Abstract
Chronic stress leads to hypofunction of the medial prefrontal cortex (mPFC), mechanisms of which remain to be determined. Enhanced activation of GABAergic of parvalbumin (PV) expressing interneurons (INs) is thought to play a role in stress-induced prefrontal inhibition. In this study, we tested whether chemogenetic inhibition of mPFC PV INs after chronic stress can rescue chronic stress-related behavioral and physiological phenotypes. Mice underwent 2 weeks of chronic variable stress (CVS) followed by a battery of behavioral tests known to be affected by chronic stress exposure, e.g. an open field (OF), novel object recognition (NOR), tail suspension test (TST), sucrose preference test (SPT), and light dark (LD) box. Inhibitory DREADDs were actuated by 3 mg/kg CNO administered 30 min prior to each behavioral test. CVS caused hyperactivity in the OF, reduced sucrose preference in the SPT (indicative of enhanced anhedonia), and increased anxiety-like behavior in the LD box. Inhibition of PV IN after stress mitigated these effects. In addition, CVS also resulted in reduced thymus weight and body weight loss, which were also mitigated by PV IN inhibition. Our results indicate that chronic stress leads to plastic changes in PV INs that may be mitigated by chemogenetic inhibition. Our findings implicate cortical GABAergic INs as a therapeutic target in stress-related diseases.
Introduction
Alterations in GABAergic circuitry and prefrontal hypoactivity are associated with generation of depression in humans as well as depression-relevant behaviors in rodent chronic stress models (Hasler et al., Citation2007; Luscher et al., Citation2011; Duman, Citation2014; McKlveen et al., Citation2016). Enhanced GABAergic transmission in the mPFC is observed following chronic stress (e.g. increased inhibitory synaptic drive, increased expression of GABA-ergic markers) , suggesting that increased interneuron (IN) activity may be involved in disruption of prefrontal cortical signaling and behavioral adaptations (McKlveen et al., Citation2016; Shepard et al., Citation2016).
GABAergic parvalbumin interneurons (PV INs) gate prefrontal pyramidal neuron output by peri-somatic inhibition (Cardin et al., Citation2009; Courtin et al., Citation2014; Tremblay et al., Citation2016; Ghosal et al., Citation2017). PV INs are well-positioned to maintain appropriate excitatory/inhibitory (E/I) balance required for efficient mPFC (encompassing prelimbic and infralimbic cortex) signaling, and thus play an important role in stress-mediated cortical hypofunction (Rymar and Sadikot, Citation2007; Sherwood et al., Citation2007; Cardin et al., Citation2009; Courtin et al., Citation2014; Tremblay et al., Citation2016; Ferguson and Gao, Citation2018). Chronic stress-induced Increases in expression of PV and enhancement of glutamatergic transmission onto PV INsare both associated with prefrontal hypofunction and anxiogenesis (Shepard et al., Citation2016; Page et al., Citation2019). Reduction in PV expression in mPFC is associated with antidepressant efficacy, and chemogenetic inhibiton of PV INs in the mPFC inhibits stress-induced immobility in the forced swim and reduces novelty suppressed feeding tests in mice (Ohira et al., Citation2013; Zhou et al., Citation2015; Page and Coutellier, Citation2019; Fogaca et al., 2021). Our recent work strongly suggest that inhibition of PV INs during chronic stress attenuates the impact of CVS in male mice (Nawreen et al., Citation2020). It is yet to be determined whether inhibition of PV neurons can mitigate chronic stress phenotypes after they have already been established.
This study was designed to investigate the role of prefrontal PV INs in driving lasting behavioral and physiological sequelae of CVS. Here, we inhibited PV INs in the mPFC prior to behavioral testing following CVS to test whether inhibition can rescue chronic stress-associated behavioral abnormalities. Our results indicate that inhibition of PV INs after CVS ameliorates CVS-induced hyperactivity in open field (OF), anhedonia in sucrose preference test (SPT), and anxiety-like behavior in light dark (LD) box. Additionally, we show that inhibition of PV INs can improve CVS-induced physiological sequelae, such as thymic atrophy and body weight loss. These data suggest that prefrontal PV INs play a role in driving behavioral and somatic adaptations following with chronic stress and supports the growing body of literature implicating PV INs in stress-related illnesses.
Materials and methods
Experimental design
The experiment was setup as a 2 × 2 study design, with stress (CVS or No CVS) and DREADD (hM4Di or control) as factors, with a sample size of n = 10 per group (see for experimental design and timeline).
Mice
Male breeders from BL6 PV-Cre knock-in homozygous mice line (Pvalbtm1(cre)Arbr, JAX stock# 017320, Jackson Laboratories Bar Harbor, ME) were bred with WT C57BL/6J females (JAX stock# 000664) to generate an in-house colony of heterozygous PV-Cre C57BL/6J at the University of Cincinnati animal housing facility. The PV-Cre mice line used in this study has been extensively validated in our lab and a in prior publication (Nawreen et al., Citation2020). Mice were maintained under standard conditions (12/12 h light/dark cycle, 22 ± 1 °C, food, and water ad libitum; 4 mice per cage) in accordance with the University of Cincinnati’s Institutional Animal Care and Use Committee, which approved all acute and chronic stress regimens employed in this proposal. All experiments were performed on adult male mice (∼4.5 months of age at surgery).
Stereotaxic viral vector injection with AAV vectors
Prior study from the lab has demonstrated that the Cre in the PV Cre mice is expressed specifically in PV INs and can be used to actuate inhibitory DREADDs following CNO infusion (Nawreen et al., Citation2020). PV-Cre mice were anesthetized with isoflourane, their scalps shaved and animals placed in a stereotaxic frame. The incision site was disinfected using chlorohexidine and 70% ethanol. An incision at the midline was made using a single-edged blade. Cre-dependent adeno-associated virus 2 (AAV2) vectors AAV2-hsyn-DIO-hM4D(Gi)-mCherry (Gift from Bryan Roth; Addgene viral prep # 44362-AAV2) and AAV2-hsyn-DIO-mcherry (Gift from Bryan Roth; Addgene viral prep # 50459-AAV2) were injected bilaterally at a volume of 300 nL (∼1012 genome copies/mL) into the mPFC. The coordinates used were as follows: (anterior/posterior range defined as +1.8 mm anterior to bregma, medial–lateral range defined as ± 0.2 mm lateral to the midsagittal suture; dorsal–ventral range defined as −2.9 mm ventral to skull (Paxinos and Franklin, Citation2008). For viral injections the needle was slowly lowered to 0.1 mm beyond the required dorsal-ventral coordinate and quickly pulled up to the original coordinate to create a pocket for injection. This was done to prevent backflow of the drug and undesirable spread into neighboring areas (Mukherjee and Caroni, Citation2018). Viruses were infused using a 2 uL Hamilton syringe at a rate of 60 nL/min for 5 min. Following infusion, the injector was kept at the site for 8 minutes to allow for the virus to diffuse. The injection site was covered with gel foam and the incision site was sutured. 2.5 mg/kg Meloxicam was administered for 3 d following surgery. Behavioral studies and stress protocols were initiated 3 weeks post injection to allow sufficient time for viral expression. A separate cohort of 8 animals was also injected with the AAV2 hM4Di (with incubation time of 3 weeks) for validation of DREADD efficacy ().
