Abstract
The combination of genetic and environmental factors determines the individual vulnerability for excessive ethanol intake, possibly leading to dependence. The environmental influences early in life represent examples of determinant factors for adult behaviour and can be protective as well as risk factors. Maternal separation is one model to examine the long-term consequences of early environmental experiences on neurochemistry and behaviour, including drug-taking behaviour in experimental animals. In the present review, findings from studies using repeated short and prolonged periods of maternal separation, with emphasis on effects on voluntary ethanol intake in rats with or without a genetic predisposition for high voluntary ethanol intake, are summarized. Despite some contradictory results, the general picture emerging shows that short periods of maternal separation during the postnatal period result in a lower adult voluntary ethanol intake in male rats. Prolonged periods of maternal separation were found to induce a high voluntary ethanol intake in male rats, including rats with a genetic predisposition for high ethanol intake. Results from the literature also show that changes were not just related to time of separation but were also related to the degree of handling. Interestingly, in terms of voluntary ethanol intake, female rats were generally not affected by postnatal maternal separation. The reasons for these sex differences need further investigation. In terms of neurobiological consequences of maternal separation, conclusive data are sparse and one of the future challenges will, therefore, be to identify and characterize underlying neurobiological mechanisms, especially in the individual animal.
Introduction
There are large individual differences in predisposition for a high intake of drugs of abuse and vulnerability to develop drug dependence. It is well acknowledged that genetic background, environmental influences, drug availability, sex, personality, history of drug use and other life events affect and contribute to these individual differences. Ethanol dependence is a heterogeneous disorder and the individual vulnerability to develop ethanol dependence is determined by the combination of genetic and environmental factors (Heath et al. Citation1999, Busto Citation2000, Gianoulakis Citation2001, Kreek Citation2001, Dick and Foroud Citation2003, Gunzerath and Goldman Citation2003, Schumann et al. Citation2003, Kreek et al. Citation2004, Schuckit et al. Citation2004). The genetic influence on alcoholism has been estimated to be 50–60% in both men and women (Enoch and Goldman Citation2001). As shown for other substance abuse disorders, co-morbidity with affective disorders and antisocial personality disorders is common (Helzer and Pryzbeck Citation1988, Deas and Thomas Citation2002, Petrakis et al. Citation2002, Kendler et al. Citation2003). Various protective factors influence the development of drug dependence, as indicated by the fact that even though many people experiment with drugs of abuse and start to occasionally use the drug, only a limited number acquire dependence as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM IV) (Bohman et al. Citation1984, DSM Citation2000, Lende and Smith Citation2002, Chambers et al. Citation2003, Vanyukov et al. Citation2003). In many countries, ethanol use is common and unfortunately the abuse of ethanol is increasing, especially in the younger population (Hibell et al. Citation2004). To counteract this trend, drug dependence prevention is essential and one approach in this work is to increase the knowledge of protective and risk factors linked to the propensity for initiation of excessive drug intake.
The environmental influence early in life represents one example of a determinant factor for adult behaviour. The role of early life experiences in the development of neuronal systems and the subsequent establishment of neurophysiological and behavioural functions has been studied extensively, as further described in a separate section. In humans, adverse experiences early in life, such as parental loss, neglect, distant parent-child relationships and physical and/or sexual abuse can result in neurobiological events with a potential to cause changes in brain development (Teicher et al. Citation2003) and enhance the vulnerability to develop adult psychopathology including depression, anxiety disorders and posttraumatic stress disorder (Canetti et al. Citation1997, De Bellis Citation2002, Gilmer and McKinney Citation2003, Langeland et al. Citation2004, Nemeroff Citation2004). Moreover, early life experiences have been shown to affect intake of drugs of abuse later in life (Langeland and Hartgers Citation1998, Spak et al. Citation2001, Gordon Citation2002, Langbehn et al. Citation2003). In recent years gene-environment interactions have gained increasing interest and human studies have shown that genotypes could moderate children's sensitivity to early life experiences and the long-term effects on later psychopathology (Caspi et al. Citation2002, Caspi et al. Citation2004, Caspi et al. Citation2003, Foley et al. Citation2004). Furthermore, it has been demonstrated that genotype and family relationships, as well as the interactions between these variables, could predict adolescent ethanol consumption (Nilsson et al. Citation2005). In non-human primates, it was revealed that genetic profiles could affect ethanol sensitivity and subsequent ethanol intake as a consequence of environmental manipulations (Barr et al. Citation2003, Barr et al. Citation2004). It can thus be concluded that besides genomic programming, environmental factors contribute to the development of the central nervous system and may influence the establishment of mental functions in the adult individual.
