Abstract
The COVID-19 pandemic and consequent lockdowns had a substantial impact on mental health. Distress and fatigue are highly correlated. However, little is known about the determinants of fatigue in the general population during the pandemic. This study aimed to examine the prevalence and predictors of fatigue during the COVID-19 pandemic in the UK population. Online surveys were completed by a UK community cohort in April 2020 (wave 1), July-September 2020 (wave 2) and November-December 2020 (wave 3). In total, 3097 participants completed the wave 1 survey, and 1385 and 1087 participants (85.4% women) completed wave 2 and 3 surveys respectively. Fatigue was assessed using the Chalder Fatigue Scale at waves 2 and 3. Hair samples were provided by 827 participants (90.6% women) at wave 1 and wave 2, which were analyzed to indicate HairE (stress hormone). The mean total fatigue score during wave 2 was 14.7 (SD = 4.7), significantly higher than pre-pandemic levels observed in the community (mean difference 0.50, p = .003). At wave 2, 614 (44.3%) participants met the case definition for fatigue, only 15.6% of whom indicated that fatigue lasted for more than 6 months (suggesting it had started prior to the pandemic). Predictors of fatigue at wave 3 included being in a risk group, depression and belief in having COVID-19, which explained 23.8% of the variability in fatigue scores. Depression at wave 1 was the only significant predictor of remaining a fatigue case at wave 3. Fatigue was highly prevalent in the UK community during the COVID-19 pandemic and limited people’s daily function. Depression and sociodemographic variables were significant predictors of fatigue.
HIGHLIGHTS
Fatigue levels between July-December 2020 were higher compared to pre-pandemic levels.
Predictors of fatigue levels 7-8 months later included being a clinical risk group, depression and belief in having had COVID-19.
HairE was not associated with fatigue.
Depression was the only significant predictor of remaining a fatigue case.
1. Introduction
COVID-19 was officially declared a pandemic on the 11th March 2020 (World Health Organization, Citation2020). This was followed by a number of protective measures in many countries to contain the virus, including mask-wearing, social distancing, and travel restrictions. Despite these and other measures, the pandemic resulted in unprecedented disruption to people’s daily lives, healthcare services and economies globally.
One of the more notable impacts of the pandemic was on mental health, with studies reporting an increase in depression, anxiety and stress (Jia et al., Citation2022; Xiong et al., Citation2020). Factors associated with increased psychological distress during the COVID-19 pandemic included female gender, younger age, unemployment, presence of chronic/psychiatric illnesses, loneliness and being at risk of COVID-19 complications (Hopf et al., Citation2022; Jia et al., Citation2020; Citation2022; Xiong et al., Citation2020).
Rises in cortisol levels were also observed during the COVID-19 pandemic (Haucke et al., Citation2022; Hopf et al., Citation2022; Rajcani et al., Citation2021). Hair cortisone (i.e. a metabolite of cortisol), specifically increased in greatest levels in those with a history of mental health difficulties and higher levels of stress (Jia et al., Citation2023).
Fatigue in the context of the COVID-19 pandemic has also received considerable interest, with studies reporting an increase in the prevalence of fatigue following COVID-19 infection (Joli et al., Citation2022; Poole-Wright et al., Citation2023). The psychological impact of the restrictions, can also increase fatigue. Cross-sectional studies found that disruption in daily life and restrictions due to the pandemic (e.g. social distancing) were positively associated with fatigue levels (Morgul et al., Citation2021; Yan et al., Citation2022). The COVID-19 pandemic was also associated with sleep disturbance (Jahrami et al., Citation2022) and psychological distress (Sun et al., Citation2021), both of which have been linked with increased fatigue (Alqahtani et al., Citation2022; Lee & Choi, Citation2022; Leung et al., Citation2022). Research also indicates that fatigue is associated with distress (Løke et al., Citation2022; Pawlikowska et al., Citation1994) and stress hormones, with a cross sectional study reporting that low salivary cortisol and a flat slope in diurnal cortisol were associated with fatigue (Kumari et al., Citation2009).
Nevertheless, little is known about the prevalence and predictors of fatigue in the general population during the COVID-19 pandemic. Existing research focuses either on fatigue subsequent to COVID-19 infection (Joli et al., Citation2022; Kedor et al., Citation2022; Stavem et al., Citation2021), or on “pandemic fatigue” (Abdul Rashid et al., Citation2023; Haktanir et al., Citation2022; Labrague & Ballad, Citation2021; Leung et al., Citation2022), which is described as a decline in adherence to lockdown rules and low motivation to follow protective behaviors (Haktanir et al., Citation2022; World Health Organization, Citation2020). To our knowledge, existing studies that assessed general fatigue during the COVID-19 pandemic used a cross-sectional design (John et al., Citation2022; Morgul et al., Citation2021; Yan et al., Citation2022) and exclusively recruited healthcare workers (Lee & Choi, Citation2022; Sagherian et al., Citation2020; Teng et al., Citation2020). These studies found that fatigue was highly prevalent and was associated with psychological distress and COVID-19 related preventive measures. However, due to their cross-sectional design, are less well able to explore potential determinants of fatigue.
Taken together, although evidence suggests that stress hormones, psychological distress and fatigue are closely related, prospective research examining these associations is scarce. The present prospective study investigated the prevalence and predictors of fatigue during the COVID-19 pandemic in the UK population. We hypothesized that (1) given the association of fatigue, with distress (Løke et al., Citation2022; Pawlikowska et al., Citation1994), cortisol (Kumari et al., Citation2009) and pandemic-related restrictions (Morgul et al., Citation2021; Yan et al., Citation2022), there will be a high prevalence of fatigue during July-December 2020; (2) demographic factors, stress hormones as well as psychological and situational factors (e.g. belief of having COVID-19) will predict fatigue levels during the COVID-19 pandemic.
2. Materials and methods
2.1. Study design and sample
The present paper draws on data collected as part of the COVID Stress and Health Study: a longitudinal cohort study examining the psychological and physical effects of the COVID-19 pandemic on the UK population. Details regarding the study design and recruitment have been described elsewhere (Jia et al., Citation2020). In brief, eligibility criteria stated that participants should be: aged 18 and over; able to give informed consent; able to read English and residing in the UK at the time of completing the survey. Recruitment occurred through a mainstream and social media campaign, keyworker professional bodies, and NHS trusts. The data presented here come from the first, second and third waves of data collection, collected between 3rd April 2020 and 30th April 2020 (wave 1), 1st July and 21st September 2020 (wave 2) and 11th November and 31st December 2020 (wave 3).
In the UK, the first national lockdown started on the 23rd March 2020, and was gradually relaxed from 11th May 2020, when the government allowed people to meet others from outside their household and reopened schools and non-essential retail venues. This remained until September 2020, aside from a local lockdown in Leicester in July. However, in November 2020, due to an increase in the number of infections and deaths, another national lockdown was declared, which continued until December 2020, when restrictions were gradually eased.
