Abstract
Sense of Okayness (SOK) is an emerging concept that describes a person’s ability to remain stable and unshaken in the face of life transitions and hardships. This quality enables effective stress regulation and heightened tolerance to uncertainty. To investigate the possible role of the parasympathetic nervous system (PNS) in mediating the relationship between SOK and stress regulation among older individuals, an analytical sample of N = 69 participants (74% women) with a mean age of 78.75 years (SD age = 6.78) was recruited for a standardized cognitive assessment and stress induction. Baseline heart rate variability (HRV), measured via electrocardiogram (ECG), and SOK assessments were conducted prior to stress induction, along with a baseline cognitive evaluation. Subsequently, participants were subjected to a psychosocial stress paradigm, followed by either a 30-minute SOK elevation intervention (n = 40) or a control condition with nature sounds (n = 29). A second cognitive assessment was administered post-intervention, with continuous HRV measurement through ECG. The results revealed significant HRV changes due to the experimental intervention, though no significant differences were observed between the SOK intervention and control groups. Interestingly, individuals with high trait SOK displayed more stable HRV trajectories, exhibiting a smaller decline during the stress intervention and a milder increase during both the stressor and SOK intervention phases. Overall, these findings do suggest a significant association between SOK, parasympathetic activity, and stress reactivity. These results prompt further investigation into whether personality patterns, such as a strong SOK, may be linked to reduced vagal reactivity and better coping in old age.
Introduction
Life transitions, particularly in old age, such as widowhood, retirement, and declining cognitive or physiological health, are often perceived as challenging and stressful (Schlossberg, Citation2011; Ong et al. Citation2006). Recognizing the substantial interindividual variability in coping with these transitions is crucial for identifying factors that can facilitate successful adaptation. One approach to understanding adaptation to stressors is through the examination of stress mechanisms. Stress can deplete cognitive and psychological resources required for coping with life transitions (Hobfoll, Citation1998), and individual differences in self-regulation have been proposed as a determinant of cognitive and psychological coping abilities during periods of stress (Wolf, Citation2009). Self-regulation refers to the capacity to modulate cognition, behavior, emotion and physiology to meet changing situational demands and inherently involves adaptation (Cole et al., Citation2019; Bonanno, Citation2004). Consequently, individuals with greater flexibility in their regulation strategies tend to exhibit better-coping skills, increased resilience, and reduced negative impact from cumulative life stress (Bonanno, Citation2004). In this context, we propose a new concept termed “sense of okayness,” which may serve to describe the specific ability to self-regulate and cope with stressful periods during life transitions.
“Sense of Okayness”
"Sense of Okayness" (SOK) is characterized by an individual’s ability to find internal stillness and calmness during stressful life transitions, enabling them to regulate negative emotions and upregulate positive emotions and thus utilize their cognitive and emotional resources effectively. As a trait, SOK refers to the ability and proficiency to reach such a state at times of stress. In the process of attaining SOK, one could find both proficiency in working with negative emotions and the know-how to evoke positive emotions of calmness, relief, joy, and happiness. This quality can be considered a psychological antidote to stress and is akin to a sense of safety when facing perceived threats (Porges, Citation2007). SOK comprises three main components: self-continuity, self-other bond, and self-acceptance, which collectively contribute to establishing a sense of safety. Self-continuity refers to the feeling that an essential part of the self persists and continues to exist even when other parts are lost, significantly altered, or damaged. The self-other bond entails the belief that one is not alone due to important connections with others (e.g. friends, partners) or external entities (e.g. a higher power). Self-acceptance involves perceiving things as they are and embracing the idea that nothing needs to be different. Transitions in life and sudden events might hinder the feeling of SOK. Being composed of those three parts allows the opportunity to manipulate and regulate each one of them in a benevolent way in order to recover and reclaim the whole feeling of SOK. This gives rise to inquiry into different targeted interventions.
Self-regulation and HRV
At the physiological level, self-regulation is believed to be associated with the activity of the vagus nerve (Porges, Citation2007; Thayer & Lane, Citation2009). Numerous studies have focused on resting vagally mediated heart rate variability (HRV) as an indicator of tonic cardiac vagal activity, which is considered a trait indicator of physiological self-regulation capacity supporting effective behavioral and cognitive regulation, as well as overall health and well-being (Thayer et al., Citation2009; Kemp & Quintana, Citation2013). HRV is particularly suited for assessing vagal activity during rest and periods of stress (e.g. Penttilä et al., Citation2001; Shaffer et al., Citation2014). However, given the constantly changing conditions of life that necessitate ongoing adaptation, it becomes crucial to understand the adaptive patterns of HRV activity.
Vagal flexibility
According to the polyvagal theory and the neurovisceral integration model (Porges, Citation1995; Thayer & Lane, Citation2000), vagal flexibility plays a critical role in helping individuals adapt to changing environmental demands (Thayer & Lane, Citation2009). Vagal flexibility refers to the dynamic regulation of vagal responses to match different contexts (Porges et al., Citation1996; Thayer & Lane, Citation2000). This modulation, known as vagal flexibility, represents the state dynamics of self-regulation, involving shifts in cardiac output and prefrontal cortex (PFC) activation in response to challenges (Porges, Citation1995; Thayer et al., Citation2012).
Previous research has shown that reduced basal HRV and hyporesponsiveness to challenge are associated with conditions such as chronic fatigue, social phobia, and symptoms of depression and aggression, suggesting a maladaptive response pattern (Paret et al., Citation2016; Scott and Weems, Citation2014). In contrast, elevated vagal tone during rest accompanied by rapid withdrawal during stress (referred to as vagal withdrawal) appears to be related to more flexible attention regulation toward threats and has been considered adaptive and health-protective (Gentzler et al., Citation2009; Muhtadie et al., Citation2015; Porges, Citation2007; Stange et al., Citation2017). Additionally, studies have demonstrated that resilience in individuals is associated with a rapid reduction in HRV during stress and quick recovery, indicating more adaptive stress-related adjustment (Spangler et al., Citation2021). Furthermore, higher HRV has been linked to more flexible cognitive strategies, attention, memory, and executive function (Colzato et al., Citation2018; Forte et al., Citation2019).