The neurobiological mechanisms underlying risk factors and protective factors for the vulnerability to develop psychopathology, including drug dependence, are not fully understood. Longitudinal studies in humans are time-consuming and studies that control for environmental stimuli are almost impossible to conduct. Primates are being used but they have a relatively long life span and experiments in rodents are sometimes favourable. Rodents are frequently used in addiction research, because they show preference for drugs of abuse, self-administer drugs in various experimental paradigms and show addiction-like behaviour (Spanagel Citation2003, Deroche-Gamonet et al. Citation2004, Rodd et al. Citation2004). Rodent models thus enable us to investigate neurobiological mechanisms related to drug addiction processes. The focus in the current review is on factors linked to the initiation of high ethanol intake, i.e. to the vulnerability to develop ethanol dependence. In these studies, rodent models are useful to further examine those environmental risk and protective factors and to identify the neurobiological mechanisms underlying the individual adult drug-taking behaviour. We are currently using maternal separation procedures in a series of experiments to examine the long-term consequences of early environmental experiences on voluntary ethanol intake, neurochemistry and behaviour in rats (Ploj et al. Citation2002, Citation2003a, Citationb, Roman et al. Citation2003, Citation2004, Roman Citation2004, Roman et al. Citation2005, Gustafsson et al. Citation2005). In the present review these findings are summarized, together with results from other studies using repeated short and prolonged periods of maternal separation, with emphasis on effects upon voluntary ethanol intake.
Brain ontogeny during the postnatal period
The present review deals with early environmental factors and, more specifically, studies in rats where manipulations of the environment occur during the postnatal period. As a background, a short description of neuronal development of relevant transmitter systems is provided with reference to extensive reviews within this area. The trajectory of brain development occurs in multiple stages and different systems and different brain regions have a unique course of ontogeny. Neuronal rearrangements occur mainly during two phases in life, immediately before birth and during the preadolescence period (Andersen Citation2003). Relative to species that give birth to well-developed offspring, the brain growth spurt in rats begins around the time for birth and lasts up to the third postnatal week. The fetal stage is characterised by a rapid increase in brain size, due to enhanced cellular proliferation and differentiation. Although central nervous system cellular proliferation and differentiation continue after birth, processes of maturation of neuronal circuitry gain increasing significance during the postnatal period. The stages of neurogenesis, migration and differentiation occur in a time- and region-specific manner in the developing brain and damage during these stages may show a variety of consequences (Kaufmann Citation2000). In rats, the period roughly lasting from postnatal day (PND) 4–14 has been identified as the stress hypo-responsive period (SHRP). During this period the developing rat pup does not show a glucocorticoid response to mild stressors. One explanation for this reduced responsiveness is that the developing brain is sensitive to damage and the SHRP may serve as a barrier protecting against high levels of circulating glucocorticoids (Sapolsky and Meaney Citation1986, Levine Citation2002b). It has also been demonstrated that the SHRP is characterised by a number of specific features underlying the function of the hypothalamic-pituitary-adrenal (HPA) axis during the postnatal period (de Kloet et al. Citation2005).
The HPA axis represents one system that is suggested to be involved in ethanol reward mechanisms (elaborated in a later section) and is, therefore, interesting with regard to early environmental influences. A detailed description of the ontogeny of the HPA axis and the programming of brain development has recently been published (de Kloet et al. Citation2005, Owen et al. Citation2005). Other transmitter systems closely related to ethanol dependence mechanisms include monoaminergic systems, opioid peptide systems as well as gamma-aminobutyric acid (GABA) and glutamate systems (described in a later section). A summary, covering development of monoamines, acetylcholine, amino acid transmitters and some neuropeptides has recently been published (Herlenius and Lagercrantz Citation2004). Dopaminergic neurons appear during the fetal stage in rats, earlier in females than in males (Herlenius and Lagercrantz Citation2004). At birth, markers of dopamine activity, including tyrosine hydroxylase, dopamine uptake sites and dopamine content are approximately 10% of adult levels. These markers increase during the postnatal period and reach adult levels between 4–5 weeks of life. The ontogeny of dopamine receptor density shows a linear increase and attains adult-like density at 4 weeks of age (Andersen Citation2003). Serotonergic neurons are among the earliest to be generated in the fetal brain, first in the raphe nucleus followed by a diffuse projection. The full maturation of the axon terminal network requires more time and is achieved after birth. An overview shows that serotonin has different target receptors at different times during development and in different tissues, but knowledge is still limited (Jacobs and Azmitia Citation1992, Gaspar et al. Citation2003).
Gene expression of glutamate receptors and transporters show distinct area and time specific expression during rat central nervous system (CNS) development. The expression of the N-methyl-D-aspartate (NMDA) receptor subunits and levels of the corresponding proteins as well as glutamate transporters differ across the postnatal period. Especially during the SHRP, the CNS exhibits enhanced susceptibility to modulation of the NMDA receptor system. Both excessive activation and disruption of the NMDA receptor system has, through different mechanisms, the potential to mediate apoptotic neurodegeneration (Haberny et al. Citation2002). It has been suggested that maternal separation has the potential to cause an insufficient NMDA receptor activation leading to increased apoptosis in the immature brain that could have long-term consequences for brain function (Anand and Scalzo Citation2000).
GABA has long been considered to be the main inhibitory neurotransmitter in the adult mammalian brain. However, during early brain development, GABA acts as a trophic factor to influence events such as proliferation, migration, differentiation, synapse maturation and cell death. GABA-releasing synaptic contacts have been identified around birth and it has been demonstrated that these synapses are anatomically as well as physiologically developed in the neonatal rat but the functional properties of GABA receptor signalling in the immature brain are different and in some ways are opposite to those found in the adult brain (Owens and Kriegstein Citation2002).