The authors assert that all procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects/patients were approved by University of Nottingham Faculty of Medicine and Health Sciences (ref: 506-2003) and the NHS Health Research Authority (ref: 20/HRA/1858). Written informed consent was obtained from all subjects/patients.
2.2. Procedure and measures
Participants completed an online survey during each study wave implemented through the JISC online survey platform. In Wave 1, the survey included questions relating to demographics, experience of COVID-19 related symptoms (i.e. persistent cough, fever, loss of taste, or loss of smell), belief of having had COVID-19 (“Do you believe you have had COVID-19 over the past 12 weeks?”) and COVID-19 tests, and social distancing behaviors. In addition, participants completed a variety of validated psychosocial measures including depression (Patient Health Questionnaire [PHQ-9]; (Kroenke et al., Citation2001); anxiety (General Anxiety Disorder-7 [GAD-7]; (Spitzer et al., Citation2006); stress (Perceived Stress Scale-4 [PSS-4]: (Cohen, Citation1988)), positive mood (Scale of Positive and Negative Experience [SPANE]; (Jovanović et al., Citation2020). Single items were used to measure perceived loneliness, perceived risk of COVID-19 complications, and whether respondents considered they were supporting other people (not including members of immediate family). In waves 2 and 3, participants completed the same measures, with the addition of the Chalder Fatigue Scale (Chalder et al., Citation1993) and a number of contextual questions relating to frequency and duration of fatigue and any related limitations on activities of daily living. The Chalder Fatigue Scale can be scored both in a Likert fashion (total range 0–33) or bimodally (range 0–11) with greater scores indicating greater fatigue. A score of ≥ 4 is considered a fatigue “case” using the bimodal method.
2.3. HairE measurement
The detailed procedure for hair sample collection and analysis is described elsewhere (Jia et al., Citation2023). In brief, hair samples were prepared for the cortisone assay following standard methods outlined by Gao et al. (Citation2013). A minimum of 7⋅5 mg of hair was obtained from each hair sample (≥ 3 cm) and samples shorter than 3 cm were not analyzed. Hair samples were analyzed to indicate HairE in the prior 3 months (Staufenbiel et al., Citation2015). Cortisone and cortisol present similar structure and are highly correlated (Stalder et al., Citation2012; Staufenbiel et al., Citation2015), whilst cortisone is less susceptible to contamination from products containing cortisol (Feeney et al., Citation2020; Raul et al., Citation2004; Wang et al., Citation2019).
2.4. Statistical analysis
Statistical analyses were performed using STATA (version 17). We first summarized fatigue across the cohort and compared mean fatigue levels to previously reported pre-pandemic levels in community settings (Cella & Chalder, Citation2010) using an independent samples t-test. Univariable linear regression analyses were used to examine the relationship between fatigue, HairE and COVID-related factors (belief of having had COVID-19 or having had a positive test for COVID-19; experience of COVID-19 related symptoms) prospectively (wave 1 responses predicting fatigue at waves 2 and 3). Hierarchical multivariable linear regression analyses were then conducted to examine whether any relationships between fatigue and COVID-related factors were independent of both demographic and psychosocial factors. To do this, demographic factors (age, gender, ethnicity, keyworker, clinical risk group [i.e. pregnant, elderly, diagnosed with a long-term health condition], living alone) were added to the COVID-related factors in step 1, followed by psychosocial factors (depression, generalized anxiety, stress, loneliness, positive mood) in step 2. Adjusted r-squared and pseudo r-squared statistics were examined to explore the explanatory value of the models.
Logistic regression analyses were performed to assess whether HairE, demographic, COVID-related and psychosocial factors at wave 1 predicted becoming or remaining a fatigue case at wave 3. Predictors of becoming a fatigue case at wave 3 were explored by only analyzing participants who were not a fatigue case at wave 2, whilst predictors of remaining a fatigue case was assessed by analyzing those who were a fatigue case at wave 2.
Assumptions of linear regression (normality and homoscedasticity of residuals, linearity with continuous variables) and presence of outliers were assessed graphically. Multicollinearity was assessed using variance inflation factors. Square root transformations were used for depression and anxiety scores to satisfy assumptions in regression analyses. Histograms showed that the distributions of mean hairE at wave 1 and wave 2 were not normally distributed, and thus log transformed scores for hairE were used the analysis.
3. Results
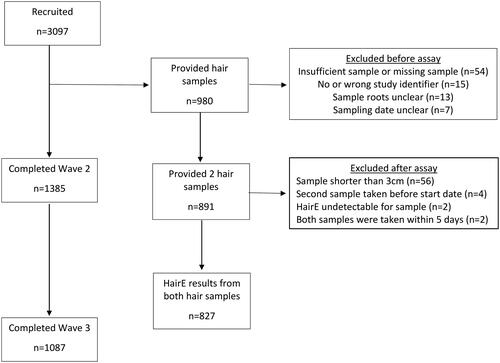
In total, 3097 eligible individuals participated in wave 1 of the study, with 1385 (44.7%) and 1087 (35.1%) completing the wave 2 and 3 surveys, respectively. 31% of our original cohort (n = 980) provided two hair samples. Of these, 89 (9%) participants were excluded for a variety of reasons, including insufficient or missing sample and unclear sample roots. The remaining 891 pairs of hair samples were assayed. Of these, 64 participants were excluded for different reasons, such as sample <3 cm and HairE being undetectable. Our final cohort consisted of 827 participants, 749 participants (90.6%) of whom were women, who provided two hair samples that were analyzed. The cohort flowchart is presented in .
shows the demographics of those participants who completed waves 2 (86.1% women) and 3 (85.4% women) of the study, as well as those who provided 2 hair samples for cortisone analysis.
Table 1. Cohort demographics at waves 2 and 3.
There were significant differences between individuals who completed both wave 1 and wave 2 surveys compared to those who only completed the wave 1 survey. These included greater dropout among younger respondents, people with BAME (i.e. Black, Asian and minority ethnic) background, keyworkers, men, and those with poorer mental health in wave 1. Full details of cohort differences can be seen in the supplementary appendix (Table S4).