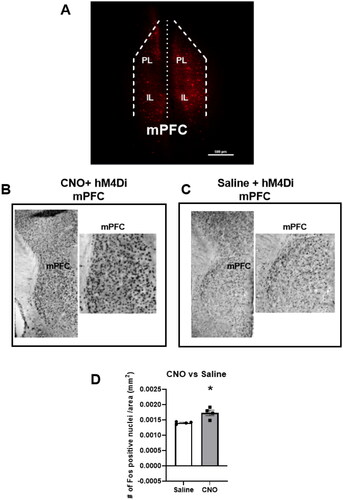
Figure 1. DREADD injection is restricted to the mPFC and CNO increases Fos activation in the mPFC of hM4Di mice.
Chronic variable stress (CVS) procedure
During the CVS procedure, mice were subjected to a series of randomly presented stressors administered twice daily over a period of 14 d, including overnight stressors. The unpredictable stressors used were as follows: restraint (1 h), swim stress (10 min), cold room exposure (45 min, 4 °C), shaker stress (1 h, 100 rpm), hypoxia (30 min, 8% oxygen, and 92% nitrogen), shallow water (30 min), strobe light (3 h), wet bedding (overnight), and cage tilt (overnight, 45°). The stress regimen was identical for all animals. Control animals were handled daily and not subjected to any additional stress.
Drug administration
Clozapine N-oxide (CNO, NIMH Chemical synthesis and Drug Supply Program) was used as the DREADD actuator to activate the inhibitory DREADD. CNO was dissolved in 5% dimethylsulfoxide (Sigma, St. Louis, MO) and then diluted with 0.9% saline and administered intraperitoneally at a dose of 3 mg/kg before all the behavioral procedures. All experimental groups received CNO prior to behavioral assessments.
Behavioral assessments
Open field test
Of 24 h after cessation of the last stressor in the CVS paradigm, animals were placed in a plastic OF apparatus measuring 44 cm in length × 23 cm in width. Mice were dosed with 3 mg/kg CNO 30 min prior to start of the OF. Mice were allowed to explore the arena for 10 min in ambient room light. Total distance traveled and velocity were measured using video tracking system (EthovisionXT-Noldus).
Novel object recognition
In the first familiarization trial phase of the novel object recognition (NOR), mice were placed in a plastic arena (same arena as the OF) with two identical plastic Lego™ tree or Lego™ block secured to the bottom of the arena. Mice were given 10 min to explore the arena and objects. After the familiarization session, animals were returned back to its home cage. After a 4 h interval (Taglialatela et al., Citation2009), mice were placed in the arena for 10 min with one block and one tree, counterbalanced to prevent bias (test phase). The time spent exploring each of these objects was measured and a discrimination index was calculated as follows: difference between time spent exploring the tree or the block divided by the total time spent exploring the tree and the block. Object exploration was defined as orientation of the nose toward the object within 2–3 cm, with active vibrissae sweeping or sniffing (Lueptow, Citation2017).
Tail suspension test
The TST (Can et al., Citation2012) was conducted to observe the effects of inhibiting PV INs on passive coping behavior. Mice were suspended 55 cm above ground using a 17 cm long tape that was attached to a suspension bar, for a total time period of 6 min. Sessions were video-recorded from the side to allow full body visualization of mice behaviors-active coping (struggling) behavior, which comprised strong shaking of the body and movement of all 4 limbs, and passive coping (immobility) behavior which comprised not making any active limb movements. Behaviors were quantified by an experimenter blinded to the group assignments using behavioral scoring software Kinoscope version 3.0.4, San Diego, CA . Behaviors during the 6 min block were reported.
Sucrose preference test
Of 24 h after commencement of the TST, mice were single-housed and given access to two identical water bottles for 48 h, one filled with regular drinking water and one with sucrose. Food was provided ad libitum. Bottles for water and sucrose were weighed at the start. Every 8 h, the position of the water and sucrose was changed. After 48 hs of training, SPT was conducted. Two hours after administration of 3 mg/kg CNO, the amount of sucrose and water consumed was quantified to calculate SPT: (mL sucrose consumed/(mL sucrose consumed + mL water consumed))*100. Following completion of the SPT mice were again group housed.
Light dark box
LD box testing was performed as previously described (Bedse et al., Citation2017). Mice were individually placed into the light side of the chambers (42 cm × 42 cm) containing dark box inserts that split the chamber into light (350 lux) and dark (5 lux) halves. Position and behavior were monitored as described above for 7 min using EthovisionXT-Noldus. Amount of time spent in the light and dark side and latency to enter the dark side were measured.
Euthanasia and tissue collection
Mice were euthanized with an overdose of sodium pentobarbital after the LD box, and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.01 M phosphate buffer (PBS), pH 7.4. Brains were removed and post-fixed in 4% paraformaldehyde at 4 °C for 24 h, then transferred to 30% sucrose in 0.01 MPBS at 4 °C until processed. Thymi and adrenal glands were collected, cleaned, and weighed from all animals.
Tissue preparation
Brains were sectioned into 30 μm coronal sections using a freezing microtome (−20 °C). Sections were collected into 12 wells (1/12) containing cryoprotectant solution (30% Sucrose, 1% Polyvinyl-pyrolidone (PVP-40), and 30% Ethylene glycol, in 0.01 M PBS). 0.01 M PBS was used to rinse brain slices before each treatment described below.
Injection site and viral spread
To determine if virus spread was restricted to the mPFC, immunohistochemistry was performed as previously described (Nawreen et al., Citation2020). Free-floating sections were incubated in 1% Sodium Borohydride for 20 min and then in 3% hydrogen peroxide in PBS for 20 min. After that, slices were incubated in blocking solution (NGS, 0.3% TritonX-100, 0.2% bovine serum albumin (BSA) in 0.01 M PBS) for 1 h. The brain sections were then incubated with a rabbit anti-mCherry (1:500, Abcam, Cambridge, MA, ab167453) for 2 h, followed by visualization with donkey anti-rabbit Cy3 conjugate (1:500, Invitrogen, Carlsbad, CA, A10520). Images were acquired using Nikon Confocal Microscope at 5X magnification. Brain bregma coordinates used for mPFC cytoarchitecture were defined in the Franklin and Paxinos mouse brain atlas.