The ontogenetic profile of the endogenous opioid system in the rat is complex with developmental patterns of the three precursors occurring, independently, and with distinct profiles for the opioid peptide products. Opioid peptide products are all detectable during the fetal stage, but full expression is not completed until well after birth, with the third postnatal week often exhibiting the most marked increases. Opioid receptor types show distinct ontogeny. Mu and kappa opioid receptor sites are present at birth while delta opioid receptor sites are absent until the second postnatal week. The maturation of mu and delta opioid receptors is completed during the third and fourth postnatal weeks, respectively. Few studies have investigated peptide and receptor development in parallel and it is, therefore, unclear whether specific peptide development is directly linked to that of a single receptor site (McDowell and Kitchen Citation1987).
Taken together, transmitter systems related to ethanol addiction processes undergo developmental changes during the postnatal period. Most studies merely report the presence of transmitter substances, transporters or receptors, whereas less is known about their function and various transmitter system interactions in the developing brain. However, since the postnatal period is the targeted time period in the maternal separation model, a plausible conclusion is that stressful experiences during this period can have consequences for ethanol taking behaviour in adult life, via actions on specific developing neural systems.
The maternal separation model
Background
Newborn mammals are dependent on their mothers (or primary care giver) not only for survival but also for normal development. Maternal factors are, for instance, critical for the maintenance of the SHRP, actively inhibiting the endocrine responses to stress during the postnatal period (Sapolsky and Meaney Citation1986, Levine Citation2002b). Maternal behaviour has also been shown to be of importance for epigenetic programming, a mechanism that can explain the long-lasting effects of maternal care on gene expression in the offspring (Weaver et al. Citation2004). The early relationship between mother and offspring is an important environmental factor during early development in mammals and separation of rat pups from their dams during the postnatal period results in a variety of physiological changes, the nature of which is dependent on the specifics of the separation experience, the environmental conditions and the duration of the separation (Hofer Citation1994, Lehmann and Feldon Citation2000, Pryce and Feldon Citation2003). In the course of normal rat mother-pup interaction in the wild, the dam shows an intense care giving behaviour, but regularly leaves the nest and the pups for short periods of time (Grota and Ader Citation1969, Fleming and Rosenblatt Citation1974). However, during semi-naturalistic conditions, subordinate females have been shown to be forced to locate nests at some distance from food and water sources, resulting in more prolonged periods of separation (Calhoun Citation1962).
Research on early environmental manipulations and the consequent effects on development, physiology and behaviour originate from early studies in rodents (e.g. Weininger Citation1954, Levine Citation1957, Denenberg and Smith Citation1963). For instance, it was found that pups that had been held in the experimenter's hand and gently stroked (gentled) for a few minutes developed differently from non-handled pups (Weininger Citation1954). It was further discovered that it was not necessary to hold the pup in the hand stroking it, as similar effects could be achieved by only separating the pups from the mother (handling) (Levine Citation1957, Denenberg and Smith Citation1963). The results of this short maternal separation, with or without stroking, were found to have profound long-term effects including an altered HPA axis response, decreased emotional reactivity and enhanced learning performance and attention abilities (Levine Citation2002a). Along with these findings, the search began for an animal model with opposite effects suitable to mimic human symptoms of depression and anxiety disorders, and for this more prolonged periods of maternal separation were used (Kuhn and Schanberg Citation1998, Arborelius et al. Citation1999, Ladd et al. Citation2000, Newport et al. Citation2002, Matthews and Robbins Citation2003).
The underlying mechanisms for the different effects of short and prolonged periods of maternal separation are not fully understood but may partly be mediated by changes in maternal behaviour. Short periods of maternal separation can be viewed as mimicking the behaviour of wild rats where the mother has to leave her pups to collect food. Upon reunion, after short periods of maternal separation, the mother shows an increased intensity of maternal behaviour with more licking and grooming of her pups (Liu et al. Citation1997, Pryce et al. Citation2001, Macri et al. Citation2004). On the other hand, contradictory findings on maternal care after prolonged periods of separation have been reported, with both decreased (Huot et al. Citation2000) as well as increased (Pryce et al. Citation2001, Macri et al. Citation2004, Marmendal et al. Citation2004) amounts of maternal care reported. Based on these findings short periods of maternal separation can be viewed as a safe environment, fairly close to the natural behaviour in the wild. Regarding longer periods of maternal separation, the interpretation of this as an adverse or stressful experience may be dependent on the duration of the separation. The more prolonged periods of repeated maternal separation might serve as an un-natural condition with a possibility to cause long-term behavioural and neurochemical alterations.
Common protocols for repeated maternal separation
Experimental protocols for short periods of repeated maternal separation involve repeated separation from the dam and sometimes also the littermates for periods ranging between 3–15 min, also referred to as handling. For instance, short periods of maternal separation result, with a relatively consistent picture, when the offspring are adult, in HPA axis hyporesponsiveness, decreased emotional reactivity, improved cognitive performance and alterations in a number of transmitter systems (Anisman et al. Citation1998, Ladd et al. Citation2000, Lehmann and Feldon Citation2000, Levine Citation2002a, Newport et al. Citation2002, Pryce and Feldon Citation2003).
