenatal stressors and any baseline or reactivity HRV metric over the infant’s first year of life. However, exposure to more stressors was associated with lower post-stressor LF HRV at both 6 (β = −.44, p = .001) and 12 (β = −.37, p = .005) months of age.
Conclusions
Findings suggest potential alterations in development of the vagally mediated baroreflex function as a result of exposure to prenatal stressors, with implications for the infants’ ability to generate a resilient recovery in response to stressors.
Introduction
Women’s prenatal stress has been associated with adverse developmental outcomes for their infants (Lautarescu et al., Citation2020; Polanska et al., Citation2017; Zietlow et al., Citation2019). These adverse effects have been attributed to the programming of fetal neurological systems, involving sustained alterations to their structure and function as a result of exposure to stress (Glover et al., Citation2018; Phillips, Citation2007). Research underlying this hypothesis has focused primarily on the hypothalamic–pituitary–adrenal (HPA) axis, a key stress response system. However, the autonomic nervous system (ANS), the other major neural pathway activated in the stress response, has received less attention as a core fetal mechanism that may be altered by exposure to prenatal stressors.
The ANS undergoes substantial development in utero, including emergence and myelination of the vagal nerve as well as formation of both the sympathetic (SNS) and parasympathetic (PNS) divisions of the ANS (Cerritelli et al., Citation2021; Longin et al., Citation2006; Mulkey & du Plessis, Citation2019). Aberrant programming of autonomic development during this time could have profound effects on the evolving trajectory of a child’s ANS system, impairing the future capacity for allostatic regulation when coping with life stressors and ongoing environmental demands.
A variety of metrics can be used to assess ANS function, such as heart rate, blood pressure, skin conductance, and secretion of alpha-amylase or catecholamines (e.g. Ali & Nater, Citation2020; Andrianome, et al., Citation2017; Christopoulos et al., Citation2019; Goldstein & Cheshire, Citation2018; Ziemssen & Siepmann, Citation2019). Among the options, heart rate variability (HRV) is a frequently used, well-established measure of cardiac ANS function that is considered a reliable marker of stress regulation (Laborde et al., Citation2017; McCraty & Shaffer, Citation2015), with evidence of its sensitivity in infancy (Aye et al., Citation2018; Hashiguchi et al., Citation2020; Oliveira et al., Citation2019). HRV is the fluctuation in length of time between successive heart beats, providing measures of sympathetic and parasympathetic function depending on the HRV metric assessed. Systematic reviews substantiate the ability of HRV to pinpoint stressors that occur, identify stimuli that induce the most stress, and specify differences in stress levels (e.g. Kim et al., Citation2018; The et al., Citation2020). Meta-analyses of neuroimaging studies also suggest that HRV is linked to cortical regions in the brain involved in appraisal of stressful situations (Thayer et al., Citation2012).
Although studies are limited to date, HRV research indicates that exposure to prenatal stressors is associated with alterations in the infant ANS. When specifically assessing parasympathetic activity, studies have shown that the number of stressors/stressful events reported by women during pregnancy is linked to lower HRV in neonates in a resting state (Jacob et al., Citation2009) and reduced infant HRV at 6 months of age in response to a stressor (Bush et al., Citation2017). However, the latter investigators found no association between maternal prenatal perceived stress and infant HRV. Finally, Rash et al. (Citation2015) noted that greater overall maternal cortisol concentration during pregnancy (a physiologic measure of stress) predicted lower infant HRV PNS activity both at rest and in response to a stressor when the infant was 6 months of age.
While previous studies lend support to the hypothesis that prenatal stress is associated with alterations in infant HRV, they have examined only HRV indices of parasympathetic activity. A broader assessment of HRV metrics that examines both SNS and PNS function has not occurred. In addition, prior studies have each looked at only one specific developmental timepoint for infants in their assessment and none through 12 months postnatal. The purpose of this study was to determine the relationship between exposure to maternal prenatal stressors during gestation and infant PNS and SNS function at 3 timepoints across the first year of life, based on HRV indices.
Methods
Design and recruitment
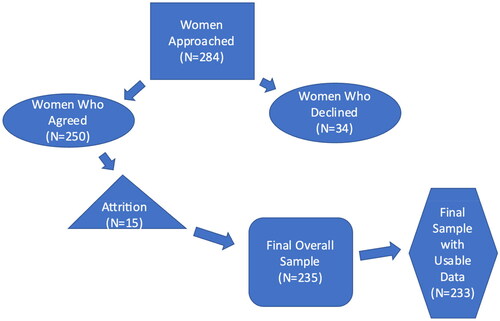
This research was part of a longitudinal study, funded by the National Institutes of Health, to examine the effect of various risk factors during pregnancy and the postpartum on stress regulation of infants over the first year of life, including regulation of emotional distress, cortisol, and heart rate variability. Participants were recruited from two obstetric clinics affiliated with a large university medical center. Inclusion criteria included English or Spanish speaking women in their third trimester of pregnancy who were 18 years or older. Women who had ongoing steroid use or a history of endocrine problems, smoked, had serious medical problems, or cognitive impairment were excluded. Infants born with chromosomal and genetic anomalies, chronic lung disease, congenital heart disease, or other major neonatal illness were also excluded. Our target sample for enrollment was 230 women, although we enrolled 250 women to account for expected attrition over the 15 months of the study. All women who met inclusion criteria at the two clinical sites were offered an opportunity to participate. Approximately, 12% of those initially recruited for the study declined, primarily because of the length of time commitment. Among those who agreed to participate (N = 250), 235 remained in the final overall sample. Based on data needed for this analysis, our sample included 233 mothers and infants (see ). Women provided informed consent and proxy consent for their infants. The study was approved by the Institutional Review Board for Human Research Protection of the University of California, San Francisco (# 14-13516;182948).
Data were collected at four timepoints over the course of the study. During the third trimester of pregnancy, women completed a sociodemographic questionnaire as well as measures of stressors experienced in their lives and their symptoms of depression. Home visits were made at 1, 6 and 12 months of infant age. At each of these visits, a “stressor” protocol was administered during which infant electrocardiographic data were collected for HRV measurement (see details below). Prior to each visit, a research assistant (RA) provided the mother with an overview of the protocol and identified a quiet time and area in the home where the visit could take place without disturbance by others living at the residence.
Additional covariate data were extracted from electronic medical records to control for potential confounders in testing of the study aims. This included information about maternal obstetric complications and neonatal morbidity.