Validation of DREADD efficacy
To determine the efficacy of the inhibitory DREADD, neuronal Fos activation was measured following injection of CNO or saline in a separate cohort of animals expressing the AAV2-hsyn-DIO-hM4D(Gi)-mCherry (Gift from Bryan Roth; Addgene viral prep # 44362-AAV2) in the mPFC. Free-floating sections were incubated in 1% Sodium Borohydride for 20 min and then in 3% hydrogen peroxide in PBS for 20 min. After that, slices were incubated in blocking solution (NGS, 0.3% TritonX-100, 0.2% BSA in 0.01 M PBS) for 1 h. Sections were then incubated with Fos rabbit polyclonal antibody (1:200, Santa Cruz, Dallas, TX, sc-52) in blocking solution overnight and was followed by incubation in secondary antibody (biotinylated goat anti-rabbit, (1:400; Vector Laboratories, Burlingame, CA, BA1000) in blocking solution for 1 h the next day. Sections were then treated with avidin-biotin horseradish peroxidase complex (1:800 in 0.01 M PBS; Vector Laboratories, PK6100) for 1 h and then developed with an 8 min incubation in DAB-Nickel solution: 10 mg 3,3′-diaminobenzidine (DAB) tablet (Sigma, DF905), 0.5 mL of a 2% aqueous nickel sulfate solution, 20 uL of 30% hydrogen peroxide in 50 mL of 0.01 M PBS. Sections were mounted on superfrost slides (Fisherbrand, Fisher, Waltham, MA), allowed to dry, dehydrated with xylene, and then coverslipped with DPX mounting medium (Sigma). Images were acquired using microscope Carl Zeiss Imager Z1 at a 5X objective. For analysis, we counted minimum of 3 bilateral sections per brain region/animal covering the prefrontal cortex (Bregma 2.10–1.34 mm) as defined in the Paxinos and Franklin atlas (Paxinos and Franklin, Citation2008). The number of Fos positive nuclei was counted using a semi-automated analysis macro in the Image J software package (National Institutes of Health, Bethesda, MD). The macro was generated using the “Analyze Particle” tool, with a defined common level of background intensity, nuclei circularity and size (previously validated manually). The relative density of the population of immunopositive cells was calculated by dividing the number of Fos positive cells by the respective brain area.
Statistical analysis
Statistical analyses for the behavioral measurements were performed using a two-way ANOVA with stress (No CVS, CVS) and DREADD (Control virus, hM4Di) as main factors. Tukey’s post-hoc test was performed in cases with significant interaction between factors. Because specific hypotheses were formed a priori on the effects of CVS within groups, planned comparisons using Fisher’s least significant difference (LSD) were performed in cases with no significant interaction effect. Data were analyzed by Graph Pad Prism version 8.1.2 (GraphPad Software, La Jolla, CA). Outliers were detected using the Grubbs’ test (GraphPad Software) and removed from analysis. After exclusion of outliers, data were assessed for normal distribution (Shapiro–Wilk) and appropriate parametric and/or non-parametric tests used. Data are presented as mean ± SEM with statistical significance set at p ≤ 0.05.
Results
CNO Gi DREADD actuation enhances Fos activation within the mPFC.
is a representative image from an animal demonstrating restriction of the virus injection to the mPFC indicated by the red mCherry staining. Our previous study extensively validated that the cells expressing the DREADD are indeed PV INs (Nawreen et al., Citation2020). We wanted to make sure that CNO was able to activate the hM4Di DREADD in inhibitory PV INs, resulting in decreased inhibition on nearby neurons and an increase in neural activation in the mPFC as measured with expression of the immediate early gene c-fos (Rogan and Roth, Citation2011; Soumier and Sibille, Citation2014). A separate cohort of PV Cre mice was injected with the AAV2 hM4Di construct and dosed with either saline or 3 mg/kg CNO (n = 4 animals/group). Compared with saline, mice dosed with CNO showed a significant increase in Fos activation in the mPFC (t = 2.8, df = 6, p = 0.04; ), consistent with inhibition of inhibitory INs responsible for limiting baseline pyramidal cell activity (Soumier and Sibille, Citation2014).
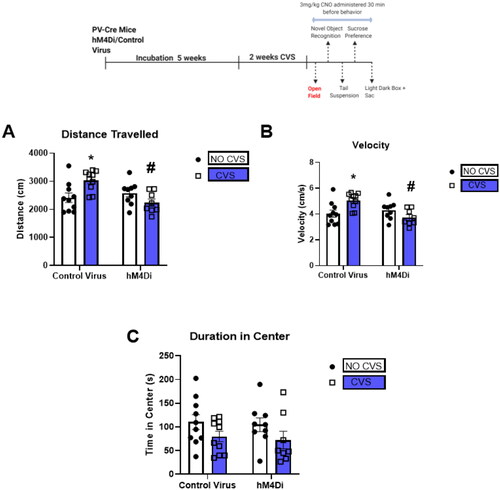
CVS causes hyperactivity and PV IN inhibition mitigates the effect
We subsequently assessed OF behavior 24 h after cessation of CVS. Our purpose was to determine the effects of CVS and subsequent inhibition of PV INs on locomotor behavior. CVS increased total distance traveled, velocity, and PV IN inhibition after CVS mitigated this effect. Two-way ANOVA of total distance traveled showed a significant stress × DREADD interaction [F(1,32) = 10.1; p = 0.003; ]. Tukey’s post hoc test indicated a significant increase in total distance traveled in the CVS group (*indicate p < 0.05 compared with Control No CVS) that was prevented by PV IN inhibition (# indicate p < 0.05 compared with Control CVS). Two-way ANOVA of velocity showed a significant stress × DREADD interaction [F(1,32) = 10.1; p = 0.003; ]. Tukey’s post hoc test indicated a significant increase in velocity in the CVS group (* indicate p < 0.05 compared with Control No CVS) that was again decreased by PV IN inhibition (# indicate p < 0.05 compared with Control CVS). No significant difference in duration of time in center was observed ().
Figure 2. CVS increases locomotion in the open field test and PV IN inhibition mitigates the effect.
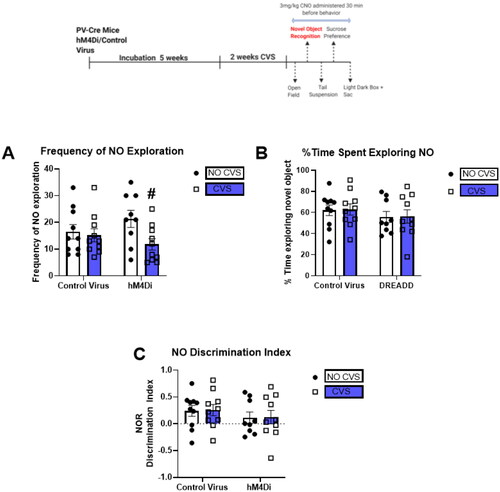
CVS and PV IN inhibition do not affect novel object recognition.