Common experimental protocols for prolonged periods of repeated maternal separation involve separations for >60 min, most often > 180 min. Interestingly, prolonged periods of maternal separation result in effects that are different, and often opposite, from those observed after short periods of maternal separation (Kuhn and Schanberg Citation1998, Anisman et al. Citation1998, Anand and Scalzo Citation2000, Ladd et al. Citation2000, Lehmann and Feldon Citation2000, Newport et al. Citation2002, Pryce and Feldon Citation2003, Cirulli et al. Citation2003). Results among studies that utilized prolonged periods of maternal separation are, however, sometimes contradictory. These conflicting results appear to be due to large variations in the experimental protocols used, such as frequency and duration of separation, individual or litter separation, temperature and light/dark cycle as well as different control groups used for comparisons. It has for instance been shown that maternal separation performed under different thermal and circadian conditions had different effects on pup body weight and on the amount of maternal care received. At weaning, pups separated under cold conditions (21°C) were of lower body weight than pups separated under warm conditions (32°C), and the pups separated under warm conditions were of lower body weight than non-handled controls. Furthermore, pups separated during the light as well as the dark period received a greater amount of maternal care at reunion relative to non-handled pups, and pups separated under cold conditions received greater care at several hours after reunion compared to pups separated under warm conditions and non-handled pups (Ruedi-Bettschen et al. Citation2004). Variations in protocols have been reviewed and discussed in excellent reviews (Lehmann and Feldon Citation2000, Pryce and Feldon Citation2003) and it is beyond the scope in the present review to give a detailed description of the various experimental protocols used. Briefly, several different protocols for maternal separation are currently in use in which the pups are separated from the dam either in litters or isolated from other littermates. Maternal separation can be performed on a single occasion (referred to as maternal deprivation), repeatedly at randomly chosen time periods, daily during parts of or the entire SHRP, as well as daily during the entire postnatal period.
Control groups in maternal separation models
Control groups for comparisons are often either non-handled or housed under normal animal facility rearing (AFR) conditions with regular cage cleaning procedures. Non-handled pups are left completely undisturbed and never handled whereas AFR pups have experienced human contact during cage cleaning. The question has been raised whether rat pups require a minimal amount of stimulation in order to develop into adult rats with a behavioural profile typical for laboratory rats and that non-handling of pups might be below this limit (Pryce and Feldon Citation2003). However, the use of AFR as a control is not without problems since different animal facilities might vary in animal husbandry (Crabbe et al. Citation1999). Few studies have investigated the influence of the choice of control groups. In a recent study investigating long-term effects of maternal separation on voluntary ethanol intake, an elegant design was used in order to control for time of separation, handling and rearing conditions. During the postnatal period, litters were assigned to MS0 (maternal separation zero min; pups were picked up by the tail and replaced), a short and a prolonged period of maternal separation, respectively, non-handled (handled once for a bedding change) and AFR. In adulthood, these rats were tested for voluntary ethanol intake. It was demonstrated that the rats subjected to a short period of maternal separation consumed significantly less ethanol compared to the rats subjected to prolonged periods of maternal separation but neither group differed from the MS0 or the AFR group of rats. In addition, the non-handled group of rats had the greatest ethanol intake and consumed significantly more than all other groups. These findings, therefore, demonstrated that the results were not simply related to time of separation but also to the degree of handling (Jaworski et al. Citation2005).
Maternal separation and reward
The findings of persistent behavioural consequences after postnatal short and prolonged maternal separation have raised the question whether maternal separation also could influence adult drug intake behaviour. That is, is this model useful in studies of neurobiological substrates for individual differences in vulnerability for drug dependence, for instance ethanol dependence?
Ethanol reward mechanisms
As previously described, a normal development of many brain transmitter systems is dependent on postnatal environmental influences, such as mother-infant interactions, suggesting that maternal separation could affect adult brain reward function. In drug reward and addiction processes, a variety of endogenous systems are involved (e.g. Di Chiara Citation1999, Koob and Le Moal Citation2001, Robinson and Berridge Citation2001, Wise Citation2004). Ethanol has a more complex mechanism of action than most other drugs of abuse. Ethanol has the ability to change the function of membrane proteins such as ligand-gated ion channels, like the GABAA receptor and the NMDA receptor, ion channels and G-proteins (Fadda and Rossetti Citation1998, Koob et al. Citation1998, Boehm II et al. Citation2005). Accordingly, ethanol can affect the function of a number of endogenous systems. Of particular interest as target systems for postnatal manipulation are those systems involved in reinforcing effects of ethanol and/or with a potential role in a predisposition for excessive ethanol intake and ethanol dependence. The endogenous opioid system (Nylander and Silberring Citation1998, Van Ree et al. Citation2000, Gianoulakis Citation2004, Oswald and Wand Citation2004) and the mesocorticolimbic dopamine system (Di Chiara et al. Citation1996, Gianoulakis Citation1996, Koob et al. Citation1998) have been shown to be involved in acquisition and maintenance of ethanol dependence. Recent gene knock-out studies confirm these findings (Kieffer and Gaveriaux-Ruff Citation2002, Savelieva et al. Citation2002). Ethanol reinforcement includes activation of opioid transmission and subsequent dopamine release and opioid antagonists, like naltrexone, reduce both ethanol-induced dopamine release and ethanol consumption (Di Chiara et al. Citation1996, Koob et al. Citation1998, Gianoulakis Citation2004, Oswald and Wand Citation2004). Currently, naltrexone is used in the