3.1. Prevalence of fatigue (n = 1385)
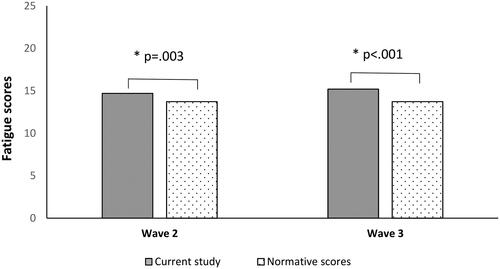
The mean total fatigue score as measured during wave 2 was 14.7 (SD = 4.7) and during wave 3 was 15.2 (SD = 5.0). An independent samples t-test indicated that fatigue scores at wave 2 [t(2998) = 2.94, p = .003, mean difference 0.50, 95% CI: 0.17, 0.84] and wave 3 [t(2700) = 5.35, p < .001, mean difference 1.00, 95% CI: 0.63, 1.37] were significantly higher than pre-pandemic levels observed in community settings (M = 14.2, SD = 4.6) (Cella & Chalder, Citation2010) (See ). For the physical fatigue subscale, the mean was 9.5 (SD = 3.4) and for the mental fatigue subscale the mean was 5.2 (SD = 1.8). Scored bimodally, the sample had a mean fatigue score of 3.5 (SD = 3.4) (wave 2) and 3.7 (SD = 3.5) (wave 3), with 614 (44.3%) and 511 (47%) participants meeting the case definition for fatigue (score ≥ 4) at waves 2 and 3 respectively. Only 428 of the 614 participants meeting the criteria for fatigue at wave 2 reported the duration of fatigue. Most of those (77.5%) (i.e. who met the criteria and indicated its duration), developed fatigue during the pandemic. At wave 2, 668 (48.2%) participants reported that fatigue had substantially limited one or more activities of daily living (including work, leisure, housework, self-care, study, exercise or family life) and 378 (27.3%) reported feeling fatigued more than 50% of the time. 556 (40.1%) participants indicated the fatigue they experienced did not get substantially better following rest. At wave 3, 969 (89.1%) participants reported that fatigue limited their daily activities, 373 (34.3%) reported feeling fatigued more than 50% of the time and 311 (28.6%) participants stated that fatigue did not substantially improve following rest.
3.2. Relationship between fatigue and belief of having COVID-19 or having a positive test for COVID-19
At wave 1, where testing was not yet widely available, only 9 (0.3%) respondents had received a positive test result for COVID-19 and 568 (18.3%) of respondents experienced at least 1 of the main COVID symptoms in the past 12 weeks (i.e. persistent cough, fever, loss of taste, or loss of smell). At wave 2, 18 respondents (1.3%) had received a positive test in the past 12 weeks and 108 (7.8%) of respondents experienced at least 1 of the classic COVID-19 symptoms in the past 12 weeks. At wave 3, only 24 (2.2%) responders reported experiencing at least 1 of the main COVID-19 symptoms in the past 12 weeks.
In prospective univariate analyses, participants who at wave 1 believed they had had COVID-19 reported higher levels of fatigue during wave 2 (B = 2.17, 95% CI: 1.51, 2.83, p < .001) and wave 3 (B = 2.43, 95% CI: 1.64, 3.22, p < .001) and were more likely to be a fatigue case than respondents who did not believe they had had COVID-19 at wave 2 (OR = 2.17, 95% CI: 1.63, 2.91, p < .001) and wave 3 (OR = 2.16, 95% CI: 1.56, 3.00, p < .001). However, belief of having COVID-19 alone only explained a relatively small proportion of variance in total fatigue scores (adj r2 = 0.03) and fatigue caseness (pseudo r2 = 0.01) at waves 2 and 3. Interestingly, testing positive for COVID-19 at wave 1 was not associated with fatigue scores (p = .58) or fatigue caseness at wave 2 (p = .25), possibly because only 9 people had received a positive test at wave 1. The association between testing positive for COVID-19 at wave 1 and fatigue scores (B = 5.77, 95% CI: .08, 11.47, p = .047) and fatigue caseness at wave 3 (OR = .88, 95% CI: .78, .99, p = .045) was on the border of statistical significance.
This pattern of results was also true in wave 2 cross-sectional analyses, for those who reported that they believed they had COVID-19 between waves 1 and 2 (n = 58) (total fatigue: B = 2.20, 95% CI: 0.97, 3.43, p < .001; caseness: OR = 1.82, 95% CI: 1.07, 3.11, p = .027).
Relatedly, those who experienced one or more of the “classic” COVID-related symptoms (persistent cough, fever, loss of taste or loss of smell) in the 12 weeks prior to wave 1, reported higher levels of fatigue at wave 2 (B = 2.01, 95% CI: 1.33, 2.68, p < .001) and wave 3 (B = 2.46, 95% CI: 1.65, 2.68, p < .001), and were more likely to be a fatigue case during wave 2 (OR = 2.06, 95% CI: 1.53, 2.77, p < .001) and 3 (OR = 2.05, 95% CI: 1.47, 2.87, p < .001) than those who did not experience these symptoms.
Cross-sectional models looking at only wave 2 and wave 3 data are presented in the supplementary file (Tables S1-S3).
Table 3. Multivariate linear regression model between sociodemographic and psychological variables at wave 1 fatigue cases at waves 2 and 3.
Table 4. Multivariate logistic regression model between variables at wave 1 and fatigue caseness at wave 3.
3.3. Predictors of fatigue at waves 2 and 3
Multivariable linear regression models were used to examine whether the observed relationships between COVID-related factors and fatigue remained independently significant when accounting for demographic and psychosocial factors (see ). Considering total fatigue score, in prospective analyses (W1 to W2 and W3), in the final model, being in a clinical risk group, depression scores, and belief in having had COVID-19 were independently significant predictors of greater fatigue at waves 2 and 3. Being a keyworker was a significant predictor of fatigue at wave 2 only. The largest proportion of variance was explained by the model that included depression, accounting for 21.6% of the variability in fatigue scores at wave 2 and 23.8% at wave 3. This is compared to 6% (W2) and 7% (W3) in the model including demographics and belief in having had COVID-19 alone. The same pattern was observed predicting fatigue caseness prospectively, at wave 2 and 3 (see ). Gender was a significant independent predictor only at wave 2—with women more likely to be a case.
Table 2. Multivariate linear regression model between sociodemographic and psychological variables at wave 1 and fatigue at waves 2 and 3.
Predictors of mental and physical fatigue subscales were also explored at waves 2 and 3, using the same variables. Belief of having had COVID-19 (B = .62, 95% CI: .38, .86, p < .001), age (B = .01, 95% CI: .00, .01, p = .043), depression (B = .49, 95% CI: .36, .62, p < .001), and generalized anxiety (B = .13, 95% CI: .01, .25, p = .032) at wave 1 predicted mental fatigue scores at wave 2. Significant wave 1 predictors of physical fatigue at wave 2, included belief of having had COVID-19 (B = .74, 95% CI: .29, 1.19, p = .001), being a keyworker (B = .44, 95% CI: .10, .78, p = .011), being in a clinical risk group (B = .94, 95% CI: .53, 1.36, p < .001) and depression (B = .88, 95% CI: .64, 1.12, p < .001).
Predictors of mental fatigue at wave 3 included belief in having had COVID-19 (B = .68, 95% CI: .39, .97, p < .001), depression (B = .47, 95% CI: .32, .63, p < .001), and stress (B = .06, 95% CI: .01, .11, p = .028) at wave 1. Predictors of physical fatigue at wave 3 were belief in having had COVID-19 (B = .87, 95% CI: .36, 1.38, p = .001), being in a clinical risk group (B = .57, 95% CI: .11, 1.04, p = .015), and depression (B = 1.00, 95% CI: .72, 1.27, p < .001) at wave 1. Loneliness was a non-significant predictor (B = .09, 95% CI: .00, .18, p = .047).