24 h after cessation of the OF, animals underwent NOR test to determine the effects of CVS and PV IN inhibition on short-term memory. No significant differences in the NO discrimination index () or % time spent exploring novel object () were observed in any of the groups tested. Two-way ANOVA on the Frequency of NO exploration indicated a main effect of CVS only [F(1,35) = 4.3; p = 0.04; ].
Figure 3. CVS and PV IN inhibition do not affect novel object recognition (NOR).
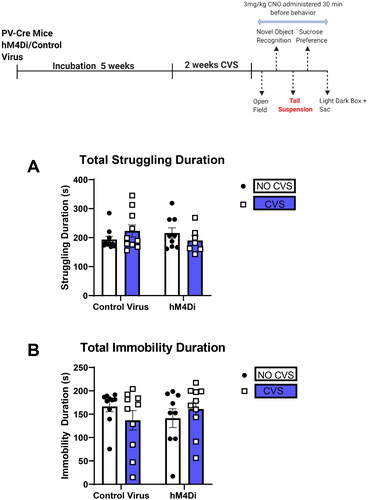
CVS and PV IN inhibition does not affect immobility in the tail suspension test
24 h after cessation of the NOR test, animals underwent tails suspension test (TST). No significant differences in any of the groups were observed ().
Figure 4. CVS and PV IN inhibition do not affect immobility in the tail suspension test
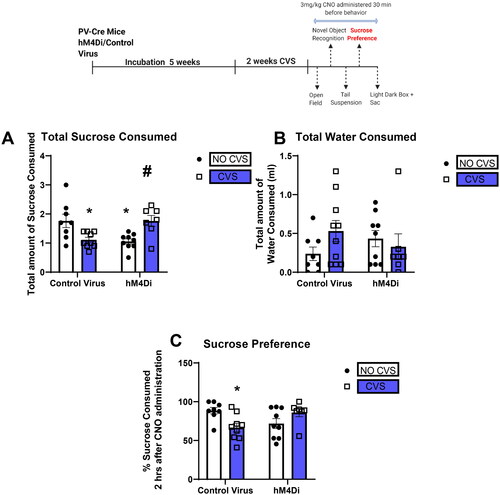
PV IN inhibition attenuates effects of CVS on sucrose preference
The SPT was conducted 24 h after the TST. There was a significant reduction in the total amount of sucrose consumed and sucrose preference in the CVS group, an effect that was prevented by PV IN inhibition. Two-way ANOVA of total amount of sucrose consumed showed a significant stress X DREADD interaction [F(1,29) = 19.2; p = 0.0001; ]. Tukey’s post hoc test showed CVS significantly reduces total sucrose intake (* indicate p < 0.05 compared with Control No CVS) an effect that was prevented by PV IN inhibition (p > 0.05 compared with Control No CVS; # indicate p < 0.05 compared with Control CVS). Interestingly, PV IN inhibition alone without CVS also reduced total sucrose consumption (* indicate p < 0.05 compared with Control No CVS). Analysis of sucrose preference revealed a significant stress X DREADD interaction [F(1,29) = 10.55; p = 0.002; ]. Tukey’s post hoc test showed CVS significantly reduced sucrose preference (* indicate p < 0.05 compared with Control No CVS) an effect that was prevented by PV IN inhibition (p > 0.05 compared with Control No CVS). No effects were seen on water consumed in any of the groups tested ().
Figure 5. PV IN inhibition attenuates effects of CVS on sucrose preference
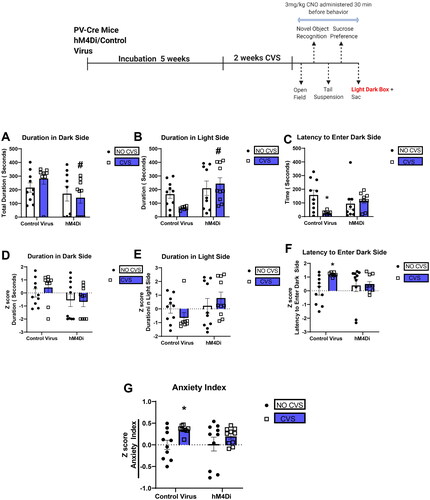
PV IN inhibition attenuates CVS-induced anxiety-like behavior in the light-dark box
The LD box was used to assess anxiety-like behavior following CVS. Two-way ANOVA showed a significant effect of DREADD for the duration of time spent in the dark side [F(1,33) = 4.6; p = 0.04; ]. Planned comparisons revealed the hM4Di CVS group spent significantly less time in the dark side compared with Control CVS group (p = 0.03). Analysis of duration in the light side showed a significant main effect of DREADD [F(1,32) = 7.7; p = 0.009; ]. Planned comparisons revealed the hM4Di CVS group spent significantly more time in the light side compared with Control CVS group (p = 0.004). There was a significant DREADD X CVS interaction in the latency to enter the dark side [F(1,32) = 6.6; p = 0.02; ]. Tukey’s post hoc test revealed only the Control CVS group to be significantly different from the Control No CVS group (p < 0.05) and PV IN inhibition prevented that effect (p > 0.05 for DREADD CVS group compared with Control No CVS). To investigate the consistency of behavioral performance in the LD box, we performed z-score analysis using the mean value of the control group as baseline (Guilloux et al., Citation2011). Average of all the z scores was used to calculate an anxiety index. Two-way ANOVA revealed a significant main effect of CVS for the Anxiety Index [F(1,35) = 6.4; p = 0.02; ]. Planned comparisons revealed the Control CVS group had a higher anxiety index compared with the Control No CVS group (p < 0.05) and PV IN inhibition after CVS mitigated that effect (p > 0.05 DREADD CVS compared to Control No CVS).
Figure 6. PV IN inhibition attenuates CVS-induced anxiety-like behavior in the light-dark box.