treatment of ethanol dependence (O'Malley et al. Citation1992, Volpicelli et al. Citation1992). It has also been demonstrated that ethanol-preferring animals have deranged opioid systems suggesting that individual differences in opioid systems may be linked to an enhanced risk for development of ethanol dependence (Nylander et al. Citation1994, Nylander et al. Citation1995, Jamensky and Gianoulakis Citation1997, Citation1999, Ploj et al. Citation2000). Another system of interest related to predisposition for ethanol dependence is the serotonergic system (Katner and Weiss Citation2001, Naranjo et al. Citation2002, Vazquez et al. Citation2002, Kelai et al. Citation2003, McBride et al. Citation2004, Oreland Citation2004). Individual differences in, for instance, monoamino-oxidase function and genes encoding for the serotonin transporter have been implicated in an increased risk for alcoholism (Barr et al. Citation2003, Barr et al. Citation2004, Oreland Citation2004, Lin et al. Citation2005). Finally, additional systems that have been shown to be involved in ethanol addiction processes, and thus interesting in studies of environmental influences on ethanol intake, are the corticotropin-releasing factor (CRF) (Valdez and Koob Citation2004, Bruijnzeel and Gold Citation2005), nociceptin/orphanin FQ (Ciccocioppo et al. Citation2000, Ciccocioppo et al. Citation2003) and neuropeptide Y (Heilig and Thorsell Citation2002, Valdez and Koob Citation2004) systems.
Stress and ethanol interactions
Several lines of evidence have shown that stress can influence ethanol addiction processes, including acquisition as well as maintenance and relapse. Firstly, the prominent separation-induced effects on HPA axis function (Lehmann and Feldon Citation2000, Levine Citation2002a, Pryce and Feldon Citation2003), and secondly the effects of glucocorticoids on brain reward systems (Piazza and Le Moal Citation1996) suggest that also ethanol intake may be affected. Stress facilitates the acquisition of ethanol intake and can also induce an increase in ethanol intake (Pohorecky Citation1990, Citation1991, Piazza and Le Moal Citation1996, Brady and Sonne Citation1999, Boehm II et al. Citation2005). Activation of CRF and subsequent adrenal corticosteroid hormone release is known to enhance voluntary ethanol intake (Piazza and Le Moal Citation1996, Fahlke and Hansen Citation1999). There is also evidence that CRF can modulate ethanol intake through mechanisms within the brain (Koob Citation1999). Thirdly, overwhelming evidence indicates a general close interaction between stress and dependence mechanisms (Piazza and Le Moal Citation1996, Kreek and Koob Citation1998, Thadani Citation2002) and altogether this suggests that early stressful experiences during the postnatal period, for instance induced by maternal separation, have the potential to influence voluntary drug intake, including ethanol intake.
Maternal separation and reward mechanisms
Hitherto, several lines of evidence for alterations in brain reward systems after maternal separation procedures have indeed been presented, both at the neurobiological and behavioural level. In studies of neurochemical consequences of maternal separation, changes in mesocorticolimbic dopamine neurons have been observed. An increase in tissue levels of dopamine (Matthews et al. Citation2001) and an enhanced K+-induced release of dopamine (Hall et al. Citation1999) in the nucleus accumbens have been described. In addition, the dopamine transporter is affected in this area, as lower densities of transporter sites were found after prolonged maternal separation (Meaney et al. Citation2002, Brake et al. Citation2004). In contrast, only minor changes have been found in dopamine receptor binding (Meaney et al. Citation2002, Ploj and Nylander Citation2003) or sensitivity (Matthews et al. Citation1999). Another monoamine of interest for vulnerability to ethanol abuse and also affected by maternal separation is serotonin (Matthews et al. Citation2001, Vazquez et al. Citation2002).
The opioid dynorphin and enkephalin systems are also changed after maternal separation. Using maternal separation protocols with the pups being isolated from each other whilst separated from the mother, long-term changes in the dynorphin system have been observed in the pituitary gland, hypothalamus, striatum and hippocampus after short periods of maternal separation (Ploj et al. Citation1999). As for the enkephalin system, alterations in tissue levels and extracellular levels of enkephalin have been described in the nucleus accumbens after prolonged maternal separation (Vazquez et al. Citation2005). Maternal separation with the litters kept intact resulted in less pronounced effects; the dynorphin levels were altered in the hypothalamus, pituitary gland, amygdala, substantia nigra and the periaqueductal gray in the adult animal, but alterations in enkephalin levels were observed only in the hypothalamus (Ploj et al. Citation2003b). The dynorphin system was affected mainly after short periods of separation while prolonged separation affected the pituitary gland and the periaqueductal gray (Ploj et al. Citation2003b). Litter separation was shown to cause long-term changes also in the nociceptin/orphanin FQ system, which was affected in the hypothalamus, medial prefrontal cortex and the periaqueductal gray (Ploj et al. Citation2002). As shown for dopamine receptors, maternal separation left opioid as well as opioid receptor-like 1 receptor binding largely unaffected (Ploj and Nylander Citation2003, Ploj et al. Citation2003a, Vazquez et al. Citation2005). The effects of maternal separation have been shown to be gender specific. In female rats, both the opioid and nociceptin/orphanin FQ peptide systems were generally less affected by maternal separation than in males (Ploj et al. Citation2001, Gustafsson et al. Citation2005). At present, with the available data it is difficult to draw any conclusions regarding the relevance of these alterations in peptide levels for the propensity to drink ethanol in the individual rat. The major obstacle is the presence of individual differences in separation-induced effects on ethanol intake (described in a later section) and the difficulty of assessing whether the animal in which an altered basal peptide level is observed would have acquired a low or high ethanol intake. That is, to better correlate separation-induced neurobiological effects, subsequent behavioural effects and ethanol intake, more research information in individual animals has to be provided. However, it is an interesting finding that maternal separation results in long-term changes in systems otherwise known to be linked to predisposition for ethanol dependence.