Expanding upon the premises of the Neurovisceral Integration Model, the Vagal Tank Theory (Laborde et al., 2017) proposes that diverse phasic responses of HRV may be considered adaptive, depending on the nature of the environmental demands encountered by the organism. According to this theory, small vagal withdrawals or even HRV increments indicate self-regulatory resource activation or effective utilization, thereby facilitating top-down control (Laborde et al., 2017). Recent findings in this domain indicate that the impact of phasic HRV changes is less evident in participants with initially high self-regulatory/processing resources but more pronounced in those with low self-regulatory/processing capacities (Schmaußer & Laborde, Citation2023). For example, in a dart-throwing game, participants with high emotional intelligence exhibited a smaller reduction in HRV than subjects with low emotional intelligence (Mosley et al. Citation2017). Another study found that when faced with a visual search task under time pressure, participants with high compared to low trait emotional intelligence displayed higher HRV during the task (Laborde et al., Citation2011). This supports the notion that some emotional traits can function as resources and facilitate better emotion regulation under pressure, which can facilitate performance. While there is strong support regarding the relationship between resting vagal activity and health, inconsistencies exist regarding what would count as an adaptive/healthy response to stress in terms of vagal activity. Would a quick withdrawal in response to stress would count as adaptive? Or rather, a stable pattern with just a minor decrease or even an increase in vagal activity would count as adaptive?
Those inconsistencies of predictions hold true also for older adults. Some studies suggest that older individuals typically exhibit exaggerated vagal withdrawal during stressful tasks like isometric exercise (Thompson et al., Citation2013), while others report minimal vagal withdrawal during psychosocial stress tasks (e.g. speech and mental arithmetic) (Uchino et al., Citation1999). Furthermore, certain studies have found similar stress-induced HRV changes between healthy young and older adults (Wood et al., Citation2002). As with other traits of mental health, SOK can promote better regulation of stress, and vagal activity can be used as an indicator for such adaptive regulation.
Description of the present study
The present project aims to examine the role of SOK in vagal and cortisol (will be reported elsewhere) responses during alternating periods of stress and relaxation in older adults. We investigated whether a short SOK-based intervention influences vagal recovery from a stressful situation and whether it acts as a buffer against the effects of stress on cognitive performance. Additionally, we examine the impact of trait SOK on vagal responses to stress and relaxation. Vagal activity is measured through HRV, in the frequency band associated with respiratory sinus arrhythmia (RSA), as a vagally mediated marker (Rajendra Acharya et al., Citation2006). Thus, this study incorporates measures of changes in vagal flexibility reflected in RSA levels.
Hypotheses of the present study
Based on the findings from previous studies as outlined above, we hypothesized that 1) HRV, as indicated by vagal activity, will be higher in the group receiving the SOK intervention than the control group. 2) Individuals with high trait SOK will exhibit higher vagal activity during stressful situations and faster recovery from stress in terms of vagal activity than those with low SOK. 3) Cognitive performance on a test-retest task will be less impaired by stress in the group receiving the SOK intervention than in the control group.
Methods
Participants
Ninety older individuals (age 62-90) residing in community-dwelling and assisted living facilities in Konstanz, Germany, were initially contacted for the study. The recruitment process involved sending a letter to facility management, followed by an introductory meeting with the residents. During the meeting, the research procedure and general purpose were presented. After the introduction, residents had the opportunity to volunteer for the study and provide their names to management. Subsequently, residents who expressed interest and gave permission were contacted by phone to schedule the group sessions. From the ninety initially contacted, eight participants declined participation due to personal reasons (e.g. illness, forgetfulness). Participant exclusion criteria included impaired cognitive functioning (scoring below 22 on the MoCA test), which would hinder the successful completion of the cognitive tasks in the experimental protocol, a history of cardiovascular or endocrine disease, and current use of heart or hormone medications that could interfere with the outcome measures assessed as part of the experimental protocol. Six subjects did not hand in the questionnaire and were thus excluded; two others had too many missing values in the SOK items and were then excluded, three others scored below 22 on the MoCA test and were equally excluded, and two others were lastly excluded because of severe artifacts in the HRV-data. The final analytical sample comprised 69 participants (mean age = 78.75, SD = 6.78, 74% women). In cases where more than 20% of items for a partucular scale were missing, that subject was exluede from the respective analysis. (see Table 1 for details). All participants provided informed consent and received cognitive feedback based on their performance on the MoCA test. The study received approval from the Ethics Committee of the University of Konstanz.
Procedure
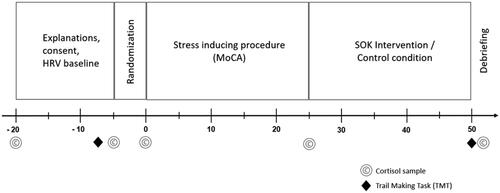
The experimental sessions were conducted in the residents’ homes, typically in group rooms, on weekdays, from either 10:00 to 11:30 am or 2:00 to 4:00 pm. Due to the daily routines at the nursery homes, participants frequently came late for the noon sessions, which often caused the extra 30 minutes. Participants were assessed together in groups of up to six individuals. Upon the arrival of the two investigators responsible for all assessments (henceforth referred to as IG and CB), participants were greeted, and a brief overview of the experimental procedures was provided. First, written informed consent was obtained from each participant. Following that, participants were instructed on the use of the heart rate variability (HRV) sensors. After attaching the heart rate sensor (Polar H7 or H10, Polar Electro Oy, Kempele, Finland) via a chest belt to the upper torso, baseline HRV measures were recorded for 10 minutes. All data was transferred in real-time to iPads running the HRV logger app for data storage. After the baseline EEG recording, participants completed a cognitive task assessing executive function and speed of processing (Trail Making Test-TMT) (Reitan & Wolfson, Citation1993). Subsequently, participants were exposed to 25 min psychosocial stress induction via a modified cognitive assessment incorporating stressful components (Moca group test, described below). The experimental group then engaged in a 30-minute intervention to bolster the sense of okayness (SOK). Finally, participants completed a second version of the cognitive test they performed at the beginning of the experimental stage. The control group followed the same procedure, with the exception of the intervention. Instead of the 30-minute SOK intervention, the control group listened to relaxing nature sounds. Throughout the experiment, participants provided eight saliva samples. depicts the experimental procedure. At the conclusion of the experiment, participants were provided with a set of self-report questionnaires to complete at home. These questionnaires included measures of demographics, perceived stress, resilience, self-efficacy, and SOK.