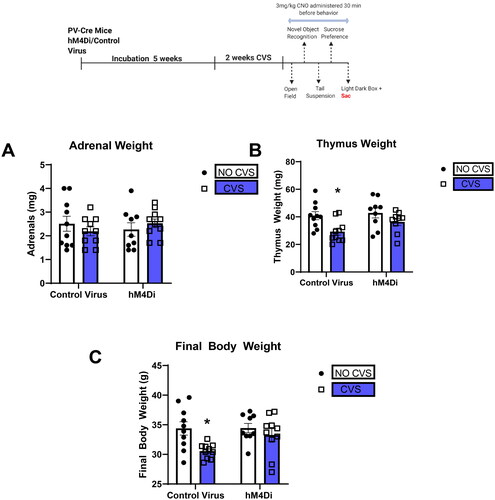
PV IN inhibition attenuates CVS-induced decreases in thymus weight and body weight gain
Organs and body weights were used to assess somatic effects of CVS. Adrenal hypertrophy and/or thymic atrophy are often observed following chronic stress and are used as indicators of repeated/chronic hypothalamic pituitary adrenal (HPA) axis activation. Here, there was a main effect of CVS [F(1,34) = 9.6; p = 0.004; ] on thymus weights. Planned comparisons indicated that CVS Control group had significantly decreased thymus weight compared to No CVS Control group (p = 0.006), which was prevented by CVS DREADD when compared to No CVS Control group (p = 0.3). Decreased body weight gain was also observed following chronic mild stress exposure in mice (Ghosal, Packard, et al., Citation2017). We observed a significant main effect of stress on final body weight [F(1,35) = 7.8; p = 0.008; ] consistent with known effects of CVS on body weight gain. Planned comparisons revealed final body weight in CVS group to be significantly lower than No CVS Control group (p = 0.02), which was attenuated by CVS hM4Di when compared to No CVS Control group (p = 0.8). No effects were seen on adrenal weight in any of the groups tested ().
Figure 7. PV IN inhibition attenuates CVS-induced decreases in thymus weight and body weight gain.
Discussion
Our studies indicate that inhibiting prefrontal GABAergic PV INs after chronic stress can ameliorate behavioral and physiological phenotypes induced by chronic stress exposure. PV IN inhibition by DREADD increased overall activation of the mPFC. CVS resulted in hyperactivity in the OF, anhedonia in the SPT (reduced sucrose intake and preference), and enhanced anxiety-related behavior in the LD box, all of which were mitigated by inhibition of prefrontal PV INs. CVS-related decreases in body and thymus weights were also attenuated by PV IN inhibition after two weeks of stress exposure. Our data indicate that PV INs play a role in inhibiting prefrontal output during chronic stress, adding to the growing body of literature implicating PV INs in driving mPFC hypofunction associated with stress.
In our study, CVS mice increased OF activity, in contrast with prior studies suggesting an opposite OF response following chronic stress (Ahrens et al., Citation2018). Our results are in agreement with a prior study (Strekalova et al., Citation2004) indicating that chronic stress can result in hyperactivity in mice when tested under bright and moderate illumination. The hyperactive behaviors were not detectable under weak illumination or after the injection of diazepam (0.25 mg/kg) (Strekalova et al., Citation2004). Our OF experiment was conducted in ambient room light and supports the above finding. Importantly, PV IN inhibition in naive control mice (without CVS) did not affect locomotor activity, indicating that hyperactivity is associated with the experience of CVS. Hyperlocomotion may be indicative of increased impulsivity, which is known to be enhancedfollowing chronic stress (Oswald et al., Citation2007). Some studies note that disruption of PV INs can lead to a hyperactive and impulsive behavior in mice (Khan et al., Citation2017) suggesting a possible role of PV INs in driving these behaviors as a consequence of stress. Based on our current finding, future studies should focus on modulation of prefrontal PV IN activity and subsequent effects on impulsivity. We did not observe any significant effect of CVS on duration of time in center in the OF test.
Our SPT data indicatethat CVS reduces sucrose preference, a behavioral response suggestive of anhedonia in mice (Liu et al., Citation2018). Decreased sucrose preference is observed with various chronic stress models and has been interpreted to reflect a depression-like phenotype (Mourlon et al., Citation2010; Zhou et al., Citation2011). Our data show that PV IN inhibition after stress can mitigate CVS-induced decrements in responsiveness to reward and coping behavior. Thus, inhibiting PV IN to promote prefrontal output may block expression of behaviors normally observed following chronic stress. We also observed that PV IN inhibition alone without CVS reduced total sucrose consumption. This result supports our previous finding where PV IN inhibition acutely (before stress) disrupted coping in the tail suspension test (TST; Nawreen et al., Citation2020). Our findings indicate that an optimum level of PV IN activity may be important for proper functioning of the mPFC. Too much or too little activity of the PV INs may disrupt the prefrontal excitation/inhibition (E/I) balance leading to alterations in behavioral endpoints.
We also explored how PV IN inhibition after CVS may affect behavioral threat assessment behaviors. Exposure to CVS reduced latency to traverse to the dark side of the LD box compared with control animals, highlighting a risk-avoidant phenotype, consistent with prior studies (Wohleb et al., Citation2018). PV IN inhibition after CVS mitigated the CVS mediated reduced latency in the LD box. Though the CVS group was not significantly different from control in the duration of time spent in the dark side, the PV IN inhibition after CVS significantly mitigated the duration of time spent on the dark side when compared to the CVS group. Moreover, analysis of the combined z scores of the different behavioral responses, showed CVS group had an increased anxiety index that was mitigated by PV IN inhibition.
GABA receptor modulators are known to have antidepressant and anxiolytic properties (Bhutada et al., Citation2010; Mehta et al., Citation2017; Zanos et al., Citation2017; Samad et al., Citation2018). The antidepressant effects of rapid-acting drugs are thought to occur via inhibition of GABA INs (Fogaca et al., 2021). Positive allosteric modulators of GABAA receptors, e.g. diazepam, also act as anxiolytics (Engin et al., Citation2016). GABAergic PV INs are well positioned to provide strong, fast-spiking inhibitory signals to pyramidal projection neurons in the PFC and reduce network excitability, and therefore could be contributing to chronic stress-mediated hypoactivity (Sarihi et al., Citation2012; Winkelmann et al., Citation2014; Tremblay et al., Citation2016). Antidepressants, such as fluoxetine and ketamine reduce PV expression in the mPFC (Ohira et al., Citation2013; Zhou et al., Citation2015; Page et al., Citation2019), and preventing the reduction in PV IN activity leads to loss of antidepressant efficacy in modulating stress-related behaviors, further suggesting that reduced activity of PV INs might be playing a role in therapeutic efficacy of antidepressants (Zhou et al., Citation2015; Page et al., Citation2019; Fogaca et al., 2021).