Depression at wave 1 was the only predictor of remaining a fatigue case at wave 3 (OR = 1.63, 95% CI: 1.13, 2.35, p = .008). Depression (OR = 1.42, 95% CI: 1.01, 1.99, p = .042) and age (OR = 0.98, 95% CI: 0.96, 0.99, p = .045) at wave 1 were the only significant predictors of becoming a fatigue case at wave 3.
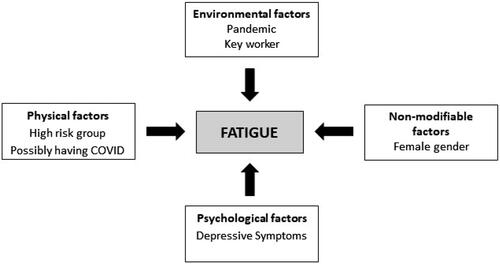
The different physical, environmental, demographic and psychological factors associated with fatigue are presented in .
3.4. Association between cortisone levels and fatigue scores
The mean HairE levels at wave 1 were 2.0 (SD = .75) and at wave 2 were 2.3 (SD = .75). HairE at wave 1 was not associated with fatigue scores or with being a fatigue case at wave 2 (p = .79 and p = .72, respectively) or wave 3 (p = .76 and p = .20, respectively). Similarly, HairE at wave 2 or change in HairE between wave 1 and wave 2 was not associated with fatigue scores (p = .73 and p = .93, respectively) or with being a fatigue case at T3 (p = .68 and p = .33, respectively). The association between HairE at wave 1 and fatigue duration at wave 2 or 3 was also not significant (p = .97 and p = .95, respectively).
Similarly, the association between fatigue and HairE did not reach statistical significance when analyzing only those in a high clinical risk group (i.e. diagnosed with cancer, severe asthma/COPD etc) (n = 16) (p = .29), or those who were a fatigue case and reported disabling fatigue lasting 6 months and more (n = 33) (p = .16).
HairE at wave 1 was significantly associated with fatigue not improving with rest at wave 2 (July-Sep) (OR = .79, p = .018). The association remained significant after controlling for baseline depression and age, however it became insignificant after gender was entered in the model (p = .058). This association was also explored in women only, but did not reach statistical significance after controlling for depression. HairE at wave 2 was not associated with fatigue not getting better at T3 (p > .05).
4. Discussion
The present prospective study aimed to explore the prevalence and predictors of fatigue during the COVID-19 pandemic in a large UK cohort (n = 3097). Findings indicate a high prevalence of fatigue during the COVID-19 pandemic, with 44.3% of the participants meeting the criteria for fatigue caseness. Fatigue significantly impaired people’s ability to perform their usual activities, such as work, exercise, housework, self-care, leisure and relationships. Predictors of fatigue at waves 2 and 3 included being in a clinical risk group (i.e. pregnant, elderly, diagnosed with a long-term health condition), depression scores and belief of having COVID-19. Being a keyworker (e.g. health, social care support worker, key public services worker; see ) also predicted greater levels of fatigue, but only at wave 2. Depression was the only significant predictor of remaining a fatigue case at wave 3. Contrary to our hypothesis, HairE was not associated with fatigue levels.
Our findings are consistent with cross-sectional studies conducted during 2020–2021 in India, Hong Kong, and Turkey that assessed the prevalence of fatigue. Although these studies used a different measure to assess fatigue (Fatigue Assessment Scale; [Michielsen et al., Citation2003]), they reported a prevalence rate of fatigue between 25.7% and 64.1% (John et al., Citation2022; Morgul et al., Citation2021; Yan et al., Citation2022).
The mean total fatigue score at waves 2 and 3 in our sample was significantly higher than pre-pandemic levels observed in the community (Cella & Chalder, Citation2010). Most participants who met the case criteria for fatigue and reported fatigue duration (77.5%) stated that fatigue lasted for less than 6 months, and therefore developed during the pandemic. These findings suggest that, to some extent, fatigue may be attributed to the context at the time, including COVID-19 lockdowns, COVID-19 infections and the impact of both on people’s lives. Cross-sectional studies indicate that greater disruption and restrictions in daily life due to the pandemic (e.g. avoidance of crowded places, isolation) were associated with increased fatigue (Morgul et al., Citation2021; Yan et al., Citation2022). In line with this, at waves 2 and 3, 28.6% and 40% of the participants respectively, mentioned that their fatigue did not improve following rest. This could be attributed to factors such as social isolation or lack of exercise as well as possible post COVID-infection sequelae, as fatigue following COVID-19 infection is a common symptom, which can persist for at least 12 months (Jahrami et al., Citation2022). The COVID-19 pandemic and lockdowns can be characterized as chronic stressors, which had a considerable impact on health and people’s financial situations (Gasteiger et al., Citation2021; Jia et al., Citation2022; Lee et al., Citation2021; Xiong et al., Citation2020). Stressors have previously been shown to dysregulate a range of physiological mechanisms (Yaribeygi et al., Citation2017), which could also subsequently influence fatigue. It is also possible that fatigue could be due to physiological changes following COVID-19 infection (Poole-Wright et al., Citation2023; Versace et al., Citation2021). Finally, there was a reduction in physical activity during lockdowns, especially among younger people (De La Vega et al., Citation2022; McCarthy et al., Citation2021), which may explain the high levels of fatigue. Nevertheless, this study only included a small number of people who had tested for COVID-19, and the association between testing positive and fatigue was on the border of statistical significance (p = .045).
Prospective analysis showed that depression was a significant predictor of fatigue scores and fatigue caseness 7-8 months later (wave 3; between November and December 2020). The association between fatigue and depression is confirmed by community-based studies (Galland-Decker et al., Citation2019; Pawlikowska et al., Citation1994). Cross-sectional studies conducted during the COVID-19 pandemic also indicate that depression was associated with fatigue in healthcare staff (Lee & Choi, Citation2022; Teng et al., Citation2020) and pandemic fatigue in the general population (Leung et al., Citation2022; Xin et al., Citation2022). Although women in our study were more likely to be a fatigue case at wave 2, this association was lost after depression was entered in the regression model. It is likely therefore that this association may be attributed to the higher prevalence of depression in women (Lim et al., Citation2018). The largest proportion of participants were women (90.6%), which also limited comparisons between genders.
Anxiety, stress and loneliness were not significant predictors of general fatigue, which contradicts findings from other studies (Doerr et al., Citation2015; Jaremka et al., Citation2014). However, in the present study, stress and generalized anxiety predicted mental fatigue specifically at waves 3 and 2, respectively. It may be possible that stress and anxiety are mainly associated with mental fatigue, which can in turn influence physical fatigue. It is also possible that chronic stressors (e.g. the COVID-19 pandemic) influence fatigue indirectly, through their association with factors such as depression.