Measures
Exposure to prenatal stressors
Our measure of stressors was the Crisis in Family Systems Questionnaire—Revised (CRISYS-R; Berry et al., Citation2001). This is a 63-item measure in which respondents identify the occurrence of specific stressors in their lives over the prior 6 months. Items assess multiple domains, such as financial, legal, safety in the home and community, medical, job-related, discrimination, and relationship stressors. Validity and reliability of the measure has been supported across diverse populations (Berry et al., Citation2001, Citation2006). The alpha reliability for our sample of women was .84. The total number of stressors reported by women served as our score for exposure to prenatal stressors.
Heart rate variability
Infant HRV data were collected as part of a standardized “stressor” protocol which infants experienced. The protocol at 1 month of infant age differed from the protocol used at 6 and 12 months to be developmentally appropriate. Both protocols had distinct baseline, stressor, and recovery periods. Prior to beginning the baseline period, the mother positioned the infant for the protocol with the assistance of the RA. A respiratory belt was then placed on the infant and the areas where electrodes were to be attached were thoroughly cleansed with preparation pads. Three pediatric Ag/AgCL electrodes were attached on the chest and abdomen of the infant in the lead-II electrode formation, with connection to a portable cardiac monitor (MindWare Technologies Ltd., Gahanna, OH). Electrocardiogram (ECG) readings were tested and calibrated for accuracy. The recording was then initiated and continued throughout baseline, stressor and recovery periods.
Stressor protocol at 1 month postnatal
This protocol involved a previously tested caregiving procedure shown to elicit a broad distribution of stress responses from infants in their first month of life (Weiss & Niemann, Citation2015). After placement of the ECG electrodes, infants were positioned on their stomach with the head turned to one side. They were left undisturbed with no social interaction for 10 min before a 5-min “baseline” period began. Then, a 15-min “stressor” period began. First, a saliva sample was acquired from the infant with a cotton swab placed inside the infant’s mouth between the cheek and gum. Next, the baby was positioned on the back and a temperature taken under the arm. The diaper was then removed, perineal care was performed, and a new diaper was applied. Finally, the baby was positioned on the stomach with the head to one side, and a second saliva sample was acquired with a cotton swab. The “post-stressor” period was then initiated. During this period, the baby was covered with a blanket and left undisturbed on the stomach for 5 min.
Stressor protocol at 6 and 12 months postnatal
The “Repeated Still Face Paradigm” was used to acquire HRV data at 6 and 12 months of infant age (Mesman et al., Citation2009; Tronick, Citation2003). The “Still Face Paradigm” is a well-established protocol shown to reliably elicit a stress response for HRV and other stress-related measures (Lester et al, Citation2018; Ritz et al., Citation2020). During this procedure, mother and infant sat facing each other about 18–24 inches apart. After placement of the electrodes, the infant was given about 5 min to adjust to wearing them. Then the protocol was initiated. The RA cued the mother to begin each of five segments: baseline spontaneous play, first still face episode, another period of spontaneous play, second still face episode, and final period of spontaneous play. In the baseline and spontaneous play segments, the mother and infant interacted as they wished by talking, singing, or touching, but they could not use any toys and the infant could not be picked up. During the still face segments, the mother sat back in her chair and maintained a neutral expression. She continued to look at her baby’s face but was instructed not to talk, sing, smile, vocalize or touch her baby. Each segment of the procedure lasted 2 min, for a total of 10 min.
Data collection and processing of HRV
Infant digitized ECG data were recorded on a memory card and transferred to Mindware BioLab Acquisition Software (MindWare Technologies Ltd.). The software employs a validated algorithm to mark each R wave and calculate the interbeat (R-R peak) interval of the QRS complex of the cardiac cycle. Inconsistent interbeat intervals compared to intervals before and after were marked as potential artifact. ECG records were then manually reviewed to confirm R peak accuracy and remove R peak artifact.
We used Fast Fourier Transformation to separate HRV into various frequency bands for analysis, including low frequency (LF), high frequency (HF), and the LF/HF ratio. Frequency-domain measures were employed based on the seminal and still prevailing recommendation of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (Citation1996) as the most appropriate HRV metrics for analyzing short term ECG recordings (1996). The following frequency-domain parameters obtained from spectral analysis were used to account for infant developmental HRV status: LF band (0.040 − 0.240 Hz) and HF band (0.240 − 1.040 Hz) (Bar-Haim et al., Citation2000; Cacioppo et al., Citation2017; Patural et al., Citation2019). The heart period time series was detrended and tapered using a Hamming window.
HF HRV is under vagal or parasympathetic nervous system control within the ANS (Laborde et al., Citation2017; Shaffer & Ginsberg, Citation2017) and is typically considered an indicator of more adaptive, optimal ANS function (Grossman & Taylor, Citation2007; McCraty & Shaffer, Citation2015; Porges, Citation2007). There is less agreement about the degree to which LF HRV and the LF/HF ratio are influenced by sympathetic versus parasympathetic stimulation. Most frequently, they are viewed as reflecting a mix of sympathetic and parasympathetic nervous system inputs (Oliveira et al., Citation2019; McCraty & Shaffer, Citation2015; Shaffer & Ginsberg, Citation2017).
Covariates
Data on six covariates were acquired to examine their potential confounding effects. Each of these variables has been previously associated with infant HRV metrics. Two were maternal variables: obstetric medical risk (Sharifi-Heris et al., Citation2023) and depressive symptoms (Jacob et al., Citation2009; Pinto et al., Citation2023). The Obstetric Medical Risk Index (Lobel et al., Citation2008) was used to extract data from the medical record regarding risks and complications related to pregnancy (e.g. placenta previa, polyhydramnios, cigarette smoking, anemia). The index is a validated and reliable tool, showing excellent predictive validity for adverse birth outcomes. Scores ranged from 0 (no risks or complications) to 38 (many risks and complications). The Patient Health Questionnaire − 9 (PHQ-9; Kroenke et al., Citation2001) was used to assess mothers’ depression. The PHQ-9 is a self-report questionnaire, based on the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria for major depression, that identifies depressive symptoms experienced over the past 2 weeks. Scores range from 0–27, with higher scores indicating more depressive symptoms and higher severity. A PHQ-9 score ≥10 has a sensitivity of 88% and a specificity of 88% for major depression.