Psychosocial stress induction
Five minutes after completing the Trail Making Test, participants were exposed to a modified version of the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., Citation2005). This modified version was designed to induce stress by incorporating elements of performance evaluation and social comparison. Specifically, participants were informed that the test was commonly used in clinical settings to evaluate the cognitive abilities of older adults. They were advised to approach the test seriously and strive to perform to the best of their abilities. The modified MoCA consisted of a series of cognitive tasks involving various domains such as memory, attention, and executive function. Some tasks were completed independently, while others were performed in a group setting. Those included memory, delayed recall, verbal fluency, attention, and sentence repetition parts. In those group parts of the test, the researcher asked the participants to perform the test in front of the rest. For a detailed description of the modified MoCA procedure, please refer to the appendix section of this study.
The SOK intervention consisted of two components: a 15-minute Loving-kindness meditation (LKM) and a 15-minute short life-review process. During the LKM intervention, participants were provided with the following instructions: (1) To sit in a comfortable position with closed eyes and focus on their breath and bodily sensations (proprioception); (2) To visualize and cultivate feelings of kindness, love, and compassion directed toward themselves and a chosen loving person or entity; and (3) To generate and extend these feelings of kindness and compassion toward themselves and the chosen person or entity.
The life-review process was adapted from the "Life Review Interview" developed by Baltes and Smith (Citation2008). Participants were asked to reflect on and select three significant life events. Subsequently, they were presented with questions aimed at fostering a sense of integration and coherence regarding these events. Examples of the questions included: "What aspects of yourself were manifested during these events?" "Which characteristics of your personality emerged or developed as a result of these events?" and "What new insights about yourself and life arose from these events?"
Measurements
Heart rate variability (HRV)
We employed continuous HRV recording using Polar H7 or H10 heart rate sensors (Polar Electro Oy, Kempele, Finland) incorporated into a two-electrode chest belt to examine autonomic regulation during the different experimental phases. The HR sensors were wirelessly connected via Bluetooth to an iPad (Apple Computer, Cupertino, CA, United States) running the HRV Logger App for iOS. This application records and stores heart rate (HR) and beat-to-beat intervals (RR intervals) from the Polar sensors who capture the ECG signal at a sampling rate of 1000 Hz. Additionally, manual event markers were set throughout the session to enable accurate segmentation of the experimental phases. Subsequently, the recorded data was transferred to local desktop computers in the laboratory for further processing.
The RR data underwent processing using R (version 4.1.0) in conjunction with the R-Studio user interface (version 1.4.1717). Ectopic beats were removed, artifacts were identified and eliminated, and missing beats were interpolated based on visual inspection. These procedures were facilitated by custom R scripts developed in-house. Two participants were excluded from the analysis due to an excessive number of missing time points. Finally, HRV parameters, specifically rMSSD and HF-HRV, were computed for analysis in intervals corresponding to the various experimental stages. This computation was performed using the RHRV package (version 4.2.7, Rodríguez-Liñares et al., Citation2011) and an additional custom script. The segmented intervals are depicted in .
Cognitive Performance
Cognitive abilities before and after the experiment were assessed using the trail-making B test administered through the "INPL Trail Making Test" application (Motus Design Group, 2016) on iPads. Participants were instructed to connect numbers and letters in ascending order while alternating between the two categories (e.g. 1-A-2-B-3-C…). The task conditions were evaluated based on completion times and the number of errors committed.
Subjective levels of current stress
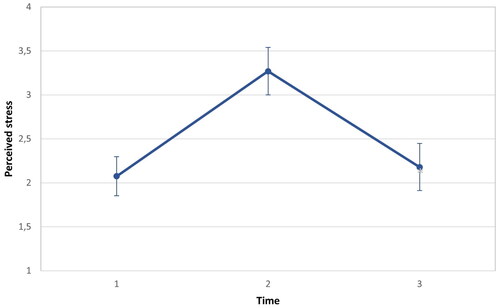
Current levels of perceived stress were assessed using a modified version of the Perceived Stress Scale (PSS-10, Cohen and Williamson, Citation1988), designed to measure key components of stress by examining individuals’ perceptions of controllability, workload, and predictability over the preceding month. A shortened version of the PSS-10 comprising three items was utilized to facilitate rapid and practical evaluations, augmented by an additional item prompting participants to report their current stress level. In addition, the temporal frame was adjusted, with participants asked to rate the applicability of each item to their current momentary (instead of the following month) experience using a 4-point Likert scale (1 = does not apply at all, 4 = does entirely apply). Higher scores indicated greater perceived stress. Subjective stress levels were assessed at three distinct time points: baseline, end of the stress-inducing situation, and end of the intervention.
SOK
The SOK construct was measured using a 14-item scale comprising 7-point Likert scales. The questionnaire was handed out to be filled at home together with other self-reported measures. This scale was developed in previous work by one of the authors (IG) (Gilo et al., in press). Example items include: (a) "I feel safe," and (b) "I feel that no matter what happens, I am okay.” Cronbach’s alpha = .825
Trait resilience
Trait resilience was evaluated using the 10-item version of the established Resilience Scale developed by Connor and Davidson (2003). The questionnaire was handed out to be filled at home together with other self-reported measures The scale measures the ability to cope with stress and adversity. Respondents rate items on a scale from 0 (“not true at all”) to 4 (“true nearly all the time”). Cronbach’s alpha = .824
Statistical analysis
Prior to conducting the statistical analyses, we performed data pre-processing procedures to ensure the integrity and validity of the recorded heart rate (HR) data. Outliers in the HR values were identified, and any values that deviated more than three standard deviations above or below the mean across all conditions and time points were winsorized. In cases where a participant omitted more than 20% of the items on a given subscale, the corresponding variable was coded as "NA" and excluded from the respective analysis. (see for details).
Table 1. Descriptive statistics of the groups
For assessing stress reactivity and stress levels, we employed the area under the curve with respect to increase (AUCi) and the area under the curve with respect to ground (AUCg) measures, which are commonly used indicators (Pruessner et al., Citation2003). Specifically, we calculated AUCi and AUCg for logarithmized HF-HRF values, here referred to as respiratory sinus arrhythmia (RSA) values (AUCi_rsa, AUCG_rsa).
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 28.0. The significance threshold was set to p < 0.05. Normal distribution was assessed using Shapiro-Wilk tests, while homogeneity of variance was evaluated using Levene’s tests. Some aspects of the analysis were preregistered on the Open Science Framework (https://osf.io/yufkq/).