HairE was not a predictor of fatigue, which is not in line with previous research reporting significant associations between stress hormones and fatigue. Previously, cross-sectional studies found that low salivary cortisol and a flat slop in diurnal cortisol were associated with fatigue in community dwelling adults (Kumari et al., Citation2009) and hair cortisone levels were associated with fatigue levels in people with HIV (Zhang et al., Citation2021). A systematic review also reported an attenuation of cortisol diurnal awakening response (CAR) in people with chronic fatigue syndrome (CFS) (Powell et al., Citation2013). Nevertheless, associations found in clinical populations (i.e. HIV, CFS) may not be generalizable to our sample. It is possible that CAR is associated with fatigue, whereas the total cortisone output is not, as it cannot capture the change in cortisol levels from waking to peak levels, which can be indicative of hypothalamic-pituitary-adrenal (HPA) axis dysfunction. The present study only measured hair cortisone, which is a metabolite of cortisol, and is considered an acceptable marker of free cortisol (Raul et al., Citation2004; Staufenbiel et al., Citation2015), but cannot assess CAR. HairE findings may also be dependent on the sensitivity of analyses conducted in the lab.
Environmental and physical factors, such as being a keyworker and a clinical risk group also predicted fatigue levels. Being a keyworker was associated with greater fatigue scores at wave 2, but not at wave 3. A significant proportion of the keyworkers included in this study were health, social care or support workers, who, especially during the early stages of the pandemic, were under significant physical and emotional strain (Sun et al., Citation2021). Nevertheless, longitudinal research suggests that a proportion of mental health professionals showed improvements in well-being during later stages of the pandemic (Kogan et al., Citation2023). This, combined with the greater drop out of those with poorer mental health in our study may explain the significant association found at wave 2 but not wave 3 data. Other studies also report high levels of fatigue among frontline staff during the first waves of the COVID-19 pandemic (Haktanir et al., Citation2022; Labrague & Ballad, Citation2021; Sun et al., Citation2021).
Although long-term health conditions have been associated with fatigue (Goërtz et al., Citation2021; Torossian & Jacelon, Citation2021), physical health comorbidities, or belief of having had COVID-19 in our sample only explained a small percentage of the variability in fatigue scores. The largest proportion of variance at wave 3 was explained when depression was added to the multivariate model (23.8%). This highlights the importance of psychological factors, especially depression in fatigue. The remaining variability in fatigue scores could be explained by other psychosocial or biological factors, such as sleep, resilience, comorbidities and dysregulation of physiological mechanisms. Fatigue is clearly a complex phenomenon which may be better understood using a holistic biopsychosocial approach.
4.1. Strengths and limitations
This study presents a few limitations that need to be considered when interpreting the findings. There was a high attrition rate, as surveys at wave 2 and 3 were completed only by 44.7% and 35.1% of the participants respectively; there was a greater dropout among younger respondents, keyworkers, men, and those with poorer mental health in wave 1. Although there was a large number of participants who completed all surveys (n = 1087), these differences could have influenced the findings. Another limitation was that fatigue was not measured during the first wave (April 2020) and therefore we were not able to compare fatigue levels between the first and second wave. Finally, data on sleep disturbance or physical activity, which can influence fatigue were not collected. The majority of participants in wave 3 were women (85.4%), which may limit the generalizability of findings. Individuals who provided two hair samples suitable for analysis were more likely to be female, older but also less stressed, anxious and depressed than the rest of the cohort. The gender differences may be explained by practical reasons involved in the collection of the hair samples, as being able to provide a hair sample at least 1 cm long was an eligibility criterion. This may have prevented men with shorter or no hair to take part in the study. Finally, other HPA axis markers, such as CAR were not assessed.
The large sample size and prospective design are the biggest strengths of the study. In addition, multiple psychosocial and demographic factors were explored as predictors of fatigue during the COVID-19 pandemic.
4.2. Clinical implications
Our findings highlight the importance of depression as a predictor of fatigue during the COVID-19 pandemic. Although interventions that target depression could improve fatigue outcomes, fatigue is a complex multi-factorial symptom which warrants a targeted biopsychosocial approach. A systematic review of randomized controlled trials suggested that moderate exercise can improve fatigue and energy levels (Wender et al., Citation2022).
5. Conclusion
This is the first prospective study to assess the prevalence and predictors of fatigue in the UK general population during the COVID-19 pandemic. Our findings indicate that fatigue was highly prevalent during 2020 and impacted people’s daily functioning. Predictors of fatigue at wave 3 (November-December 2020) included depression, being in a high-risk group, and belief of having COVID-19 in wave 1 (April 2020). Depression was the only significant predictor of remaining and becoming a fatigue case 7-8 months later. Nevertheless, HairE was not a predictor of fatigue. It is reasonable to deduce that chronic stressors such as the COVID-19 pandemic, have a significant psychological impact which is subsequently associated with increased fatigue levels.
Author contributions
K.A., R.J., K.V., C.C. and T.C. contributed to the study design. K.A., R.J. and K.V. were responsible for coordination and management of recruitment. K.A. and M.K. conducted the statistical analysis. K.A., M.K., R.J., C.C., K.V. and T.C. interpreted the data. K.A. and M.K. drafted the first version of manuscript. R.J., C.C., K.V. and T.C. substantially contributed to revisions of the final manuscript.
Supplemental Material
Download MS Word (26 KB)Acknowledgements
For the purposes of open access, the authors have applied a Creative Commons Attribution (CC BY) licence to any Accepted Author Manuscript version arising from this submission.
Disclosure statement
TC is the author of self-help books on chronic fatigue for which she has received royalties; has received ad hoc payments for workshops carried out in long-term conditions; has received travel expenses and accommodation costs of attending Conferences; is in receipt of grants from Guy’s and St Thomas’ Charity, NIHR and UKRI.
Data availability statement
Data will be deposited in the University of Nottingham data archive. Access to this dataset will be embargoed for a period of 12 months to permit planned analyses of the dataset. Afterward, it may be shared with the consent of the Chief Investigator.
Additional information
Funding
Notes on contributors
Michail Kalfas
Michail Kalfas MSc, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London, UK. Michael is interested in psychiatric conditions and in the interplay between physical and mental health.
Kieran Ayling
Kieran Ayling PhD, University of Nottingham, Nottingham, UK. Research interests: Interactions between psychological, behavioural, and biological factors as they pertain to health. Teaching: Kieran has supervised academic clinical fellows, MSc. Health Psychology project students and PhD students.
Ru Jia
Ru Jia PhD, Dr Ru Jia is a researcher working on behavioural interventions that help improve population health at the University of Oxford.
Carol Coupland
Carol Coupland PhD, University of Nottingham. Expertise: Medical statistics in primary care, statistical analysis of primary care research databases, design and analysis of epidemiological studies and cluster randomised trials.