In addition, 4 infant covariates were examined: gestational age (GA) of the infant at birth (Fyfe et al., Citation2015), preterm (<37weeks gestation) versus full term birth status (Aye et al., Citation2018), neonatal morbidity (Chiera et al., Citation2020), and baseline salivary cortisol at 1, 6 and 12 months postnatal (Hashiguchi et al., Citation2020). Data for the first 3 variables were taken from the electronic medical record. The Neonatal Morbidity Assessment Index for Newborns (Verma et al., Citation2005) was used to measure severity of an infant’s health problems. This validated index contains 47 binary items that identify complications during delivery and health problems experienced during the first month of life (e.g. hyperbilirubinemia or intraventricular hemorrhage; Fu et al., Citation2023). A final score was computed by aggregating the number of problems an infant experienced. Salivary specimens were collected from infants prior to initiating each stressor protocol using a SalivaBio infant swab and assayed for cortisol at the Salimetrics bioscience laboratory (Weiss et al., Citation2023).
Data analysis
We used descriptive statistics to delineate sample characteristics and Spearman correlations to examine preliminary relationships between potential covariates and HRV metrics. All HRV scores were log transformed to address skew. Because we used a different stressor protocol at 1 month of infant age than at 6 and 12 months (to assure developmental suitability), we assessed whether the one-month caregiving stressor elicited comparable HRV reactivity to the still face stressor used at 6 and 12 months postnatal. We employed multilevel regression to assess potential differences in reactivity to the stressor across time. We also used multilevel regression to test our primary aim, the effects of prenatal stressors on infant HRV over 12 months postnatal, controlling for the effects of identified covariates. Dependent variables (DVs) in separate regression models included infant resting state LF, HF and LF/HF ratio prior to the stressor, infant post-stressor LF, HF and LF/HF ratio, and the change in scores for each of the HRV variables from baseline to the post-stressor period (reactivity). These DVs were examined at 1 month, 6 months, and 12 months postnatal. Predictor variables were grand-mean centered, improving our ability to interpret the intercept values. Full Information Maximum Likelihood (FIML) was employed to achieve unbiased estimates of the effects over time for data missing at random. We applied a Bonferroni adjustment to the confidence intervals (98.33%) to account for multiple comparisons, resulting in an alpha requirement of ≤ 0.0167 for significance. Covariates showing a significant relationship (p ≤ .05) in preliminary analysis were included in the final regression models. We performed statistical analyses with Stata 16.
Results
Sample characteristics
The sample included 233 mothers and infants whose characteristics are shown in . In brief, women’s average age was 33.56, ranging from 21 to 47. 64.4% were racially and ethnically diverse, including 24% Hispanic/Latinx and 21.8% African American/Black. Women were well-educated, with 56.6% having a college degree. Overall, women had a mean (M) of 3.37 medical complications or risks during pregnancy (out of a possible 37), with a range from 0–9. Assessment of depression indicated that women, on average, had mild depression (M = 5.53), although 18% met the criteria for clinical depression (score ≥10). Infants were evenly split by sex. On average, their gestational age at birth was 36.93 weeks, ranging from 27.3 to 47 weeks. Infants as a group had minimal physical morbidity (M = 137.45) extending from no physical health problems to having a score of 1,813 on the morbidity measure, indicating severe morbidity.
Table 1. Characteristics of women and infants.
The number of prenatal stressors reported by mothers ranged from 0 to 39 (M = 6.51) out of a possible 63. As shown in , housing problems represented the domain of stressors experienced by the greatest percentage of women (44.8%). Within this domain, loss of housing (21%) and pests (rodents, insects) in the home (14%) were most frequently reported. Work challenges represented the second stressor domain most frequently reported by women (16%), with lack of work/loss of employment (30%) representing the greatest stressor in that domain. The domain involving financial strains was also prominent (11.6%), with 25% of the women indicating inadequate or decreased income and 15% reporting they were ‘deep in debt’.
Table 2. Percent of women who reported stressful events in various stressor domains.
HRV characteristics
Infant HRV scores (log transformed) for baseline, the post-stressor timepoint and reactivity (baseline to post-stressor change) are shown in . Baseline, post-stressor, and reactivity values for LF HRV increased incrementally from 1 to 12 months postnatal, as did HF Baseline and Post-Stressor HRV. Baseline LF/HF HRV ratio showed no clear trajectory, although the ratio for post-stressor response declined from 1 to 12 months postnatal and the reactivity ratio appeared to increase over time. Bivariate correlations between baseline HRV metrics showed a consistent, positive relationship between LF and HF at each of the 3 timepoints over the first year of life (r = .69, r = .68, r = .75), all significant at p = .001. LF was not related to the LF/HF ratio but HF was consistently, negatively associated (p = .001) with the ratio at all timepoints (r = −.58, r = −.43, r = −.54). In addition, as shown in Supplemental Table 1, the extent of HRV reactivity to the caregiving stressor at 1 month postnatal did not differ significantly from reactivity to the still face stressor at 6 and 12 months postnatal. The p-values for differences between 1 month and 6- or 12-month reactivity of LF, HF, and the LF/HF ratio ranged from .15 to .65.
Table 3. Mean (SE) infant HRV for low frequency (LF), high frequency (HF), and the LF/HF ratio for resting state, reactivity to the stressor, and the post-stressor period at 1, 6, and, 12 months postnatal.
Correlations between covariates and HRV metrics
In preliminary bivariate correlations, women’s obstetric risk was not related to any HRV baseline, post-stressor, or reactivity metric, ranging from −.01 to .14 (NS). For infant covariates, we found no differences between infants born term and preterm in any HRV measure, with p-values for differences ranging from .19 to .39. Similarly, there were no significant correlations between any baseline salivary cortisol measure and any HRV measure. The largest association was between infant baseline cortisol and baseline HF HRV at one month postnatal (−.047, p = .60). In contrast, women’s depression was associated with LF, HF and the LF/HF ratio at diverse timepoints, with correlations ranging from −.01 to .33 (p = .001). Both the infant’s gestational age (GA) and neonatal morbidity showed significant relationships to various HRV scores. GA correlations ranged from −.06 to .31 (p = .01) while neonatal morbidity had correlations ranging from .00 to .48 (p = .001). Because of the strong collinearity between GA and morbidity (r = −.63, p = .000) and the robust correlations between morbidity and HRV metrics, we elected to include only neonatal morbidity in our multilevel regression models, along with women’s depression.
HRV regression models for baseline, post-stressor, and reactivity to the stressor
As shown in and , there were no significant associations between prenatal stressors and a) any baseline, resting state HRV metric at 1, 6 or 12 months postnatal, or b) reactivity to the stressor for any HRV metric at 1, 6 or 12 months postnatal. However, data in indicate that exposure to more prenatal stressors was associated with lower LF HRV in response to stressors (post-stressor) during the first year of life (coefficient = −.023, p=.001). Although the post-stressor LF HRV ratio was also negatively associated with exposure to prenatal stressors (−.011, p = .04), the strength of the association did not meet the stringent 98.33% CI criteria we set to adjust for multiple testing.