To account for potential person-related covariates that might have influenced the main outcomes of the study, we conducted one-way Analysis of Variances (ANOVAs). Condition (experimental versus control) served as the independent variable, while age, medication usage, baseline measures, baseline RSA, and baseline subjective stress were included as dependent variables. Variables that exhibited unequal distributions across the groups were considered potential covariates, and their effects were assessed by incorporating them in subsequent analyses.
Next, we examined the influence of design-related factors on HRV levels at baseline. We conducted a Welch two-sample t-test to assess the effect of session start time on HF-HRV baseline. Additionally, an Analysis of Covariance (ANCOVA) was performed to investigate whether session start timehad an impact on HRV baseline while controlling for the influence of the experimental condition.
Subsequently, manipulation checks were conducted to assess the effectiveness of the stress induction and intervention. To this end, two separate one-way repeated measure ANOVAs were performed with time as the repeated measures factor and either the subjective stress levels (at three-time points), or the HRV levels (at four-time points) as dependent variables.
Finally, we tested our hypotheses based on the preregistered models available on the Open Science Framework (https://osf.io/pfxe8/). A series of repeated measures ANCOVAs were conducted to investigate how the trajectories of HRV levels were influenced by the experimental condition and levels of trait sense of okayness (SOK). Post hoc analyses were conducted to explore observed differences further and elucidate specific directions and relationships. Mixed regressions were used to examine whether differences in SOK will predict vagal activity (AUCg and AUCi levels) during the stress situation.
Results
Sample characteristics
The final sample consisted of N = 69 participants, divided into experimental group (n = 40) and control group (n = 29). summarizes the descriptive statistics for the groups and the results of the analyses conducted to identify covariates associated with the experimental condition.
Stress manipulation check
Subjective stress was analyzed at three time points: at baseline, end of the stress situation, and second baseline. A one-way repeated measures within-subjects ANOVA with time revealed a significant difference in subjective stress scores over time, F(1,66) = 25.185, p < 0.001, partial η2 = 0.276
HRV levels were analyzed at four time points: at baseline, at the end of the stress situation, during the intervention, and at the second baseline. A one-way repeated measures within-subjects ANOVA with time revealed a significant cubic effect in HRV levels over time, F(1,65) = 11.255, p = 0.001, partial η2 = 0.15
2and 3illustrate the changes in subjective stress ratings and HRV levels across the experiment.
Heart rate variability
To examine the hypothesis that HRV levels, were higher after the Sense of Okayness (SOK) intervention in the experimental group compared to the control group, logarithmized HF-HRV (RSA) was analyzed over 10 time points (t1: baseline, t2: trail-making test, t3-5: stress situation, t5-8: SOK intervention or nature sounds; t9: second Trail making test, t10: second baseline). A two-factor mixed-design Analysis of Covariance (ANCOVA) was conducted, with age as a covariate. We observed a significant main effect of time on RSA, F(4.7,239.69) = 5.458, p < 0.001, but no significant time by group interaction effect, F(4.7,249) = 0.41, p = 0.83. Figure 4 presents the RSA levels of the experimental and control groups over the ten-time points. Post hoc T-test analysis reveals that the experimental group exhibited a significant decline in RSA during the stress situation (t(38) = 2.172, p < 0.036), while the control group exhibited no significant change (t(28) = 1.784, p < 0.086). Both experimental and control groups exhibited significant increases during the intervention phase: t(28) = 2.707, p = 0.012 and t(38) = 2.324, p = 0.026, respectively ().
Figure 3. Changes in HRV levels across the experiment (n=66). The values represent means plus/minus standard error. Time represents the within-subject factor in the Anova.
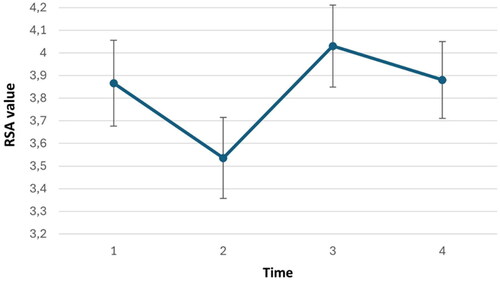
Figure 4. Changes inHRV over time (t1: baseline, t2: trail making test, t3-5: stress situation, t5-8: SOK intervention or nature sounds; t9: second Trail making test, t10: second baseline). For Experimental (n = 38) and control (n = 28) groups.
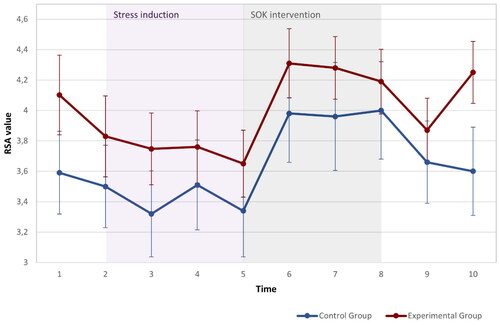
To be able to investigate the diverging HRV values more closely at the end of the experiment, we conducted an additional repeated measures ANOVA only with time points 9 and 10, which revealed a significant group-by-time interaction for the period following the second cognitive test, F(1,51) = 4.650, p = 0.036. The experimental group showed increased HRV levels, t(27) = 7.32, p<.05, while the control group showed no change t(26) = 0.75. Overall, we observed a main effect of the stress situation and intervention on RSA levels. However, except for the post-intervention period (points 9-10), we did not find significant interaction effects for the two groups. Therefore, we can only partially support the hypothesis that HRV levels are higher after the SOK intervention in the experimental group compared to the control group, as the effect seemed to be specific to the post-intervention period.
To explore the hypotheses regarding trait SOK and HRV levels, we divided the sample based on the median (5.5) into high and low trait SOK groups. This method was used also in comparing high/low trait emotional intelligence in athlete (Laborde et al., 2011). A repeated measures ANCOVA demonstrated an interaction effect between cubic time and group on RSA, F(1,52) = 4.7, p = 0.035. illustrates the HRC time course of the high and low trait SOK groups over the ten-time points.