Kavita Vedhara
Kavita Vedhara PhD. Health psychologist at Cardiff University with expertise in the inter-relationships between psychological factors and health and disease outcomes. Kavita’s research spans three main areas. The first is concerned with psychological influences on the body and this work has shown that negative moods such as stress and depression can make us more vulnerable to infections, including COVID19; can slow the healing of wounds, such as diabetic foot ulcers; and can also make vaccines work less well. The second area is concerned with psychological influences on behaviour and has explored the factors influencing people’s engagement with health interventions such as screening, mask wearing and vaccinations. The third area is concerned with interventions and how we can ‘harness’ the power of these psychological influences to improve the health and well-being of patients and the public.
Trudie Chalder
Trudie Chalder PhD, King’s College London. Research interests: Epidemiological and aetiological studies of fatigue and distress in adolescents and adults with long term conditions; evaluating efficacy of CBT.
References
- Abdul Rashid, M. R., Syed Mohamad, S. N., Tajjudin, A. I. A., Roslan, N., Jaffar, A., Mohideen, F. B. S., Addnan, F. H., Baharom, N., & Ithnin, M. (2023). COVID-19 pandemic fatigue and its sociodemographic, mental health status, and perceived causes: A cross-sectional study nearing the transition to an endemic phase in Malaysia. International Journal of Environmental Research and Public Health, 20(5), 1. https://doi.org/10.3390/ijerph20054476
- Alqahtani, J. S., AlRabeeah, S. M., Aldhahir, A. M., Siraj, R., Aldabayan, Y. S., Alghamdi, S. M., Alqahtani, A. S., Alsaif, S. S., Naser, A. Y., & Alwafi, H. (2022). Sleep quality, insomnia, anxiety, fatigue, stress, memory and active coping during the COVID-19 pandemic. International Journal of Environmental Research and Public Health, 19(9), 4940. https://doi.org/10.3390/ijerph19094940
- Cella, M., & Chalder, T. (2010). Measuring fatigue in clinical and community settings. Journal of Psychosomatic Research, 69(1), 17–12. https://doi.org/10.1016/j.jpsychores.2009.10.007
- Chalder, T., Berelowitz, G., Pawlikowska, T., Watts, L., Wessely, S., Wright, D., & Wallace, E. P. (1993). Development of a fatigue scale. Journal of Psychosomatic Research, 37(2), 147–153. https://doi.org/10.1016/0022-3999(93)90081-p
- Cohen, S. (1988). Perceived stress in a probability sample of the United States. In The social psychology of health. The Claremont Symposium on Applied Social Psychology (pp. 31–67). Sage Publications, Inc.
- De La Vega, R., Almendros, L. J., Barquín, R. R., Boros, S., Demetrovics, Z., & Szabo, A. (2022). Exercise addiction during the COVID-19 Pandemic: An international study confirming the need for considering passion and perfectionism. International Journal of Mental Health and Addiction, 20(2), 1159–1170. https://doi.org/10.1007/s11469-020-00433-7
- Doerr, J. M., Ditzen, B., Strahler, J., Linnemann, A., Ziemek, J., Skoluda, N., Hoppmann, C. A., & Nater, U. M. (2015). Reciprocal relationship between acute stress and acute fatigue in everyday life in a sample of university students. Biological Psychology, 110, 42–49. https://doi.org/10.1016/j.biopsycho.2015.06.009
- Feeney, J. C., O’Halloran, A. M., & Kenny, R. A. (2020). The association between hair cortisol, hair cortisone, and cognitive function in a population-based cohort of older adults: Results from the irish longitudinal study on ageing. The Journals of Gerontology, 75(2), 257–265. https://doi.org/10.1093/gerona/gly258
- Galland-Decker, C., Marques-Vidal, P., & Vollenweider, P. (2019). Prevalence and factors associated with fatigue in the Lausanne middle-aged population: A population-based, cross-sectional survey. BMJ Open, 9(8), e027070. https://doi.org/10.1136/bmjopen-2018-027070
- Gao, W., Stalder, T., Foley, P., Rauh, M., Deng, H., & Kirschbaum, C. (2013). Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. Journal of Chromatography, 928, 1–8. https://doi.org/10.1016/j.jchromb.2013.03.008
- Gasteiger, N., Vedhara, K., Massey, A., Jia, R., Ayling, K., Chalder, T., Coupland, C., & Broadbent, E. (2021). Depression, anxiety and stress during the COVID-19 pandemic: results from a New Zealand cohort study on mental well-being. BMJ Open, 11(5), e045325. https://doi.org/10.1136/bmjopen-2020-045325
- Goërtz, Y. M. J., Braamse, A. M. J., Spruit, M. A., Janssen, D. J. A., Ebadi, Z., Van Herck, M., Burtin, C., Peters, J. B., Sprangers, M. A. G., Lamers, F., Twisk, J. W. R., Thong, M. S. Y., Vercoulen, J. H., Geerlings, S. E., Vaes, A. W., Beijers, R. J. H. C. G., van Beers, M., Schols, A. M. W. J., Rosmalen, J. G. M., & Knoop, H. (2021). Fatigue in patients with chronic disease: Results from the population-based Lifelines Cohort Study. Scientific Reports, 11(1), 20977. https://doi.org/10.1038/s41598-021-00337-z
- Haktanir, A., Can, N., Seki, T., Kurnaz, M. F., & Dilmaç, B. (2022). Do we experience pandemic fatigue? Current state, predictors, and prevention. Current Psychology, 41(10), 7314–7325. https://doi.org/10.1007/s12144-021-02397-w
- Haucke, M., Golde, S., Saft, S., Hellweg, R., Liu, S., & Heinzel, S. (2022). The effects of momentary loneliness and COVID-19 stressors on hypothalamic-pituitary adrenal (HPA) axis functioning: A lockdown stage changes the association between loneliness and salivary cortisol. Psychoneuroendocrinology, 145, 105894. https://doi.org/10.1016/j.psyneuen.2022.105894
- Hopf, D., Schneider, E., Aguilar-Raab, C., Scheele, D., Morr, M., Klein, T., Ditzen, B., & Eckstein, M. (2022). Loneliness and diurnal cortisol levels during COVID-19 lockdown: the roles of living situation, relationship status and relationship quality. Scientific Reports, 12(1), 15076. https://doi.org/10.1038/s41598-022-19224-2
- Jahrami, H. A., Alhaj, O. A., Humood, A. M., Alenezi, A. F., Fekih-Romdhane, F., AlRasheed, M. M., Saif, Z. Q., Bragazzi, N. L., Pandi-Perumal, S. R., BaHammam, A. S., & Vitiello, M. V. (2022). Sleep disturbances during the COVID-19 pandemic: A systematic review, meta-analysis, and meta-regression. Sleep Medicine Reviews, 62, 101591. https://doi.org/10.1016/j.smrv.2022.101591