Table 4. Multilevel regression for the association of prenatal stressors with low frequency (LF), high frequency (HF) and LF/HF ratio HRV during a baseline, resting state across the first year of lifeTable Footnote*.
Table 5. Multilevel regression for the association of prenatal stressors with low frequency (LF), high frequency (HF) and the LF/HF ratio HRV during a post-stressor period across the first year of lifeTable Footnote*.
Table 6. Multilevel regression for the association of prenatal stressors with HRV reactivity to a stressor for low frequency (LF), high frequency (HF) and the LF/HF ratio across the first year of lifeTable Footnote*.
Results in provide more detailed data regarding the effects of prenatal stressors on post-stressor LF HRV in separate regression models at the three developmental timepoints. Data show that exposure to more prenatal stressors was associated with lower post-stressor LF HRV at both 6 (β= −.44, p = .001) and 12 (β = −.37, p = .005) months of infant age. Both these beta coefficients reflect a medium effect size. Although neonatal morbidity had a significant effect, no association between prenatal stressors and post-stressor LF HRV was found at 1 month postnatal.
Table 7. Linear regression for the association of prenatal stressors with low frequency heart rate variability at 1 month, 6 months and 12 months postnatalTable Footnote*.
Discussion
Results suggest that fetal exposure to prenatal stressors may influence development of the infant cardiac ANS over the first year of life, as measured by HRV. Exposure to a greater number of stressors was associated with lower LF HRV at 6 and 12 months of life. We found no significant effects for LF HRV at 1 month postnatal nor for other HRV frequency bands (i.e. HF, the LF/HF ratio). In addition, effects were not observed for baseline HRV activity or HRV reactivity scores, but only during the post-stressor period following the standardized stressor.
Potential fetal programming of the ANS
Findings suggest that exposure to prenatal stressors may induce a fetal programming effect that shapes the way in which the cardiac ANS recovers from stressors as the infant develops, affecting the ability to regulate recovery from the HRV response to environmental demands (Mulkey & du Plessis, Citation2019; Phillips, Citation2007). Mulkey and du Plessis (Citation2019) describe these regulatory difficulties as impairments in homeostatic or allostatic plasticity, whereby the nervous system cannot effectively return to an optimal state after an environmental stressor has passed or been resolved. The mechanism through which gestational exposure to stressors may influence the ANS is not yet known. However, this could involve maternal secretion of norepinephrine and acetylcholine, neurotransmitters (NTs) released by the SNS and PNS respectively and which directly influence HRV. These NTs are known to be neuromodulators during fetal development (Horackova et al., Citation2022; Wu et al., Citation2015). The placenta plays a key role in preventing or enabling elevated NT levels from the mother’s neuronal secretion into the fetal-placental unit. Even transient disruptions of NT modulation during gestation have been associated with permanent changes in fetal structure and function (Piquer et al., Citation2017; Rosenfeld, Citation2021). Thus, maternal norepinephrine or acetylcholine perturbations as a result of exposure to stressors could trigger fetal HRV alterations through placental mediation.
Another potential mechanism through which prenatal stressors may affect infant HRV is via the impact of a mother’s HPA axis on the infant’s ANS. In both human and animal studies, prenatal HPA axis hormones of the mother (cortisol and cortisone) have been associated with alterations in fetal heart rate, HRV and the cardiac conduction system (Antolic et al., Citation2018; Hunter et al., Citation2021; Turan & Kaya, Citation2023). Other studies report that administration of prenatal synthetic corticosteroids to mothers also elicits changes in fetal heart rate parameters (Noben et al., Citation2019; Verdurmen et al., Citation2013). Based on their systematic review, Verdurmen and colleagues (Citation2013) proposed that persistent exposure to corticosteroids and related vasoconstriction may potentiate a vagally mediated baroreceptor response which triggers reflex inhibition of the sympathetic branch and activation of the parasympathetic branch to influence fetal heart activity. Perturbations in the fetal HPA axis, enabled by transplacental mediation of maternal perturbations, would ultimately impact the cardiac ANS since the ANS and the HPA axis are reciprocally innervated (Bleker et al., Citation2020; Rotenberg & McGrath, Citation2016).
Our finding that HRV alterations were specific to the infant’s response to stress, rather than the baseline/resting state, is congruent with previous research (Bush et al., Citation2017; Suurland et al., Citation2017). Together, the data from our studies suggest that exposure to prenatal stressors or adversity may influence the infant’s regulation of the stress response rather than basal or ongoing activation of the ANS when stressors are not occurring. Although Jacob et al. (Citation2009) did find an association between prenatal stressors and HRV changes during the basal, resting state, they studied newborns in their first week of life, not infants who were at least 6 months of age as assessed by these other studies.
An effect of prenatal stressors later versus earlier in development
We found the presence of significant associations between prenatal stressors and LF HRV at both 6 and 12 months of age, but not earlier in development when assessed at 1 month postnatal. Research indicates that LF HRV develops steadily over the first year of life (Patural et al., Citation2019), incrementally increasing in its power. Diminished maturity of the LF HRV band at 1 month of age may be the reason why no relationship between stressors and LF HRV was found at that point in time.
However, it is important to note that the caregiving stressor we used at 1 month of age was different than the still face stressor used at 6 and 12 months postnatal. Although our assessment of the reactivity of the two stressor protocols indicated no differences in the HRV reactivity they elicited, it is possible that the stronger socioemotional nature of the still face paradigm induced unique effects on the LF HRV band that were not induced by the more procedurally-oriented caregiving stressor.
The research of Patural and colleagues (Citation2019) may shed light on why we found that LF HRV was sensitive to the effects of stressors while HF HRV was not. They reported that, over the first year of life, the LF band represents 12-13% of total HRV power (i.e. overall autonomic activity) while the HF band reflects only 6%–7%. Although development of the HF band progresses more rapidly to exceed LF values by 2 years of age, HF HRV may not be as sensitive an indicator of cardiac ANS function during the first year of life.