Chemogenetic inhibition of PV INs in the mPFC attenuate behavioral effects of stress on forced swim and novelty-suppressed feeding tests, and activation of PV IN abolishes the behavioral effects of scopolamine (Fogaca et al., 2021). Prefrontal PV INs also mediate threat-assessment behavior, and chronic activation of PV INs lead to increased threat-avoidance behaviors in female mice (Page et al., Citation2019). Our recent work showed that inhibition of PV IN during chronic stress can attenuate some of the behavioral and physiological deficits associated with chronic stress. Results from this study further demonstrate that inhibition of PV, after stress-mediated plastic changes have been manifest, can also mitigate and attenuate the behavioral effects of chronic stress. It should be noted that inhibition of PV IN without stress may lead to opposite behavioral effects (Soumier and Sibille, Citation2014; Nawreen et al., Citation2020). This suggests that an optimum level of E/I balance need be maintained in the PFC appropriate function, and that PV INs play a crucial role in maintaining that balance (Ferguson and Gao, Citation2018). Chronic stress can disrupt the E/I balance leading to over inhibition by increasing the activity of PV INs, resulting in maladaptive behavioral outcomes (Page and Coutellier, Citation2019; Page et al., Citation2019). Here, we demonstrate that by acutely inhibiting PV IN after stress-mediated plasticity has taken place, we can mitigate anxiety effects.
Our experiments indicate that inhibition of PV IN after stress can prevent chronic stress-induced decreases in thymus and body weight. The thymus glands are highly sensitive to repeated stress, and it is believed that decreased thymus size is linked to increases in corticosterone secretion and stress response (Herman et al., Citation2005; Flak et al., Citation2012; Herman, Citation2013). Blockade of thymic atrophy suggests that PV INs participate in control of the central limb of HPA axis activation and demonstrates that PV IN inhibition may play a role in control of stress-induced glucocorticoid sensitivity and autonomic activation. Inhibition of PV IN also attenuated CVS-induced reduction in final body weight, suggesting that repeated DREADD activation may accelerate recovery from stress exposure.
There are a few caveats to this study that must be considered in the interpretation of the data. 1) It is notable that effects of PV IN inhibition did not mitigate all behaviors related to CVS, e.g. center time in the OF, TST-related immobility. These data suggest that the role of the mPFC in CVS-induced changes is behavior-dependent, or that some behaviors tested in our experiment are not dependent on PV IN activity. 2) Our design tests outcomes for several days following removal from CVS, which may result in adaptations over time. 3) Our paradigm also involved repeated injections of CNO over several behavioral testing days, causing repeated modulation of ongoing PV IN activity. Despite these caveats, in all cases PV IN inhibition attenuated effects of chronic stress, supporting a role for enhanced PV activity in stress-related behavioral and physiological changes. 4) The SPT was conducted before the light-dark box test, meaning that mice experience a brief social isolation stress (in order to measure intake) prior to measuring anxiety-related behavior. 5) Finally, this study was conducted only in male mice. Because PV IN modulation may have sex-specific effects (Shepard et al., Citation2016; Page et al., Citation2019), it would be important to next examine the effects of PV IN modulation in females.
In conclusion, our study shows plasticity in prefrontal PV INs play a role in driving behavioral and somatic adaptations associated with chronic stress. Chronic stress-mediated hypoactivity, anhedonia, and anxiety-like responses may be mediated partly via plastic changes in PV IN function and may play a role in expression of stress-related behavioral changes (e.g. as occur in depression and PTSD). We show that inhibiting PV INs increases overall activation of the mPFC. Our data indicate that chemogenetic inhibition of PV INs after chronic stress likely mitigates over-inhibition of glutamatergic neurons, which is sufficient to attenuate chronic stress-mediated increased hyperactivity, decreased sucrose preference, and increased anxiety-like behavior and in preventing thymic atrophy and body weight loss. Taken together, our findings suggest that reducing the activity of PV INs in the mPFC may facilitate output of prefrontal neurons and could provide therapeutic benefits for stress-related disorders.
Authors’ contribution
NN (PhD), JH (PhD): Conceptualization, Methodology, Software.
NN, KO (graduate student), BC(PhD), MS (PhD): Data collection, analysis. NN, JH: Writing – Reviewing and Editing.
Acknowledgments
The authors would like to thank members of the Herman lab for assistance with this project.
Disclosure statement
The authors declare that this study was conducted in the absence of any financial or commercial relationships that could be considered as a potential conflict of interest.
Additional information
Funding
Notes on contributors
Nawshaba Nawreen
Nawshaba Nawreen is currently a research scientist at Eli Lilly at Company. She is currently leading toxicology drug development efforts in Neuroscience. She earned a PhD in Neuroscience in 2023 from the University of Cincinnati. Her dissertation focused on understanding the role of prefrontal cortex in stress related disorders.
Kristen Oshima
Kristen Oshima is a graduate student in the Neuroscience Graduate Program at the University of Cincinnati. She received her bachelor’s degree in Neurobiology in 2020 from the University of Cincinnati.
James Chambers
James Chambers is a research associate at the University of Cincinnati in the Department of Pharmacology and Systems Physiology. He completed his master’s degree in Experimental Psychology (1998) and PhD in Neuroscience (2002) at Florida State University.
Marissa Smail
Marissa Smail is a postdoctoral scholar in the Department of Psychology at the Ohio State University. She earned a PhD in Neuroscience in 2023 from the University of Cincinnati. Her research interests center around investigating the molecular mechanisms underlying how animals respond to stress and various environmental factors.
James P. Herman
James P. Herman is Flor van Maanen Chair and Professor at the University of Cincinnati. He is an internationally recognized researcher in the area of stress biology. His interestsb revolve around understanding the neurobiological basis of stress integration and stress-related diseases.
References
- Ahrens, S., Wu, M. V., Furlan, A., Hwang, G. R., Paik, R., Li, H., Penzo, M. A., Tollkuhn, J., & Li, B. (2018). A central extended amygdala circuit that modulates anxiety. The Journal of Neuroscience, 38(24), 1–14. https://doi.org/10.1523/JNEUROSCI.0705-18.2018
- Bedse, G., Hartley, N. D., Neale, E., Gaulden, A. D., Patrick, T. A., Kingsley, P. J., Uddin, M. J., Plath, N., Marnett, L. J., & Patel, S. (2017). Functional redundancy between canonical endocannabinoid signaling systems in the modulation of anxiety. Biological Psychiatry, 82(7), 488–499. https://doi.org/10.1016/j.biopsych.2017.03.002
- Bhutada, P., Mundhada, Y., Bansod, K., Ubgade, A., Quazi, M., Umathe, S., & Mundhada, D. (2010). Reversal by quercetin of corticotrophin releasing factor induced anxiety- and depression-like effect in mice. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 34(6), 955–960. https://doi.org/10.1016/j.pnpbp.2010.04.025
- Can, A., Dao, D. T., Terrillion, C. E., Piantadosi, S. C., Bhat, S., & Gould, T. D. (2012). The tail suspension test. Jove- Journal of Visualized Experiments, 29, e3638.