To examine the hypothesis that individuals with high-trait SOK will exhibit a stronger rebound at the end of the stressor and during the SOK intervention, compared to the low-trait SOK, we focused on time points T5-T8. A repeated measures ANCOVA demonstrated an interaction effect between time and group on RSA, F(1,52) = 4.732, p = 0.034. However, contrary to our hypothesis, individuals with a high trait SOK demonstrated a smaller rebound in HRV at the end of stress and during the intervention ().
Figure 5. Changes in HRV across the experiment(t1: baseline, t2: trail making test, t3-5: stress situation, t5-8: SOK intervention or nature sounds; t9: second Trail making test, t10: second baseline) for high (n = 34) and low (n = 34) trait SOK groups. The values represent means.
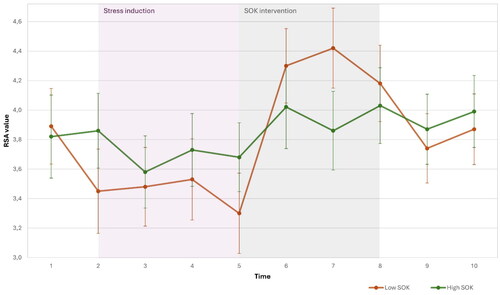
We then examined the hypothesis that individuals with high trait SOK would exhibit higher parasympathetic activity during stressful situations than individuals with low SOK. We focused on time points T1-T5, representing the period of stress. The method of multiple linear regression was used to test if trait SOK and age significantly predicted AUCg and AUCi(area under the curve of HRV)., The model did not yield a significant result for AUCg (F(2,53) = 1.894, p = 0.161) and for AUCi F(2,5253) = 1.642, p = 0.203), indicating that there was no significant effect of trait SOK on HRV levels during the stress period. Thus, we had to reject the hypothesis that individuals with high trait SOK exhibit higher parasympathetic activity during stressful situations compared to individuals with low SOK.
To investigate the hypothesis that the experimental group would be less affected by the stress situation due to the buffering effect of the SOK intervention, we created a new variable called Trail Making Test (TMT) change. TMT change represents the absolute difference between TMT scores at the first and second baseline. Subsequently, an independent t-test was conducted for the two groups. However, we found no significant interaction effect (T(64) = 0.624, p = 0.535), leading us to reject the hypothesis that the experimental group would exhibit a different response to the stress situation based on the buffering effect of the SOK intervention.
Discussion
SOK is an emerging concept that describes a person’s ability to stand right amid turbulence, challenge, or transition and not to be shaken or fall apart by it. The current study aimed to explore the acute stress recovery mechanisms through an SOK-based intervention in older adults, specifically focusing on the role of the parasympathetic nervous system (PNS) in mediating the relationship between SOK and stress regulation. Our findings first indicate that using a modified version of the Moca Test acted as a stressor, and successfully induced both an increase in subjective stress as well as a significant impact on PNS activity, as evidenced by decreased HRV levels.]l.
In relation to the SOK-based intervention, our hypothesis posited that participants receiving the intervention would demonstrate higher vagal activity measured by HRV after the stress situation, compared to the group that did not receive such an intervention. While both groups experienced a significant decline in HRV during the stress situation, followed by a significant increase in HRV during the intervention, we did not observe a significant interaction effect between the different groups, except for the post-intervention period. This prediction was based on the Vagal tank theory, which predicts that heightened emotional regulation will lead to greater recovery from stress, evident in higher levels of HRV. Upon reflection, the choice of nature sounds as the control treatment might have introduced an unintended bias. Prior research has suggested that exposure to nature sounds can expedite PNS recovery from stress. For instance, subjects exposed to nature sounds after a stressful mental arithmetic task exhibited faster recovery from sympathetic activation compared to those not exposed to such sounds (Alvarsson et al., Citation2010). Similarly, another study reported decreased sympathetic activation after exposure to forest sounds but not city sounds (Hyunju, 2019). Thus, a more suitable design would allow the control group to experience spontaneous recovery without any intervention.
Interestingly, we observed a post-intervention interaction effect (t9-t10) of group and time on HRV activity. While the experimental group’s HRV activity increased, the control group’s HRV showed no change. This delayed effect could perhaps be attributed to novelty, creating a positive impact regardless of the nature of the innovation. As time progresses, the novelty effect tends to diminish, and subsequent data may reveal that the intervention alone is only moderately effective or even ineffective (Björkman et al., Citation2019). Our study administered both interventions after the stress situation, representing novel stimuli. Given this line of thought, one possible interpretation of those results could be that the effect of the SOK intervention persisted over time while the effect of nature sounds waned more quickly.
Regarding trait SOK, combining the assumptions of the neurovisceral integration model and Vagal tank theories (Thayer & Lane, Citation2009; Laborde et al., 2017), we hypothesized that individuals with high trait SOK would exhibit higher vagal activity during stressful situations and recover more quickly from stress in terms of vagal activity compared to individuals with low trait SOK. Surprisingly, subjects with low SOK exhibited more profound recovery than those with high trait SOK. However, we found no significant difference in HRV levels between high and low SOK individuals during stress.
Those findings regarding the recovery phase partly stand in contrast to the promise of the vagal tank theory regarding stress recovery. The theory defines fast restoration levels of HRV after a stressful event as adaptive. Fast restoration reflects having sufficient resources to recover and effectively meet potential subsequent stressful events (Laborde et al., 2017). Therefore, individuals with more resources (high SOK) are expected to recover faster from stress than those with fewer resources (low SOK). Findings supporting that promise showed that women with greater difficulties in regulating their emotions showed smaller HRV recovery after stress compared to women with smaller regulation difficulties (Berna et al., Citation2014). However, in this study, the low SOK individuals showed greater HRV recovery after stress. One explanation for this surprising finding could be that in this study, the recovery phase was not a spontaneous recovery but rather an intervention designed to increase recovery. It appears that the intervention encouraged recovery more for the low-trait SOK individuals than for the high-SOK individuals. The low SOK individuals experienced a greater reduction in HRV, and their levels of HRV were lower (though not significantly) at the beginning of the intervention. A similar pattern was found in a meta-analysis by Hofmann et al., (Citation2010) examining the effectiveness of relaxation techniques for stress reduction and found that individuals with higher baseline stress demonstrated larger effect sizes in response to relaxation interventions compared to those with lower baseline stress levels.
Our Findings here, then, are aligned with the literature stating that Individuals with lower resources benefit more from a stress relief-designed intervention. However, it may be more beneficial to look at the complete trajectory pattern of HRV along the design (baseline, response to stress, recovery) than focusing separately on the recovery phase.