As indicated by the neurochemical studies, maternal separation also induces changes in the response to reward-related stimuli. The responsiveness of the mesocorticolimbic dopamine neurons was, for instance, altered. An increased sensitivity to psychostimulant-induced behavioural effects (Zimmerberg and Shartrand Citation1992, Meaney et al. Citation2002), and an enhanced dopamine release in response to both stress and psychostimulants (Kehoe et al. Citation1996, Kehoe et al. Citation1998, Hall et al. Citation1999, Brake et al. Citation2004), represent examples of consequences after prolonged maternal separation.
Maternal separation and drug self-administration
All these consequences of maternal separation indicate that postnatal environmental influences may result in an altered liability to use drugs of abuse in adult life. Alterations in drug-taking behaviour have also been described after maternal separation in rats. Several studies have focused on early environmental influence on adult cocaine self-administration, where results indicate gender-specific and dose-dependent effects (e.g. Matthews et al. Citation1999, Kosten et al. Citation2004, Marquardt et al. Citation2004, Lynch et al. Citation2005, Zhang et al. Citation2005). Furthermore, an increased morphine self-administration has been reported in rats subjected to prolonged maternal separation (Vazquez et al. Citation2005). These results and other results concerning effects of environmental stress on opiate and psychostimulant self-administration as well as place preference are summarized in a recent review (Lu et al. Citation2003).
Taking the results from the above studies together, maternal separation in rats directly affects brain areas involved in ethanol reward as evidenced by long-term neurochemical changes in these areas and as an altered responsiveness to rewarding stimuli. Maternal separation also indirectly affects brain reward mechanisms by inducing alterations in systems regulating reward circuits and by inducing changes in behaviours that, in turn, can affect drug-taking behaviours. These results thus show that experimental manipulation of the environment during the postnatal period in rats may serve as a useful model in studies of long-term effects of early environmental factors on ethanol intake; in the next section, focus is on voluntary ethanol intake after maternal separation.
Maternal separation and voluntary ethanol intake
A number of studies have assessed the impact of different protocols for maternal separation during the postnatal period on later voluntary ethanol intake in rats, as summarized in .
Table I. A summary of different protocols used for investigation of the effects of short and prolonged periods of maternal separation and the effects on voluntary ethanol intake in rats. For detailed information see the original articles.
Short periods of maternal separation and voluntary ethanol intake
To our knowledge, only two studies have used only short periods of maternal separation in comparison with control groups (Weinberg Citation1987, Lancaster Citation1998). When comparing individual pup separation for 3 min during PND 1–14 with non-handled controls and a control group consisting of pups that were weighed once weekly, a lower ethanol intake and preference was found in the separated male and female rats in comparison with the weighed control rats while non-handled rats were not different from either group (Weinberg Citation1987). Another protocol used individual separation for 15 min during PND 1–7 and non-handled controls in male and female Long-Evans rats. From PND 7–21 all litters remained undisturbed. Starting on PND 25, voluntary intake of beer (containing 5% ethanol) was investigated until PND 85. In the male rats it was shown that the separated rats had a greater ethanol intake during the peripubertal period (PND 32–43) in comparison with non-handled males. This period was followed by a period of 30 days with no differences between separated and non-handled males, except five random days during PND 73–83 when the separated rats again had a higher ethanol intake than the male controls. In the female rats, only occasional differences in voluntary ethanol intake were detected. The non-handled females had a greater intake in comparison with separated females on PND 32 and on three random days the intake was greater in the separated females compared to the non-handled rats (Lancaster Citation1998).
Short and prolonged periods of maternal separation and voluntary ethanol intake
Most studies have utilized repeated short and prolonged periods of maternal separation and various control groups in order to investigate long-term effects on ethanol intake. One exception is the study by Marmendal et al. (Citation2004), in which male and female Wistar rats experiencing 240 min maternal separation during PND 1–15 were compared with control litters that experienced 5 min of maternal separation. An investigation of voluntary ethanol intake at approximately PND 100 revealed no differences between the two groups, either in males or in female rats (Marmendal et al. Citation2004). As compared to the other protocols, this is, to our knowledge, the only study that performed the separations during the dark period of the light/dark cycle. Furthermore, the rats were frequently handled during behavioural tests prior to access to ethanol. These experimental factors may have affected the results and possibly explain the absence of maternal separation-induced effects on voluntary ethanol intake, effects that have been observed in other studies.