LF HRV as a potential marker of vagally mediated baroreflex function
There is growing evidence that LF HRV is associated with parasympathetic, vagal function. In his seminal work, Porges (Citation2007) proposed that, while sympathetic influences might be involved in triggering neural circuits involved in LF HRV, the final pathway for the LF frequency band is vagal. Hashiguchi et al. (Citation2020) reported that both HF and LF had a negative correlation with newborn salivary cortisol levels, suggesting that LF HRV may reflect decreased SNS activity. Although based on a study with adults, Thomas et al. (Citation2019) found that LF was not correlated with electrodermal reactivity in response to stressors (a measure of sympathetic arousal) but that LF reactivity did correlate with HF reactivity during baroreflex modulation. Based on their findings, investigators proposed that LF does not represent sympathetic activity but may instead reflect vagally mediated baroreflex effects. Similarly, Goldstein et al. (Citation2011) reported a strong association between LF and HF power. They also suggested that LF power is likely an index of baroreflex function rather than sympathetic tone; baroreflex-induced changes are mediated by both parasympathetic and sympathetic nerves. Akin to the findings of these studies, we found significant, positive correlations between LF and HF HRV values for infants at 1, 6 and 12 months of age. It is possible that exposure to prenatal stressors could be contributing to an alteration in vagally mediated baroreflex function in the infant’s cardiac ANS, evidenced in the association between exposure to more stressors and reduced LF HRV. Reduced baroreflex function (LF HRV) could result in less inhibition of the sympathetic nervous system (Thomas et al., Citation2019), enabling greater sympathetic arousal in the infant’s response to a stressor. However, it is important to note that this hypothesis is speculative and requires further research, using more specific measures of baroreflex function, before assuming such an interpretation is valid.
Limitations
Certain characteristics of our sample limit generalizability. Overall, women were well-educated, had mild depression, and incurred minimal obstetric risks. Infants were healthy on average, with no major physical morbidity. Thus, results may not reflect the stressors experienced by women and infants at higher social, psychological and medical risk or the ways in which their stressors are associated with HRV. For instance, extremely premature infants who spend longer periods of time in the Neonatal Intensive Care Unit may have unique profiles of HRV response as a result of their more stressful postnatal experience. Another limitation was our lack of HRV assessment in the week after birth, precluding a fuller understanding of the postnatal trajectory.
Nonetheless, our study had numerous strengths. The sample was racially and ethnically diverse. Our measure of stressor types was broad in scope (e.g. environmental, financial, health). We examined HRV at three longitudinal timepoints across the first year of life, rather than the cross-sectional approach of previous studies, as well as two different bands of HRV.
Implications
Longitudinal studies (traversing gestation, shortly after birth, and throughout the first year of infant life) are needed to fully understand the trajectory of alterations in ANS development associated with prenatal stressors. Careful assessment of the relationships among various HRV metrics will be critical to better understand interactive developmental effects. This should include assessment of frequency-domain metrics such as those we have examined as well as time-domain indices in which variability in the interbeat interval is sampled (e.g. SDNN—the standard deviation of all beat-to-beat intervals) rather than the amount of signal energy or power within frequency bands. Various indices of HRV or their interactions could capture uniquely different effects of prenatal stressors. Research to explore the relationship of HRV metrics to other measures of SNS and PNS function (e.g. cortisol and alpha-amylase, skin conductance, blood pressure) is also essential. Further studies of LF HRV as a potential indicator of baroreflex function should be undertaken to determine the validity of this emerging hypothesis. More specific measures of baroreflex function (Javorka et al., Citation2021; Lin & Diedrich, Citation2022) should also be examined to support the justifiability of any hypothesized links between prenatal stressors and infant baroreflex function. There is a need to assess whether specific stressors (e.g. financial insecurity, neighborhood violence) may be linked to HRV alterations differently or in some universal way. Further research is necessary to evaluate the effects of prenatal stressors within specific populations of varied racial and cultural backgrounds as well as those having a range of medical and psychosocial risk. Finally, it will be important to understand the potential moderating effects of maternal sensitivity or other parenting characteristics in buffering any postnatal effect of prenatal stressors on infant HRV. Maternal sensitivity has been associated with increased PNS during infant HRV response to a stressor (Conradt & Ablow, Citation2010) while insensitive behavior predicts greater SNS activation and impaired PNS activation of infants during periods of stress (Bosquet Enlow et al., Citation2014; Köhler-Dauner et al., Citation2022). Such findings suggest that more sensitive parenting by mothers might protect the infant from any adverse HRV alterations in managing stress that result from exposure to prenatal stressors. Mothers’ early assistance with external regulation of the infants’ sympathetic arousal could foster improved self-regulation as development proceeds.
Although further research is needed to confirm our results, they expand the understanding of how prenatal stressors may impact cardiac ANS development and inform future research. Our results indicate a particular influence on the infant’s ability to mount a resilient ANS recovery after a stressor. HRV impairment in the allostatic modulation of stress has been linked to many mental and physical health problems, with its detrimental impact on immune function and emotion regulation as core features that may underlie broad-spectrum health effects (Cubillo et al., Citation2023; Jung et al., Citation2019; Kemp & Quintana, Citation2013). Our findings can ultimately help in identifying valid, reliable HRV markers for assessing stress-related disorders in early life as well as fruitful HRV targets for early interventions to prevent and treat impairments in stress regulation.
Author contributions
S. Weiss was responsible for project administration, funding acquisition, project conceptualization, design of the methodology, data analysis, and writing of the original draft. C. Leung contributed to project conceptualization, data collection, oversight and management of heart rate variability spectral analysis, and review of the manuscript. B. Cooper performed statistical analysis and reviewed the manuscript.
Supplemental Material
Download MS Word (12.7 KB)Acknowledgments
The authors are grateful to Sandra Niemann, PhD, for her contributions to project management and to both Sandra Niemann, PhD, and Nina Ahlers, MPH, for their contributions to data collection and data management.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Sandra J. Weiss
Sandra J. Weiss, PhD, DNSc is a Professor and the Eschbach Endowed Chair in the Department of Community Health Systems at the University of California, San Francisco. Dr. Weiss studies: 1) women’s depression and stress, especially during pregnancy and the postpartum, and 2) biopsychosocial factors that shape fetal development of the nervous system and the microbiome as foundations for mental health in infancy and early childhood.
Bruce Cooper
Bruce Cooper, PhD is a biostatistician and data analyst in the Department of Community Health Systems at the University of California, San Francisco. Dr. Cooper has expertise in advanced statistical methods, including structural regression modeling, latent class analysis, and procedures involving complex longitudinal designs.
Cherry Leung
Cherry Leung, PhD, RN is an Associate Professor in the Department of Community Health Systems at the University of California, San Francisco. Dr. Leung’s research focuses on environmental and biological risk factors associated with infant, child, and adolescent mental health. She has a particular interest in the role of the gut microbiome in development and treatment of adolescent depression.