The overall analysis indicates that individuals with high trait SOK demonstrated a smaller reduction in HRV during the stress intervention (though only a trend and not significant) and a smaller increase after the end of the stress and during the SOK intervention. In other words, they showed a more stable pattern of HRV levels across the different conditions.
According to the vagal flexibility perspective, greater reactivity and recovery from stress represent a healthy adaptation to the dynamic range of challenges (Porges et al., Citation1996; Thayer & Lane, Citation2000). Previous research has suggested that vagal flexibility promotes self-regulation and contributes to better functioning under stress (Spangler, Citation2020). However, it is important to note that these findings are not conclusive. For example, studies on resilience in firefighters have shown no relationship between trait resilience and vagal flexibility during stressful operations (Andreas, 2019). Furthermore, the vagal tank theory distinguishes between situations requiring a low and high level of activity. It suggests that a smaller vagal withdrawal is adaptive when the task requires a low activity level and relies on executive functioning and top-down control (Laborde et al., Citation2018). Our findings support the Vagal tank theory, showing that in a cognitively demanding stress situation, having more resources (high sok) correlates with HRV stability.
Nevertheless, another possible explanation could be that individuals with high SOK did not perceive the Moca (stress situation) as stressful, which might account for their relatively stable HRV levels. This interpretation aligns with Lazarus and Folkman’s theory of stress appraisal, which suggests that individuals evaluate a situation as threatening or non-threatening, influencing the activation of the stress response (Lazarus & Folkman, Citation1984). Therefore, further studies are needed to examine further the differences in HRV response to different kinds of stressors.
Our study contributes to the understanding of vagal response patterns in older adults, elucidating aspects that have received relatively less attention in previous research. While some investigations have established certain findings regarding older adults and vagal activity, the trajectory of vagal response and recovery remains underexplored in this population. For instance, existing literature indicates that older adults with higher resting HRV tend to employ more adaptive emotion regulation strategies, facilitating effective management and modulation of emotional experiences, thereby leading to superior emotional regulation outcomes (Smith et al., Citation2019). Moreover, higher HRV in older adults has been linked with greater psychological resilience, as evidenced by their enhanced emotional stability and ability to navigate life challenges more effectively (Brown & Brown, Citation2014).
However, understanding of stress response and recovery patterns in older adults is still limited. One study by Crowley et al. (Citation2015) found that faster vagal recovery following a cognitive challenge was associated with reduced deficits in executive function among older individuals, suggesting a potential protective mechanism. Another study highlighted the differential effects of deep breathing on vagal recovery between older and younger adults, indicating that this technique may be more beneficial for older adults in restoring vagal levels after stress (Magnon et al., Citation2021). Our findings contribute to this body of research by demonstrating that in older adults, greater psychological resources (high SOK) is associated with higher Vagal stability in cognitively demanding tasks, emphasizing the importance of understanding individual differences in stress response and recovery mechanisms among older populations.
More broadly, our study holds particular significance for older adults, as understanding effective stress management becomes increasingly vital with age. By shedding light on acute stress recovery mechanisms in this population, specifically focusing on Sense of Okayness (SOK) and its impact on parasympathetic nervous system (PNS) activity, our findings provide valuable insights into targeted interventions for enhancing stress resilience and promoting well-being. This research underscores the importance of tailored approaches that consider individual differences in stress response and recovery mechanisms among older adults, contributing to their overall mental health and well-being.
Limitations
Several limitations should be considered when considering the findings of this study. First, the sample size was relatively small, which might limit the generalizability of the results. A larger and more diverse sample would provide a more comprehensive understanding of the relationship between SOK, stress regulation, and PNS activity. Second, choosing nature sounds as the control treatment might have introduced confounding factors, given the potential for nature sounds to promote PNS recovery. Using a neutral control condition without any intervention would have provided a clearer contrast. Third, the study focused on acute stress recovery and did not assess long-term effects or the durability of the intervention. Future research should investigate the sustained impact of SOK-based interventions over an extended period. Moreover, because trait SOK was measured sometime following the experiment, we might expect a potential intervention effect on the SOK measurement. Therefore, future design should try to measure baseline levels of trait SOK as well. Finally, without a placebo-stress group, it becomes challenging to discern whether the observed changes in HRV following stress and intervention are solely due to the stressor itself or influenced by other factors like participant expectations or attention from the experimenter. Thus, we have limited ability to isolate and interpret the precise effects of the stress induction procedure and intervention accurately.
Future studies
Despite the contributions of this study, several avenues for future research should be explored. First, replication studies with larger and more diverse samples are needed to validate the current findings and enhance their generalizability. Second, longitudinal designs could investigate the long-term effects of SOK-based interventions on stress regulation and cognitive performance in older adults. Such studies would provide insights into the sustainability and durability of the intervention effects. Third, exploring the underlying mechanisms linking SOK, stress regulation, and PNS activity is crucial. Future research could investigate potential mediators, such as cognitive appraisals or coping strategies, that may explain the relationship between SOK and stress outcomes. Moreover, examining potential moderators, such as age or gender, could help identify individual differences in the effectiveness of SOK-based interventions. Lastly, incorporating more objective measures of stress, such as physiological markers and neuroimaging techniques, would provide a more comprehensive understanding of the physiological processes involved in stress regulation and the impact of SOK-based interventions.
In summary, the findings of this study emphasize the feasibility and effectiveness of Sense of Okayness (SOK) interventions in reducing subjective stress levels among elderly individuals. The results also highlight an intriguing observation regarding the more pronounced and fluctuating parasympathetic activity among individuals with lower levels of SOK when transitioning between relaxation and stress states. However, this unexpected finding challenges our initial expectations and warrants further investigation, as it contradicts existing research and theories. Additionally, while this study provides valuable insights into the relationship between SOK, stress, and parasympathetic activity, the underlying mechanisms remain elusive and require continued exploration. Future research should delve deeper into unraveling the intricate pathways and mechanisms through which SOK influences the stress response system. By gaining a more comprehensive understanding of these mechanisms, we can develop targeted interventions that enhance stress resilience and well-being in older adults.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Itai Gilo
Mr. Itai Gilo, PhD candidate, Department of Gerontology, Haifa, research interests stress, Sense of Okayness.