- Cardin, J. A., Carlén, M., Meletis, K., Knoblich, U., Zhang, F., Deisseroth, K., Tsai, L. H., & Moore, C. I. (2009). Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature, 459(7247), 663–667. https://doi.org/10.1038/nature08002
- Courtin, J., Chaudun, F., Rozeske, R. R., Karalis, N., Gonzalez-Campo, C., Wurtz, H., Abdi, A., Baufreton, J., Bienvenu, T. C., & Herry, C. (2014). Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature, 505(7481), 92–96. https://doi.org/10.1038/nature12755
- Duman, R. S. (2014). Pathophysiology of depression and innovative treatments: Remodeling glutamatergic synaptic connections. Dialogues in Clinical Neuroscience, 16(1), 11–27. https://doi.org/10.31887/DCNS.2014.16.1/rduman
- Engin, E., Smith, K. S., Gao, Y., Nagy, D., Foster, R. A., Tsvetkov, E., Keist, R., Crestani, F., Fritschy, J. M., Bolshakov, V. Y., Hajos, M., Heldt, S. A., & Rudolph, U. (2016). Modulation of anxiety and fear via distinct intrahippocampal circuits. eLife, 5, e14120. https://doi.org/10.7554/eLife.14120
- Ferguson, B. R., & Gao, W. J. (2018). PV interneurons: Critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Frontiers in Neural Circuits, 12, 37. https://doi.org/10.3389/fncir.2018.00037
- Flak, J. N., Solomon, M. B., Jankord, R., Krause, E. G., & Herman, J. P. (2012). Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain. The European Journal of Neuroscience, 36(4), 2547–2555. https://doi.org/10.1111/j.1460-9568.2012.08161.x
- Fogaça, M. V., Wu, M., Li, C., Li, X. Y., Picciotto, M. R., & Duman, R. S. (2021). Inhibition of GABA interneurons in the mPFC is sufficient and necessary for rapid antidepressant responses. Molecular Psychiatry, 26(7), 3277–3291. https://doi.org/10.1038/s41380-020-00916-y
- Ghosal, S., Hare, B., & Duman, R. S. (2017). Prefrontal cortex GABAergic deficits and circuit dysfunction in the pathophysiology and treatment of chronic stress and depression. Current Opinion in Behavioral Sciences, 14, 1–8. https://doi.org/10.1016/j.cobeha.2016.09.012
- Ghosal, S., Packard, A. E. B., Mahbod, P., McKlveen, J. M., Seeley, R. J., Myers, B., Ulrich-Lai, Y., Smith, E. P., D’Alessio, D. A., & Herman, J. P. (2017). Disruption of glucagon-like peptide 1 signaling in sim1 neurons reduces physiological and behavioral reactivity to acute and chronic stress. Journal of Neuroscience, 37, 184–193.
- Guilloux, J. P., Seney, M., Edgar, N., & Sibille, E. (2011). Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: Relevance to emotionality and sex. Journal of Neuroscience Methods, 197(1), 21–31. https://doi.org/10.1016/j.jneumeth.2011.01.019
- Hasler, G., van der Veen, J. W., Tumonis, T., Meyers, N., Shen, J., & Drevets, W. C. (2007). Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Archives of General Psychiatry, 64(2), 193–200. https://doi.org/10.1001/archpsyc.64.2.193
- Herman, J. P. (2013). Neural control of chronic stress adaptation. Frontiers in Behavioral Neuroscience, 7, 61. https://doi.org/10.3389/fnbeh.2013.00061
- Herman, J. P., Ostrander, M. M., Mueller, N. K., & Figueiredo, H. (2005). Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 29(8), 1201–1213. https://doi.org/10.1016/j.pnpbp.2005.08.006
- Khan, A., de Jong, L. A., Kamenski, M. E., Higa, K. K., Lucero, J. D., Young, J. W., Behrens, M. M., & Powell, S. B. (2017). Adolescent GBR12909 exposure induces oxidative stress, disrupts parvalbumin-positive interneurons, and leads to hyperactivity and impulsivity in adult mice. Neuroscience, 345, 166–175. https://doi.org/10.1016/j.neuroscience.2016.11.022
- Liu, M. Y., Yin, C. Y., Zhu, L. J., Zhu, X. H., Xu, C., Luo, C. X., Chen, H., Zhu, D. Y., & Zhou, Q. G. (2018). Sucrose preference test for measurement of stress-induced anhedonia in mice. Nature Protocols, 13(7), 1686–1698. https://doi.org/10.1038/s41596-018-0011-z
- Lueptow, L. M. (2017). Novel object recognition test for the investigation of learning and memory in mice. Journal of Visualized Experiments: JoVE, 126, 55718
- Luscher, B., Shen, Q., & Sahir, N. (2011). The GABAergic deficit hypothesis of major depressive disorder. Molecular Psychiatry, 16(4), 383–406. https://doi.org/10.1038/mp.2010.120
- McKlveen, J. M., Moloney, R. D., Scheimann, J. R., Myers, B., & Herman, J. P. (2019). "Braking" the prefrontal cortex: The role of glucocorticoids and interneurons in stress adaptation and pathology. Biological Psychiatry, 86(9), 669–681. https://doi.org/10.1016/j.biopsych.2019.04.032
- McKlveen, J. M., Morano, R. L., Fitzgerald, M., Zoubovsky, S., Cassella, S. N., Scheimann, J. R., Ghosal, S., Mahbod, P., Packard, B. A., Myers, B., Baccei, M. L., & Herman, J. P. (2016). Chronic stress increases prefrontal inhibition: A mechanism for stress-induced prefrontal dysfunction. Biological Psychiatry, 80(10), 754–764. https://doi.org/10.1016/j.biopsych.2016.03.2101
- Mehta, V., Parashar, A., & Udayabanu, M. (2017). Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiology & Behavior, 171, 69–78. https://doi.org/10.1016/j.physbeh.2017.01.006
- Mourlon, V., Baudin, A., Blanc, O., Lauber, A., Giros, B., Naudon, L., & Daugé, V. (2010). Maternal deprivation induces depressive-like behaviours only in female rats. Behavioural Brain Research, 213(2), 278–287. https://doi.org/10.1016/j.bbr.2010.05.017
- Mukherjee, A., & Caroni, P. (2018). Infralimbic cortex is required for learning alternatives to prelimbic promoted associations through reciprocal connectivity. Nature Communications, 9(1), 2727. https://doi.org/10.1038/s41467-018-05318-x
- Nawreen, N., Cotella, E. M., Morano, R., Mahbod, P., Dalal, K. S., Fitzgerald, M., Martelle, S., Packard, B. A., Franco-Villanueva, A., Moloney, R. D., & Herman, J. P. (2020). Chemogenetic inhibition of infralimbic prefrontal cortex GABAergic parvalbumin interneurons attenuates the impact of chronic stress in male mice. eNeuro, 7(5), ENEURO.0423-19.2020. https://doi.org/10.1523/ENEURO.0423-19.2020
- Ohira, K., Takeuchi, R., Iwanaga, T., & Miyakawa, T. (2013). Chronic fluoxetine treatment reduces parvalbumin expression and perineuronal nets in gamma-aminobutyric acidergic interneurons of the frontal cortex in adult mice. Molecular Brain, 6, 43. https://doi.org/10.1186/1756-6606-6-43
- Oswald, L. M., Wong, D. F., Zhou, Y., Kumar, A., Brasic, J., Alexander, M., Ye, W., Kuwabara, H., Hilton, J., & Wand, G. S. (2007). Impulsivity and chronic stress are associated with amphetamine-induced striatal dopamine release. NeuroImage, 36(1), 153–166. https://doi.org/10.1016/j.neuroimage.2007.01.055
- Page, C. E., & Coutellier, L. (2019). Prefrontal excitatory/inhibitory balance in stress and emotional disorders: Evidence for over-inhibition. Neuroscience and Biobehavioral Reviews, 105, 39–51. https://doi.org/10.1016/j.neubiorev.2019.07.024
- Page, C. E., Shepard, R., Heslin, K., & Coutellier, L. (2019). Prefrontal parvalbumin cells are sensitive to stress and mediate anxiety-related behaviors in female mice. Scientific Reports, 9(1), 19772. https://doi.org/10.1038/s41598-019-56424-9
- Paxinos, G., & Franklin, K. B. J. (2008). Mouse brain in stereotaxic coordinates. Compact ( 3rd ed.). Academic.