References
- Ali, N., & Nater, U. (2020). Salivary alpha-amylase as a biomarker of stress in behavioral medicine. International Journal of Behavioral Medicine, 27(3), 1–10. https://doi.org/10.1007/s12529-019-09843-x
- Andrianome, S., Gobert, J., Hugueville, L., Stéphan-Blanchard, E., Telliez, F., & Selmaoui, B. (2017). An assessment of the autonomic nervous system in the electrohypersensitive population: A heart rate variability and skin conductance study. Journal of Applied Physiology (Bethesda, Md.: 1985), 123(5), 1055–1062. https://doi.org/10.1152/japplphysiol.00229.2017
- Antolic, A., Wood, C. E., & Keller-Wood, M. (2018). Chronic maternal hypercortisolemia in late gestation alters fetal cardiac at birth. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 314(3), R342–R352. https://doi.org/10.1152/ajpregu.00296.2017
- Aye, C., Lewandowski, A., Oster, J., Upton, R., Davis, E., Kenworthy, Y., Boardman, H., Yu, G., Siepmann, T., Adwani, S., McCormick, K., Sverrisdottir, Y., & Leeson, P. (2018). Neonatal autonomic function after pregnancy complications and early cardiovascular development. Pediatric Research, 84(1), 85–91. https://doi.org/10.1038/s41390-018-0021-0
- Bar-Haim, Y., Marshall, P. J., & Fox, N. A. (2000). Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology, 37(1), 44–56. https://doi.org/10.1002/1098-2302(200007)37:1<44::AID-DEV6>3.0.CO;2-7
- Berry, C. A., Quinn, K. A., Portillo, N., & Shalowitz, M. U. (2006). Reliability and validity of the Spanish version of the crisis in family systems–revised. Psychological Reports, 98(1), 123–132. https://doi.org/10.2466/pr0.98.1.123-132
- Berry, C. A., Quinn, K., Shalowitz, M., & Wolf, R. (2001). Validation of the crisis in family systems–revised: A contemporary measure of life stressors. Psychological Reports, 88(3 Pt 1), 713–724. https://doi.org/10.2466/pr0.2001.88.3.713
- Bleker, L., van Dammen, L., Leeflang, M., Limpens, J., Roseboom, T., & de Rooij, S. (2020). Hypothalamic-pituitary-adrenal axis and autonomic nervous system reactivity in children prenatally exposed to maternal depression: A systematic review of prospective studies. Neuroscience and Biobehavioral Reviews, 117, 243–252. https://doi.org/10.1016/j.neubiorev.2018.05.033
- Bosquet Enlow, M., King, L., Schreier, H., Howard, J., Rosenfield, D., Ritz, T., & Wright, R. (2014). Maternal sensitivity and infant autonomic and endocrine stress responses. Early Human Development, 90(7), 377–385. https://doi.org/10.1016/j.earlhumdev.2014.04.007
- Bush, N., Jones-Mason, K., Coccia, M., Caron, Z., Alkon, A., Thomas, M., Coleman-Phox, K., Wadhwa, P., Laraia, B., Adler, N., & Epel, E. (2017). Effects of pre- and postnatal maternal stress on infant temperament and autonomic nervous system reactivity and regulation in a diverse, low-income population. Development and Psychopathology, 29(5), 1553–1571. https://doi.org/10.1017/S0954579417001237
- Cacioppo, J. T., Tassinary, L. G., & Berntson, G. G. (2017). Handbook of psychophysiology (4th ed., pp. 197–198). Cambridge University Press.
- Cerritelli, F., Frasch, M., Antonelli, M., Viglione, C., Vecchi, S., Chiera, M., & Manzotti, A. (2021). A review on the vagus nerve and autonomic nervous system during fetal development: Searching for critical windows. Frontiers in Neuroscience, 15, 721605. https://doi.org/10.3389/fnins.2021.721605
- Chiera, M., Cerritelli, F., Casini, A., Barsotti, N., Boschiero, D., Cavigioli, F., Corti, C., & Manzotti, A. (2020). Heart rate variability in the perinatal period: A critical and conceptual review. Frontiers in Neuroscience, 2514, 561186. PMID: 33071738; PMCID: PMC7544983. https://doi.org/10.3389/fnins.2020.561186
- Christopoulos, G., Uy, M., & Yap, W. (2019). The body and the brain: Measuring skin conductance responses to understand the emotional experience. Organizational Research Methods, 22(1), 394–420. https://doi.org/10.1177/1094428116681073
- Conradt, E., & Ablow, J. (2010). Infant physiological response to the still-face paradigm: Contributions of maternal sensitivity and infants’ early regulatory behavior. Infant Behavior & Development, 33(3), 251–265. https://doi.org/10.1016/j.infbeh.2010.01.001
- Cubillo, A., Tkalcec, A., Oldenhof, H., Unternaehrer, E., Raschle, N., Kohls, G., Nauta-Jansen, L., Hervas, A., Fernandez-Rivas, A., Konrad, K., Popma, A., Freitag, C., de Brito, S., Fairchild, G., & Stadler, C. (2023). Linking heart rate variability to psychological health and brain structure in adolescents with and without conduct disorder. Frontiers in Psychiatry, 2714, 1101064. https://doi.org/10.3389/fpsyt.2023.1101064
- Fu, M., Song, W., Yu, G., Yu, Y., & Yang, Q. (2023). Risk factors for length of NICU stay of newborns: A systematic review. Frontiers in Pediatrics, 11, 1121406. https://doi.org/10.3389/fped.2023.1121406
- Fyfe, K., Yiallourou, S., Wong, F., Odoi, A., Walker, A., & Horne, R. (2015). The effect of gestational age at birth on post-term maturation of heart rate variability. Sleep, 38(10), 1635–1644. https://doi.org/10.5665/sleep.5064
- Glover, V., O’Donnell, K., O’Connor, T., & Fisher, J. (2018). Prenatal maternal stress, fetal programming, and mechanisms underlying later psychopathology—A global perspective. Development and Psychopathology, 30(3), 843–854. https://doi.org/10.1017/S095457941800038X
- Goldstein, D., Bentho, O., Park, M., & Sharabi, Y. (2011). Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Experimental Physiology, 96(12), 1255–1261. https://doi.org/10.1113/expphysiol.2010.056259
- Goldstein, D., & Cheshire, W. (2018). Roles of catechol neurochemistry in autonomic function testing. Clinical Autonomic Research: Official Journal of the Clinical Autonomic Research Society, 28(3), 273–288. https://doi.org/10.1007/s10286-018-0528-9