Hilakivi-Clarke et al. (Citation1991) used a protocol in male rats consisting of 15 min handling, during which time the pups were held in the hand of an investigator for 3 min, or a mixed litter/individual separation for 60 min during PND 5–20. Those two groups were compared with control rats that experienced cage cleaning once weekly and control non-handled pups, which were housed with the handled pups. At PND 80 voluntary intake of 5% ethanol was assessed during a period of 12 days. It was found that handled rats consumed significantly less ethanol than the control rats that experienced cage cleaning, the control non-handled rats and the isolated rats. The control non-handled group was the group with the greatest ethanol intake, and consumed significantly more than both the controls that experienced cage cleaning and the isolated rats (Hilakivi-Clarke et al. Citation1991).
Another study compared daily 15 min maternal separation (MS15) and 180 min maternal separation (MS180) in litters during PND 2–14 and AFR litters in Long-Evans rats (Huot et al. Citation2001). In adulthood, the rats had access to 8% ethanol in 2.5% sucrose and 2.5% sucrose alone. The results showed a reduced sucrose intake and a greater ethanol intake in male MS180 rats compared to MS15 and AFR rats, while no differences were found between MS15 and AFR rats in either sucrose intake or ethanol intake. Interestingly, treatment with the selective serotonin reuptake inhibitor paroxetine reduced ethanol consumption in MS180 rats compared with untreated MS180 rats, while not affecting consumption in MS15 and AFR rats (Huot et al. Citation2001).
A recent study using Long-Evans rats employed an interesting design. During PND 2–14, litters were assigned to MS0 (maternal separation zero; pups were picked up by the tail and replaced), MS15, MS180, non-handled (handled once for a bedding change) and AFR. In adulthood, these rats had access to 8% ethanol in 2.5% sucrose and 2.5% sucrose water for five days. It was demonstrated that MS15 rats consumed significantly less ethanol compared to MS180 rats but neither group differed from the MS0 or the AFR group of rats. In addition, the non-handled group of rats had the greatest ethanol intake and consumed significantly more than all other groups (Jaworski et al. Citation2005). These results demonstrate that the observed changes were not just related to the time of separation but also to the degree of handling.
In a series of experiments, the same experimental protocol was used, comprising daily repeated maternal separation in litters for 15 min (MS15) or 360 min (MS360) during PND 1–21, with AFR controls as a comparison group. Of special interest in these studies was the impact of environmental factors on the individual differences in acquisition of ethanol intake. The long-term effects on acquisition of voluntary ethanol intake and subsequent ethanol intake using increasing concentrations of ethanol were, therefore, investigated. In brief, the adult rats were housed individually from the first day of access to ethanol enabling measurement of individual ethanol intake. A bottle of an ethanol solution was offered in addition to the water bottle i.e. a two-bottle free choice paradigm with continuous access to ethanol. The ethanol concentration was gradually increased from 2% ethanol (v/v) in tap water, to 4, 6, 8% and in two studies 10% ethanol during a period of approximately two weeks. Male and female rats were studied and Wistar rats were maintained on 8% ethanol (Ploj et al. Citation2003a, Roman et al. Citation2004, Gustafsson et al. Citation2005), ethanol-preferring AA (Alko, Alcohol) rats were maintained on 10% ethanol (Roman et al. Citation2003, Citation2005) and ethanol-avoiding ANA (Alko, Non-Alcohol) rats finally had access to 6% ethanol (Roman et al. Citation2005).
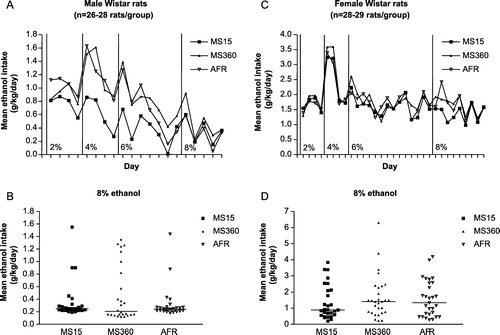
Analysis of ethanol intake (g/kg/day) in adult male Wistar rats (n = 26–28 rats/group) showed that MS15 rats consumed less ethanol than MS360 and AFR rats throughout the period of 26 days of voluntary ethanol consumption ( A). However, a large variation in intake was found within the groups ( B) and statistical analyses of ethanol intake at the different ethanol concentrations (2, 4, 6 and 8%, respectively) revealed no significant differences in ethanol intake or preference (percentage of total fluid intake). In the MS360 group of rats, a larger number of animals initiated drinking and had a greater ethanol intake, indicating the presence of subgroups with different inter-individual susceptibility to the effects of maternal separation ( B). Therefore, the ethanol intake after maternal separation in those males that initiated drinking and had the highest ethanol intake in each group (n = 8 rats/group) was compared and it was found that male MS15 rats consumed significantly less ethanol than MS360 and AFR rats at 2, 4 and 6% ethanol and significantly less than MS360 rats at 8% ethanol. At 8% ethanol, the AFR rats reduced their intake whereas the MS360 rats consumed significantly more 8% ethanol than both MS15 and AFR rats (Ploj et al. Citation2003a). Interestingly, no long-term differences between the three experimental groups were found for effects of maternal separation on adult voluntary ethanol intake in female Wistar rats ( C), and the individual differences were not as pronounced in female rats ( D) as in males (Roman et al. Citation2004, Gustafsson et al. Citation2005).