- Jaremka, L. M., Andridge, R. R., Fagundes, C. P., Alfano, C. M., Povoski, S. P., Lipari, A. M., Agnese, D. M., Arnold, M. W., Farrar, W. B., Yee, L. D., Carson, W. E., Bekaii-Saab, T., Martin, E. W., Schmidt, C. R., & Kiecolt-Glaser, J. K. (2014). Pain, depression, and fatigue: Loneliness as a longitudinal risk factor. Health Psychology, 33(9), 948–957. https://doi.org/10.1037/a0034012
- Jia, R., Ayling, K., Chalder, T., Massey, A., Broadbent, E., Morling, J. R., Coupland, C., & Vedhara, K. (2020). Young people, mental health and COVID-19 infection: The canaries we put in the coal mine. Public Health, 189, 158–161. https://doi.org/10.1016/j.puhe.2020.10.018
- Jia, R., Ayling, K., Chalder, T., Massey, A., Gasteiger, N., Broadbent, E., Coupland, C., & Vedhara, K. (2022). The prevalence, incidence, prognosis and risk factors for symptoms of depression and anxiety in a UK cohort during the COVID-19 pandemic. BJPsych Open, 8(2), e64. https://doi.org/10.1192/bjo.2022.34
- Jia, R., Ayling, K., Coupland, C., Chalder, T., Massey, A., Nater, U., Broadbent, E., Gasteiger, N., Gao, W., Kirschbaum, C., & Vedhara, K. (2023). Increases in stress hormone levels in a UK population during the COVID-19 pandemic: A prospective cohort study. Psychoneuroendocrinology, 148, 105992. https://doi.org/10.1016/j.psyneuen.2022.105992
- John, B., Marath, U., Valappil, S. P., Mathew, D., & Renjitha, M. (2022). Sleep pattern changes and the level of fatigue reported in a community sample of adults during COVID-19 pandemic. Sleep and Vigilance, 6(2), 297–312. https://doi.org/10.1007/s41782-022-00210-7
- Joli, J., Buck, P., Zipfel, S., & Stengel, A. (2022). Post-COVID-19 fatigue: A systematic review. Frontiers in Psychiatry, 13, 947973. https://doi.org/10.3389/fpsyt.2022.947973
- Jovanović, V., Lazić, M., Gavrilov-Jerković, V., & Molenaar, D. (2020). The Scale of Positive and Negative Experience (SPANE): Evaluation of measurement invariance and convergent and discriminant validity. European Journal of Psychological Assessment, 36(4), 694–704. https://doi.org/10.1027/1015-5759/a000540
- Kedor, C., Freitag, H., Meyer-Arndt, L., Wittke, K., Hanitsch, L. G., Zoller, T., Steinbeis, F., Haffke, M., Rudolf, G., Heidecker, B., Bobbert, T., Spranger, J., Volk, H.-D., Skurk, C., Konietschke, F., Paul, F., Behrends, U., Bellmann-Strobl, J., & Scheibenbogen, C. (2022). A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nature Communications, 13(1), 5104. https://doi.org/10.1038/s41467-022-32507-6
- Kogan, C. S., Garcia-Pacheco, J. A., Rebello, T. J., Montoya, M. I., Robles, R., Khoury, B., Kulygina, M., Matsumoto, C., Huang, J., Medina-Mora, M. E., Gureje, O., Stein, D. J., Sharan, P., Gaebel, W., Kanba, S., Andrews, H. F., Roberts, M. C., Pike, K. M., Zhao, M., … Reed, G. M. (2023). Longitudinal impact of the COVID-19 pandemic on stress and occupational well-being of mental health professionals: An international study. The International Journal of Neuropsychopharmacology, 26(10), 747–760. https://doi.org/10.1093/ijnp/pyad046
- Kroenke, K., Spitzer, R. L., & Williams, J. B. (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. https://doi.org/10.1046/j.1525-1497.2001.016009606.x
- Kumari, M., Badrick, E., Chandola, T., Adam, E. K., Stafford, M., Marmot, M. G., Kirschbaum, C., & Kivimaki, M. (2009). Cortisol secretion and fatigue: Associations in a community based cohort. Psychoneuroendocrinology, 34(10), 1476–1485. https://doi.org/10.1016/j.psyneuen.2009.05.001
- Labrague, L. J., & Ballad, C. A. (2021). Lockdown fatigue among college students during the COVID-19 pandemic: Predictive role of personal resilience, coping behaviors, and health. Perspectives in Psychiatric Care, 57(4), 1905–1912. https://doi.org/10.1111/ppc.12765
- Lee, E. P. X., Man, R. E. K., Gan, T. L. A., Fenwick, E. K., Aravindhan, A., Ho, K. C., Sung, S. C., Wong, T. Y., Ho, C. S. H., Gupta, P., & Lamoureux, E. L. (2021). The longitudinal psychological, physical activity, and financial impact of a COVID-19 lockdown on older adults in Singapore: The PIONEER-COVID population-based study. International Journal of Geriatric Psychiatry, 37(1), 45. https://doi.org/10.1002/gps.5645
- Lee, H., & Choi, S. (2022). Factors affecting fatigue among nurses during the COVID-19 Pandemic. International Journal of Environmental Research and Public Health, 19(18), 11380. https://doi.org/10.3390/ijerph191811380
- Leung, H. T., Gong, W.-J., Sit, S. M. M., Lai, A. Y. K., Ho, S. Y., Wang, M. P., & Lam, T. H. (2022). COVID-19 pandemic fatigue and its sociodemographic and psycho-behavioral correlates: A population-based cross-sectional study in Hong Kong. Scientific Reports, 12(1), 16114. https://doi.org/10.1038/s41598-022-19692-6
- Lim, G. Y., Tam, W. W., Lu, Y., Ho, C. S., Zhang, M. W., & Ho, R. C. (2018). Prevalence of depression in the community from 30 Countries between 1994 and 2014. Scientific Reports, 8(1), 2861. https://doi.org/10.1038/s41598-018-21243-x
- Løke, D., Løvstad, M., Andelic, N., Andersson, S., Ystrom, E., & Vassend, O. (2022). The role of pain and psychological distress in fatigue: a co-twin and within-person analysis of confounding and causal relations. Health Psychology and Behavioral Medicine, 10(1), 160–179. https://doi.org/10.1080/21642850.2022.2033121
- McCarthy, H., Potts, H. W. W., & Fisher, A. (2021). Physical activity behavior before, during, and after COVID-19 restrictions: Longitudinal Smartphone-tracking study of adults in the United Kingdom. Journal of Medical Internet Research, 23(2), e23701. https://doi.org/10.2196/23701
- Michielsen, H. J., De Vries, J., & Van Heck, G. L. (2003). Psychometric qualities of a brief self-rated fatigue measure: The Fatigue Assessment Scale. Journal of Psychosomatic Research, 54(4), 345–352. https://doi.org/10.1016/s0022-3999(02)00392-6