- Grossman, P., & Taylor, E. (2007). Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology, 74(2), 263–285. https://doi.org/10.1016/j.biopsycho.2005.11.014
- Hashiguchi, K., Kuriyama, N., Koyama, T., Matsui, D., Ozaki, E., Hasegawa, T., Tokuda, S., Niwa, F., Iwasa, K., Watanabe, I., Teramukai, S., Kitawaki, J., Watanabe, Y., Uehara, R., & Hosoi, H. (2020). Validity of stress assessment using heart-rate variability in newborns. Pediatrics International: Official Journal of the Japan Pediatric Society, 62(6), 694–700. https://doi.org/10.1111/ped.14149
- Horackova, H., Karahoda, R., Vachalova, V., Turkova, H., Abad, C., & Staud, F. (2022). Functional characterization of dopamine and norepinephrine transport across the apical and basal plasma membranes of the human placental syncytiotrophoblast. Scientific Reports, 12(1), 11603. https://doi.org/10.1038/s41598-022-15790-7
- Hunter, S., Freedman, R., Law, A., Christians, U., Holzman, J., Johnson, Z., & Hoffman, M. (2021). Maternal corticosteroids and depression during gestation and decreased fetal heart rate variability. Neuroreport, 32(14), 1170–1174. https://doi.org/10.1097/WNR.0000000000001711
- Jacob, S., Byrne, M., & Keenan, K. (2009). Neonatal physiological regulation is associated with perinatal factors: A study of neonates born to healthy African American women living in poverty. Infant Mental Health Journal, 30(1), 82–94. https://doi.org/10.1002/imhj.20204
- Javorka, K., Haskova, K., Czippelova, B., Zibolen, M. & Javorka, M. (2021). Blood pressure variability and baroreflex sensitivity in premature newborns - An effect of postconceptional and gestational age. Frontiers in Pediatrics, 9, 653573. https://doi.org/10.3389/fped.2021.653573.
- Jung, W., Jang, K., & Lee, S. (2019). Heart and brain interaction of psychiatric illness: A review focused on heart rate variability, cognitive function, and quantitative electroencephalography. Clinical Psychopharmacology and Neuroscience: The Official Scientific Journal of the Korean College of Neuropsychopharmacology, 17(4), 459–474. https://doi.org/10.9758/cpn.2019.17.4.459
- Kemp, A., & Quintana, D. (2013). The relationship between mental and physical health: Insights from the study of heart rate variability. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 89(3), 288–296. https://doi.org/10.1016/j.ijpsycho.2013.06.018
- Kim, H., Cheon, E., Bai, D., Lee, Y., & Koo, B. (2018). Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investigation, 15(3), 235–245. https://doi.org/10.30773/pi.2017.08.17
- Köhler-Dauner, F., Roder, E., Gulde, M., Mayer, I., Fegert, J. M., Ziegenhain, U., & Waller, C. (2022). Maternal sensitivity modulates child’s parasympathetic mode and buffers sympathetic activity in a free play situation. Frontiers in Psychology, 1913, 868848. PMID: 35529563; PMCID: PMC9068013. https://doi.org/10.3389/fpsyg.2022.868848
- Kroenke, K., Spitzer, R. L., & Williams, J. B. (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. https://doi.org/10.1046/j.1525-1497.2001.016009606.x
- Laborde, S., Mosley, E., & Thayer, J. (2017). Heart rate variability and cardiac vagal tone in psychophysiological research: Recommendations for experiment planning, data analysis, and data reporting. Frontiers in Psychology, 8, 213. https://doi.org/10.3389/fpsyg.2017.00213
- Lautarescu, A., Craig, M., & Glover, V. (2020). Prenatal stress: Effects on fetal and child brain development. International Review of Neurobiology, 150, 17–40. https://doi.org/10.1016/bs.irn.2019.11.002
- Lester, B., Conradt, E., Lagasse, L., Tronick, E., Padbury, J., & Marsit, C. (2018). Epigenetic programming by maternal behavior in the human infant. Pediatrics, 142(4), e20171890. https://doi.org/10.1542/peds.2017-1890
- Lin, Y., & Diedrich, A. (2022). Methods on the assessment of human baroreflex function. Frontiers in Neuroscience, 2816, 965406. PMID: 35837120; PMCID: PMC9274245. https://doi.org/10.3389/fnins.2022.965406
- Lobel, M., Cannella, D. L., Graham, J. E., DeVincent, C., Schneider, J., & Meyer, B. A. (2008). Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychology: official Journal of the Division of Health Psychology, American Psychological Association, 27(5), 604–615. https://doi.org/10.1037/a0013242
- Longin, E., Gerstner, T., Schaible, T., Lenz, T., & König, S. (2006). Maturation of the autonomic nervous system: Differences in heart rate variability in premature vs. term infants. Journal of Perinatal Medicine, 34(4), 303–308. https://doi.org/10.1515/JPM.2006.058
- McCraty, R., & Shaffer, F. (2015). Heart rate variability: New perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Global Advances in Health and Medicine, 4(1), 46–61. https://doi.org/10.7453/gahmj.2014.073
- Mesman, J., van Ijzendoorn, M. H., & Bakermans-Kranenburg, M. J. (2009). The many faces of the still-face paradigm: A review and meta-analysis. Developmental Review, 29(2), 120–162. https://doi.org/10.1016/j.dr.2009.02.001
- Mulkey, S., & Du Plessis, A. (2019). Autonomic nervous system development and its impact on neuropsychiatric outcome. Pediatric Research, 85(2), 120–126. https://doi.org/10.1038/s41390-018-0155-0
- Noben, L., Verdurmen, K., Warmerdam, G., Vullings, R., Oei, S., & van Laar, J. (2019). The fetal electrocardiogram to detect the effects of betamethasone on fetal heart rate variability. Early Human Development, 130, 57–64. https://doi.org/10.1016/j.earlhumdev.2019.01.011
- Oliveira, V., von Rosenberg, W., Montaldo, P., Adjei, T., Mendoza, J., Shivamurthappa, V., Mandic, D., & Thayyil, S. (2019). Early postnatal heart rate variability in healthy newborn infants. Frontiers in Physiology, 10, 922. https://doi.org/10.3389/fphys.2019.00922
- Patural, H., Pichot, V., Flori, S., Giraud, A., Franco, P., Pladys, P., Beuchée, A., Roche, F., & Barthelemy, J. (2019). Autonomic maturation from birth to 2 years: Normative values. Heliyon, 75(3), e01300. https://doi.org/10.1016/j.heliyon.2019.e01300