- Rogan, S. C., & Roth, B. L. (2011). Remote control of neuronal signaling. Pharmacological Reviews, 63(2), 291–315. https://doi.org/10.1124/pr.110.003020
- Rymar, V. V., & Sadikot, A. F. (2007). Laminar fate of cortical GABAergic interneurons is dependent on both birthdate and phenotype. The Journal of Comparative Neurology, 501(3), 369–380. https://doi.org/10.1002/cne.21250
- Samad, N., Saleem, A., Yasmin, F., & Shehzad, M. A. (2018). Quercetin protects against stress-induced anxiety- and depression-like behavior and improves memory in male mice. Physiological Research, 67(5), 795–808. https://doi.org/10.33549/physiolres.933776
- Sarihi, A., Mirnajafi-Zadeh, J., Jiang, B., Sohya, K., Safari, M. S., Arami, M. K., Yanagawa, Y., & Tsumoto, T. (2012). Cell type-specific, presynaptic LTP of inhibitory synapses on fast-spiking GABAergic neurons in the mouse visual cortex. The Journal of Neuroscience, 32(38), 13189–13199. https://doi.org/10.1523/JNEUROSCI.1386-12.2012
- Shepard, R., Page, C. E., & Coutellier, L. (2016). Sensitivity of the prefrontal GABAergic system to chronic stress in male and female mice: Relevance for sex differences in stress-related disorders. Neuroscience, 332, 1–12. https://doi.org/10.1016/j.neuroscience.2016.06.038
- Sherwood, C. C., Raghanti, M. A., Stimpson, C. D., Bonar, C. J., de Sousa, A. A., Preuss, T. M., & Hof, P. R. (2007). Scaling of inhibitory interneurons in areas v1 and v2 of anthropoid primates as revealed by calcium-binding protein immunohistochemistry. Brain, Behavior and Evolution, 69(3), 176–195. https://doi.org/10.1159/000096986
- Soumier, A., & Sibille, E. (2014). Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology, 39(9), 2252–2262. https://doi.org/10.1038/npp.2014.76
- Strekalova, T., Spanagel, R., Bartsch, D., Henn, F. A., & Gass, P. (2004). Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology, 29(11), 2007–2017. https://doi.org/10.1038/sj.npp.1300532
- Taglialatela, G., Hogan, D., Zhang, W. R., & Dineley, K. T. (2009). Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behavioural Brain Research, 200(1), 95–99. https://doi.org/10.1016/j.bbr.2008.12.034
- Tremblay, R., Lee, S., & Rudy, B. (2016). GABAergic interneurons in the neocortex: From cellular properties to circuits. Neuron, 91(2), 260–292. https://doi.org/10.1016/j.neuron.2016.06.033
- Winkelmann, A., Maggio, N., Eller, J., Caliskan, G., Semtner, M., Häussler, U., Jüttner, R., Dugladze, T., Smolinsky, B., Kowalczyk, S., Chronowska, E., Schwarz, G., Rathjen, F. G., Rechavi, G., Haas, C. A., Kulik, A., Gloveli, T., Heinemann, U., & Meier, J. C. (2014). Changes in neural network homeostasis trigger neuropsychiatric symptoms. The Journal of Clinical Investigation, 124(2), 696–711. https://doi.org/10.1172/JCI71472
- Wohleb, E. S., Terwilliger, R., Duman, C. H., & Duman, R. S. (2018). Stress-induced neuronal colony stimulating factor 1 provokes microglia-mediated neuronal remodeling and depressive-like behavior. Biological Psychiatry, 83(1), 38–49. https://doi.org/10.1016/j.biopsych.2017.05.026
- Zanos, P., Nelson, M. E., Highland, J. N., Krimmel, S. R., Georgiou, P., Gould, T. D., & Thompson, S. M. (2017). A negative allosteric modulator for alpha5 subunit-containing GABA receptors exerts a rapid and persistent antidepressant-like action without the side effects of the NMDA receptor antagonist ketamine in mice. eNeuro, 4(1), ENEURO.0285-16.2017. https://doi.org/10.1523/ENEURO.0285-16.2017
- Zhou, Q. G., Zhu, L. J., Chen, C., Wu, H. Y., Luo, C. X., Chang, L., & Zhu, D. Y. (2011). Hippocampal neuronal nitric oxide synthase mediates the stress-related depressive behaviors of glucocorticoids by downregulating glucocorticoid receptor. Journal of Neuroscience, 31, 7579–7590. https://doi.org/10.1523/JNEUROSCI.0004-11.2011
- Zhou, Z., Zhang, G., Li, X., Liu, X., Wang, N., Qiu, L., Liu, W., Zuo, Z., & Yang, J. (2015). Loss of phenotype of parvalbumin interneurons in rat prefrontal cortex is involved in antidepressant- and propsychotic-like behaviors following acute and repeated ketamine administration. Molecular Neurobiology, 51(2), 808–819. https://doi.org/10.1007/s12035-014-8798-2