- Morgul, E., Bener, A., Atak, M., Akyel, S., Aktaş, S., Bhugra, D., Ventriglio, A., & Jordan, T. R. (2021). COVID-19 pandemic and psychological fatigue in Turkey. The International Journal of Social Psychiatry, 67(2), 128–135. https://doi.org/10.1177/0020764020941889
- Pawlikowska, T., Chalder, T., Hirsch, S., Wallace, P., Wright, D., & Wessely, S. (1994). Population based study of fatigue and psychological distress. BMJ, 308(6931), 763–766. https://doi.org/10.1136/bmj.308.6931.763
- Poole-Wright, K., Guennouni, I., Sterry, O., Evans, R. A., Gaughran, F., & Chalder, T. (2023). Fatigue outcomes following COVID-19: A systematic review and meta-analysis. BMJ Open, 13(4), e063969. https://doi.org/10.1136/bmjopen-2022-063969
- Powell, D. J., Liossi, C., Moss-Morris, R., & Schlotz, W. (2013). Unstimulated cortisol secretory activity in everyday life and its relationship with fatigue and chronic fatigue syndrome: a systematic review and subset meta-analysis. Psychoneuroendocrinology, 38(11), 2405–2422. https://doi.org/10.1016/j.psyneuen.2013.07.004
- Rajcani, J., Vytykacova, S., Solarikova, P., & Brezina, I. (2021). Stress and hair cortisol concentrations in nurses during the first wave of the COVID-19 pandemic. Psychoneuroendocrinology, 129, 105245. https://doi.org/10.1016/j.psyneuen.2021.105245
- Raul, J. S., Cirimele, V., Ludes, B., & Kintz, P. (2004). Detection of physiological concentrations of cortisol and cortisone in human hair. Clinical Biochemistry, 37(12), 1105–1111. https://doi.org/10.1016/j.clinbiochem.2004.02.010
- Sagherian, K., Steege, L. M., Cobb, S. J., & Cho, H. (2020). Insomnia, fatigue and psychosocial well-being during COVID-19 pandemic: A cross-sectional survey of hospital nursing staff in the United States. Journal of Clinical Nursing, 32(15-16), 5382–5395. https://doi.org/10.1111/jocn.15566
- Spitzer, R. L., Kroenke, K., Williams, J. B., & Löwe, B. (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166(10), 1092–1097. https://doi.org/10.1001/archinte.166.10.1092
- Stalder, T., Steudte, S., Miller, R., Skoluda, N., Dettenborn, L., & Kirschbaum, C. (2012). Intraindividual stability of hair cortisol concentrations. Psychoneuroendocrinology, 37(5), 602–610. https://doi.org/10.1016/j.psyneuen.2011.08.007
- Staufenbiel, S. M., Penninx, B. W., de Rijke, Y. B., van den Akker, E. L., & van Rossum, E. F. (2015). Determinants of hair cortisol and hair cortisone concentrations in adults. Psychoneuroendocrinology, 60, 182–194. https://doi.org/10.1016/j.psyneuen.2015.06.011
- Stavem, K., Ghanima, W., Olsen, M. K., Gilboe, H. M., & Einvik, G. (2021). Prevalence and determinants of fatigue after COVID-19 in non-hospitalized subjects: A population-based study. International Journal of Environmental Research and Public Health, 18(4), 2030. https://doi.org/10.3390/ijerph18042030
- Sun, P., Wang, M., Song, T., Wu, Y., Luo, J., Chen, L., & Yan, L. (2021). The psychological impact of COVID-19 pandemic on health care workers: A systematic review and meta-analysis. Frontiers in Psychology, 12, 626547. https://doi.org/10.3389/fpsyg.2021.626547
- Teng, Z., Wei, Z., Qiu, Y., Tan, Y., Chen, J., Tang, H., Wu, H., Wu, R., & Huang, J. (2020). Psychological status and fatigue of frontline staff two months after the COVID-19 pandemic outbreak in China: A cross-sectional study. Journal of Affective Disorders, 275, 247–252. https://doi.org/10.1016/j.jad.2020.06.032
- Torossian, M., & Jacelon, C. S. (2021). Chronic illness and fatigue in older individuals: A systematic review. Rehabilitation Nursing, 46(3), 125–136. https://doi.org/10.1097/rnj.0000000000000278
- Versace, V., Sebastianelli, L., Ferrazzoli, D., Romanello, R., Ortelli, P., Saltuari, L., D’Acunto, A., Porrazzini, F., Ajello, V., Oliviero, A., Kofler, M., & Koch, G. (2021). Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19. Clinical Neurophysiology, 132(5), 1138–1143. https://doi.org/10.1016/j.clinph.2021.03.001
- Wang, X., Busch, J. R., Banner, J., Linnet, K., & Johansen, S. S. (2019). Hair testing for cortisol by UPLC-MS/MS in a family: External cross-contamination from use of cortisol cream. Forensic Science International, 305, 109968. https://doi.org/10.1016/j.forsciint.2019.109968
- Wender, C. L. A., Manninen, M., & O’Connor, P. J. (2022). The effect of chronic exercise on energy and fatigue states: A systematic review and meta-analysis of randomized trials. Frontiers in Psychology, 13, 907637. https://doi.org/10.3389/fpsyg.2022.907637
- World Health Organization. (2020). Pandemic fatigue -Reinvigorating the public to prevent COVID-19. Retrieved from: https://apps.who.int/iris/bitstream/handle/10665/335820/WHO-EURO-2020-1160-40906-55390-eng.pdf
- World Health Organization. (2020). WHO Director-General’s opening remarks at the media briefing on COVID-19. WHO.
- Xin, L., Wang, L., Cao, X., Tian, Y., Yang, Y., Wang, K., Kang, Z., Zhao, M., Feng, C., & Wang, X. (2022). Prevalence and influencing factors of pandemic fatigue among Chinese public in Xi’an city during COVID-19 new normal: a cross-sectional study. Public Health Front, 10, 971115. https://doi.org/10.3389/fpubh.2022.971115
- Xiong, J., Lipsitz, O., Nasri, F., Lui, L. M. W., Gill, H., Phan, L., Chen-Li, D., Iacobucci, M., Ho, R., Majeed, A., & McIntyre, R. S. (2020). Impact of COVID-19 pandemic on mental health in the general population: A systematic review. Journal of Affective Disorders, 277, 55–64. https://doi.org/10.1016/j.jad.2020.08.001
- Yan, E., Ng, H. K. L., Lai, D. W. L., & Lee, V. W. P. (2022). Physical, psychological and pandemic fatigue in the fourth wave of COVID-19 outbreak in Hong Kong: population-based, cross-sectional study. BMJ Open, 12(12), e062609. https://doi.org/10.1136/bmjopen-2022-062609
- Yaribeygi, H., Panahi, Y., Sahraei, H., Johnston, T. P., & Sahebkar, A. (2017). The impact of stress on body function: A review. EXCLI Journal, 16, 1057–1072. https://doi.org/10.17179/excli2017-480
- Zhang, Q., Li, X., Qiao, S., Liu, S., Shen, Z., & Zhou, Y. (2021). Association between hair cortisol, hair cortisone, and fatigue in people living with HIV. Stress, 24(6), 772–779. https://doi.org/10.1080/10253890.2021.1919616