- Phillips, D. (2007). Programming of the stress response: A fundamental mechanism underlying the long-term effects of the fetal environment? Journal of Internal Medicine, 261(5), 453–460. https://doi.org/10.1111/j.1365-2796.2007.01801.x
- Pinto, T., Nogueira-Silva, C., & Figueiredo, B. (2023). Fetal heart rate variability and infant self-regulation: The impact of mother’s prenatal depressive symptoms. Journal of Reproductive and Infant Psychology. Advance online publication. https://doi.org/10.1080/02646838.2023.2257730
- Piquer, B., Fonseca, J. L., & Lara, H. E. (2017). Gestational stress, placental norepinephrine transporter and offspring fertility. Reproduction (Cambridge, England), 153(2), 147–155. https://doi.org/10.1530/REP-16-0312
- Polanska, K., Krol, A., Merecz-Kot, D., Jurewicz, J., Makowiec-Dabrowska, T., Chiarotti, F., Calamandrei, G., & Hanke, W. (2017). Maternal stress during pregnancy and neurodevelopmental outcomes of children during the first 2 years of life. Journal of Paediatrics and Child Health, 53(3), 263–270. https://doi.org/10.1111/jpc.13422
- Porges, S. W. (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. https://doi.org/10.1016/j.biopsycho.2006.06.009
- Rash, J., Campbell, T., Letourneau, N., & Giesbrecht, G. (2015). Maternal cortisol during pregnancy is related to infant cardiac vagal control. Psychoneuroendocrinology, 54, 78–89. https://doi.org/10.1016/j.psyneuen.2015.01.024
- Ritz, T., Schulz, S. M., Rosenfield, D., Wright, R. J., & Bosquet Enlow, M. (2020). Cardiac sympathetic activation and parasympathetic withdrawal during psychosocial stress exposure in 6-month-old infants. Psychophysiology, 57(12), e13673. https://doi.org/10.1111/psyp.13673
- Rosenfeld, C. (2021). The placenta-brain-axis. Journal of Neuroscience Research, 99(1), 271–283. https://doi.org/10.1002/jnr.24603
- Rotenberg, S., & McGrath, J. (2016). Inter-relation between autonomic and HPA axis activity in children and adolescents. Biological Psychology, 117, 16–25. https://doi.org/10.1016/j.biopsycho.2016.01.015
- Shaffer, F., & Ginsberg, J. P. (2017). An overview of heart rate variability metrics and norms [Review]. Frontiers in Public Health, 5, 258. https://doi.org/10.3389/fpubh.2017.00258
- Sharifi-Heris, Z., Rahmani, A., Axelin, A., Rasouli, M., & Bender, M. (2023). Heart rate variability and pregnancy complications: Systematic review. Interactive Journal of Medical Research, 12, e44430. PMID: 37276013 PMCID: 10280337 https://doi.org/10.2196/44430
- Suurland, J., van der Heijden, K. B., Smaling, H., Huijbregts, S., van Goozen, S., & Swaab, H. (2017). Infant autonomic nervous system response and recovery: Associations with maternal risk status and infant emotion regulation. Development and Psychopathology, 29(3), 759–773. https://doi.org/10.1017/S0954579416000456
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation, 93(5), 1043–1065. https://doi.org/10.1161/01.CIR.93.5.1043
- Thayer, J., Ahs, F., Fredrikson, M., Sollers, J., & Wager, T. (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews, 36(2), 747–756. https://doi.org/10.1016/j.neubiorev.2011.11.009
- The, A., Reijmerink, I., van der Laan, M., & Cnossen, F. (2020). Heart rate variability as a measure of mental stress in surgery: A systematic review. International Archives of Occupational and Environmental Health, 93(7), 805–821. https://doi.org/10.1007/s00420-020-01525-6
- Thomas, B., Claassen, N., Becker, P., & Viljoen, M. (2019). Validity of commonly used heart rate variability markers of autonomic nervous system function. Neuropsychobiology, 78(1), 14–26. https://doi.org/10.1159/000495519
- Tronick, E. Z. (2003). Things still to be done on the still-face effect. Infancy, 4(4), 475–482. https://doi.org/10.1207/S15327078IN0404_02
- Turan, A., & Kaya, C. (2023). Effect of maternal cortisol levels on fetal heart rate patterns in primiparous pregnant women in the third trimester. Revista da Associacao Medica Brasileira (1992), May 1969(5), e20221610. https://doi.org/10.1590/1806-9282.20221610
- Verdurmen, K., Renckens, J., van Laar, J., & Oei, S. (2013). The influence of corticosteroids on fetal heart rate variability: A systematic review of the literature. Obstetrical & Gynecological Survey, 68(12), 811–824. https://doi.org/10.1097/OGX.0000000000000007
- Verma, A., Weir, A., Drummond, J., & Mitchell, B. F. (2005). Performance profile of an outcome measure: Morbidity Assessment Index for Newborns. Journal of Epidemiology and Community Health, 59(5), 420–426. https://doi.org/10.1136/jech.2003.019109
- Weiss, S., Keeton, V., Richoux, S., Cooper, B., & Niemann, S. (2023). Exposure to antenatal corticosteroids and infant cortisol regulation. Psychoneuroendocrinology, 2023 147, 105960., PMC9968454, https://doi.org/10.1016/j.psyneuen.2022.105960
- Weiss, S. J., & Niemann, S. (2015). Effects of antenatal corticosteroids on cortisol and heart rate reactivity of preterm infants. Biological Research for Nursing, 17(5), 487–494. https://doi.org/10.1177/1099800414564860
- Wu, W., Adams, C., Stevens, K., Chow, K., Freedman, R., & Patterson, P. (2015). The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain, Behavior, and Immunity, 46, 192–202. https://doi.org/10.1016/j.bbi.2015.02.005
- Ziemssen, T., & Siepmann, T. (2019). The investigation of the cardiovascular and sudomotor autonomic nervous system: A review. Frontiers in Neurology, 1210, 53. PMID: 30809183; PMCID: PMC6380109. https://doi.org/10.3389/fneur.2019.00053
- Zietlow, A.-L., Nonnenmacher, N., Reck, C., Ditzen, B., & Müller, M. (2019). Emotional stress during pregnancy–Associations with maternal anxiety disorders, infant cortisol reactivity, and mother–child interaction at pre-school age. Frontiers in Psychology, 10, 2179. https://doi.org/10.3389/fpsyg.2019.02179