Carla Biegert
Mrs. Carla Biegert, M.Sc. Psychology, University of Konstanz, research interests stress, cortisol.
Dikla Segel-Karpas
Dikla Segel-Karpas is an Associate Professor, currently acting as the Chair of the Department of Gerontology at the University of Haifa, Israel. Her research interests include interpersonal relationships, health, and well-being in older adulthood.
Annika Benz
Dr. Annika Benz, PhD Psychology, University of Konstanz, research interests trauma, heart-rate variability, cortisol.
Maria Meier
Dr. Maria Meier, PhD Psychology, University of Konstanz, research interests energy metabolism, stress regulation, fNIRS.
Yuval Palgi
Yuval Palgi is a full Professor, in the Department of Gerontology at the University of Haifa, Israel. His research interests include posttraumatic stress disorder among older adults and views of aging.
Jens Pruessner
Prof. Dr. Jens Pruessner, PhD Psychology, University of Konstanz, research interests physiological synchronisation, autonomic nervous system, neurodegeneration.
References
- Alvarsson, J. J., Wiens, S., & Nilsson, M. E. (2010). Stress recovery during exposure to nature sound and environmental noise. International Journal of Environmental Research and Public Health, 7(3), 1–12. https://doi.org/10.3390/ijerph7031036 20617017
- Baltes, P. B., & Smith, J. (2008). The fascination of wisdom: Its nature, ontogeny, and function. Perspectives on Psychological Science: a Journal of the Association for Psychological Science, 3(1), 56–64. https://doi.org/10.1111/j.1745-6916.2008.00062.x
- Berna, G., Ott, L., & Nandrino, J.-L. (2014). Effects of emotion regulation difficulties on the tonic and phasic cardiac autonomic response. PloS One, 9(7), e102971. https://doi.org/10.1371/journal.pone.0102971
- Björkman, A. S., Spångeus, A., & Woisetschläger, M. (2019). Mobile learning device increased study efficiency for radiology residents but with risk of temporary novelty effect. Acta Radiologica Open, 8(11), 2058460119889871. https://doi.org/10.1177/2058460119889871
- Bonanno, G. A. (2004). Loss, trauma, and human resilience: Have we underestimated the human capacity to thrive after extremely aversive events? The American Psychologist, 59(1), 20–28. https://doi.org/10.1037/0003-066X.59.1.20
- Brown, J. S., & Brown, L. S. (2014). Psychological resilience in older adults: The role of heart rate variability. Journal of Aging and Health, 26(4), 1350–1365.
- Cohen, S., & Williamson, G. (1988). Perceived stress in a probability sample of the United States. In S. Spacapan & S. Oskamp (Eds.), The social psychology of health (pp. 31–68). Sage.
- Cole, P. M., Hall, S. E., & Hajal, N. J. (2019). What we know: Self-regulation development. Frontiers in Psychology, 10, 1885. https://doi.org/10.3389/fpsyg.2019.01885
- Colzato, L. S., Jongkees, B. J., de Wit, M., van der Molen, M. J. W., & Steenbergen, L. (2018). Variable heart rate and a flexible mind: Higher resting-state heart rate variability predicts better task-switching. Cognitive, Affective & Behavioral Neuroscience, 18(4), 730–738. https://doi.org/10.3758/s13415-018-0600-x
- Crowley, O. V., Kimhy, D., McKinley, P. S., Burg, M. M., Schwartz, J. E., Lachman, M. E., Tun, P. A., Ryff, C. D., Seeman, T. E., & Sloan, R. P. (2015). Vagal recovery from cognitive challenge moderates age-related deficits in executive functioning. Research on Aging, 38(4), 504–525. https://doi.org/10.1177/0164027515593345
- Forte, G., Favieri, F., & Casagrande, M. (2019). Heart rate variability and cognitive function: A systematic review. Frontiers in Neuroscience, 13, 710. https://doi.org/10.3389/fnins.2019.00710
- Gentzler, A. L., Santucci, A. K., Kovacs, M., & Fox, N. A. (2009). Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biological Psychology, 82(2), 156–163. https://doi.org/10.1016/j.biopsycho.2009.07.002
- Hobfoll, S. E., & Wells, J. D. (1998). Conservation of resources, stress, and aging. In Handbook of aging and mental health (pp. 121–134). Springer.
- Hofmann, S. G., Sawyer, A. T., Witt, A. A., & Oh, D. (2010). The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of Consulting and Clinical Psychology, 78(2), 169–183. https://doi.org/10.1037/a0018555
- IBM Corp. (2021). IBM SPSS statistics for Windows, Version 28.0.
- Kemp, A. H., & Quintana, D. S. (2013). The relationship between mental and physical health: Insights from the study of heart rate variability. International Journal of Psychophysiology: official Journal of the International Organization of Psychophysiology, 89(3), 288–296. https://doi.org/10.1016/j.ijpsycho.2013.06.018
- Laborde, S., Brüll, A., Weber, J., & Anders, L. S. (2011). Trait emotional intelligence in sports: A protective role against stress through heart rate variability? Personality and Individual Differences, 51(1), 23–27. https://doi.org/10.1016/j.paid.2011.03.003
- Laborde, S., Mosley, E., & Mertgen, A. (2018). Vagal tank theory: the three rs of cardiac vagal control functioning–resting, reactivity, and recovery. Frontiers in Neuroscience, 12, 458. https://doi.org/10.3389/fnins.2018.00458
- Lazarus, R., & Folkman, S. (1984). Stress, appraisal, and coping. Springer.
- Magnon, V., Dutheil, F., & Vallet, G. T. (2021). Benefits from one session of deep and slow breathing on vagal tone and anxiety in young and older adults. Scientific Reports, 11(1), 19267. https://doi.org/10.1038/s41598-021-98410-7
- Mosley, E., Laborde, S., & Kavanagh, E. (2017). The contribution of coping related variables and cardiac vagal activity on the performance of a dart throwing task under pressure. Physiology & Behavior, 179, 116–125. https://doi.org/10.1016/j.physbeh.2017.05.030
- Muhtadie, L., Koslov, K., Akinola, M., & Mendes, W. B. (2015). Vagal flexibility: A physiological predictor of social sensitivity. Journal of Personality and Social Psychology, 109(1), 106–120. https://doi.org/10.1037/pspp0000016 25545841.
- Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., Cummings, J. L., & Chertkow, H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
- Ong, A. D., Bergeman, C. S., Bisconti, T. L., & Wallace, K. A. (2006). Psychological resilience, positive emotions, and successful adaptation to stress in later life. Journal of Personality and Social Psychology, 91(4), 730–749. https://doi.org/10.1037/0022-3514.91.4.730
- Paret, C., Ruf, M., Gerchen, M. F., Kluetsch, R., Demirakca, T., Jungkunz, M., Bertsch, K., Schmahl, C., & Ende, G. (2016). fMRI neurofeedback of amygdala response to aversive stimuli enhances prefrontal-limbic brain connectivity. NeuroImage, 125, 182–188. https://doi.org/10.1016/j.neuroimage.2015.10.027 26481674.
- Penttilä, J., Helminen, A., Jartti, T., Kuusela, T., Huikuri, H. V., Tulppo, M. P., Coffeng, R., & Scheinin, H. (2001). Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: Effects of various respiratory patterns. Clinical Physiology (Oxford, England), 21(3), 365–376. https://doi.org/10.1046/j.1365-2281.2001.00330.x
- Porges, S. W. (1995). Cardiac vagal tone: A physiological index of stress. Neuroscience and Biobehavioral Reviews, 19(2), 225–233. https://doi.org/10.1016/0149-7634(94)00066-a
- Porges, S. W., Doussard‐Roosevelt, J. A., Portales, A. L., & Greenspan, S. I. (1996). Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology, 29(8), 697–712. https://doi.org/10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O
- Porges, S. W. (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. https://doi.org/10.1016/j.biopsycho.2006.06.009
- Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. https://doi.org/10.1016/s0306-4530(02)00108-7 12892658.
- Rajendra Acharya, U., Paul Joseph, K., Kannathal, N., Lim, C. M., & Suri, J. S. (2006). Heart rate variability: a review. Medical & Biological Engineering & Computing, 44(12), 1031–1051. https://doi.org/10.1007/s11517-006-0119-0 17111118.
- Reitan, R. M., & Wolfson, D. (1993). The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation (2nd ed.). Neuropsychology Press.
- Rodríguez-Liñares, L., Méndez, A. J., Lado, M. J., Olivieri, D. N., Vila, X. A., & Gómez-Conde, I. (2011). An open source tool for heart rate variability spectral analysis. Computer Methods and Programs in Biomedicine, 103(1), 39–50. https://doi.org/10.1016/j.cmpb.2010.05.012 20674067.
- Schlossberg, N. K. (2011). The challenge of change: The transition model and its applications. Journal of Employment Counseling, 48(4), 159–162. https://doi.org/10.1002/j.2161-1920.2011.tb01102.x
- Schmaußer, M., & Laborde, S. (2023). Tonic and phasic cardiac vagal activity predict cognitive-affective processing in an emotional stop-signal task. International Journal of Psychophysiology: official Journal of the International Organization of Psychophysiology, 191, 9–18. https://doi.org/10.1016/j.ijpsycho.2023.06.008
- Scott, B. G., & Weems, C. F. (2014). Resting vagal tone and vagal response to stress: associations with anxiety, aggression, and perceived anxiety control among youths. Psychophysiology, 51(8), 718–727. https://doi.org/10.1111/psyp.12218 24708059.
- Shaffer, F., McCraty, R., & Zerr, C. L. (2014). A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Frontiers in Psychology, 5(2014), 1040. https://doi.org/10.3389/fpsyg.2014.01040
- Smith, A. M., Scott, W. A., Wartenberg, C. E., & Abernethy, B. (2019). Heart rate variability and resilience: The connection between HRV and age. Journal of Gerontology: Psychological Sciences, 74(7), 1136–1144.
- Spangler, D. P., & McGinley, J. J. (2020). Vagal flexibility mediates the association between resting vagal activity and cognitive performance stability across varying socioemotional demands. Frontiers in Psychology, 11, 2093. https://doi.org/10.3389/fpsyg.2020.02093
- Spangler, D. P., Cox, K. R., Thayer, J. F., Brooks, J. R., & Friedman, B. H. (2021). Interplay between state anxiety, heart rate variability, and cognition: An ex-Gaussian analysis of response times. International Journal of Psychophysiology : official Journal of the International Organization of Psychophysiology, 159, 60–70. https://doi.org/10.1016/j.ijpsycho.2020.08.018 33069780.
- Stange, J. P., Alloy, L. B., & Fresco, D. M. (2017). Inflexibility as a vulnerability to depression: A systematic qualitative review. Clinical Psychology: Science and Practice, 24(3), 245. https://doi.org/10.1037/h0101744.
- Thayer, J. F., & Lane, R. D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. https://doi.org/10.1016/s0165-0327(00)00338-4
- Thayer, J. F., Ahs, F., Fredrikson, M., Sollers, J. J., & Wager, T. D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews, 36(2), 747–756. https://doi.org/10.1016/j.neubiorev.2011.11.009 22178086.
- Thayer, J. F., Hansen, A. L., Saus-Rose, E., & Johnsen, B. H. (2009). Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine: a Publication of the Society of Behavioral Medicine, 37(2), 141–153. https://doi.org/10.1007/s12160-009-9101-z
- Thayer, J. F., & Lane, R. D. (2009). Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews, 33(2), 81–88. https://doi.org/10.1016/j.neubiorev.2008.08.004
- Thompson, B. J., Ryan, E. D., Sobolewski, E. J., Conchola, E. C., & Cramer, J. T. (2013). Age related differences in maximal and rapid torque characteristics of the leg extensors and flexors in young, middle-aged and old men. Experimental Gerontology, 48(2), 277–282. https://doi.org/10.1016/j.exger.2012.10.009 23142518.
- Uchino, B. N., Uno, D., & Holt-Lunstad, J. (1999). Social support, physiological processes, and health. Current Directions in Psychological Science, 8(5), 145–148. https://doi.org/10.1111/1467-8721.00034.
- Wolf, O. T. (2009). Stress and memory in humans: Twelve years of progress? Brain Research, 1293, 142–154. https://doi.org/10.1016/j.brainres.2009.04.013
- Wood, R., Maraj, B., Lee, C. M., & Reyes, R. (2002). Short-term heart rate variability during a cognitive challenge in young and older adults. Age and Ageing, 31(2), 131–135. https://doi.org/10.1093/ageing/31.2.131 11937476.