Figure 1 Maternal separation and long-term effects on voluntary ethanol intake in Wistar rats. A and C. Mean daily voluntary ethanol intake (g/kg/day) during continuous access to the different ethanol concentrations used in adult male (A) and female (C) Wistar rats subjected to maternal separation for 15 min (MS15), or 360 min (MS360), or normal animal facility rearing (AFR) during postnatal days (PND) 1–21. Note the different values on the, respective, y-axis. B and D. Mean ethanol intake (g/kg/day) during the period of 8% ethanol availability in each individual male (B) and female (D) Wistar rat. The horizontal lines indicate group medians. Note the different values on the respective y-axis. The figure is based on male rat data in Ploj et al. (Citation2003a) and female rat data in Gustafsson et al. (Citation2005).
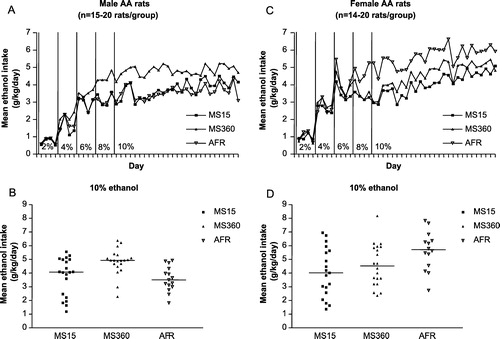
The use of the ethanol-preferring AA and ethanol-avoiding ANA rats afforded an opportunity to study the impact of early environmental factors in rats with an inherited high and low voluntary ethanol intake, respectively (Roman et al. Citation2003, Citation2005). In a first study, male AA rats were used, the number of animals was limited and the period of access to ethanol was relatively short. The respective ethanol intake pattern indicated that the MS15 rats had a delayed acquisition phase and thus, it took longer to reach a high ethanol intake in MS15 rats compared to both MS360 and AFR rats. The AFR rats reached a high, stable ethanol intake in a pattern similar to that generally seen in AA rats at 8% ethanol, whereas MS360 rats reached a stabilized intake already at 6%, and MS15 rats did not stabilise intake until 10% ethanol was available. The statistical analyses revealed that the MS15 rats had a significantly lower ethanol intake at 8% ethanol in comparison with both MS360 and AFR rats and at 10% ethanol compared to MS360 rats, while the MS360 rats showed a trend towards a greater ethanol intake compared to AFR rats (Roman et al. Citation2003). In a second, more extensive study, the impact of MS15 and MS360 on acquisition of voluntary ethanol intake and the subsequent ethanol intake in male and female AA and ANA rats was investigated. In the male AA rats ( A), the statistical analyses revealed that the AA MS15 rats had a significantly lower ethanol intake at 8% and 10% ethanol in comparison with AA MS360 rats. In the male AA MS360 rats, a statistically significant greater ethanol intake was found during 8% ethanol as well as during 10% ethanol availability compared to AA AFR rats. In contrast, both separation procedures were shown to reduce ethanol intake in female AA rats, i.e. the AFR rats had the greatest intake, as illustrated in C. In male and female ANA rats, no major separation-induced effects were detected (Roman et al. Citation2005), despite the fact that the ANA line of rats is suggested to be more susceptible to various stressors than AA rats (e.g. Tuominen et al. Citation1990, Möller et al. Citation1997, Overstreet et al. Citation1997). However, it is also possible that the main factor controlling ethanol consumption in ANA rats is the accumulation of acetaldehyde during ethanol exposure (Eriksson Citation1973, Hilakivi et al. Citation1984, Sinclair et al. Citation1989), which may limit the effect of any manipulation that would elevate ethanol intake in other rats without this metabolic trait.
Figure 2 Maternal separation and long-term effects on voluntary ethanol intake in ethanol-preferring AA (Alko, Alcohol) rats. A and C. Mean daily voluntary ethanol intake (g/kg/day) during continuous access to the different ethanol concentrations used in adult male (A) and female (C) (AA) rats subjected to maternal separation for 15 min (MS15), or 360 min (MS360), or normal animal facility rearing (AFR) during postnatal days (PND) 1–21. B and D. Mean ethanol intake (g/kg/day) during the period of 10% ethanol availability in each individual male (B) and female (D) AA rat. The horizontal lines indicate group medians. The figure is based on data in Roman et al. (Citation2005).
Taken together, these results indicate that most studies on effects of 15 min postnatal separations result in lower ethanol intake in male rats (Weinberg Citation1987, Hilakivi-Clarke et al. Citation1991, Ploj et al. Citation2003a, Roman et al. Citation2003, Jaworski et al. Citation2005), independent of whether the control groups are non-handled or AFR. Along with the results from a short period of maternal separation, prolonged periods of maternal separation show fairly good consistency. Studies on the effects of the MS180 (Huot et al. Citation2001) and MS360 paradigms (Ploj et al. Citation2003a, Roman et al. Citation2005) in male rats showed greater voluntary ethanol intake in comparison with AFR rats. In two studies, the non-handled group of animals had the greatest voluntary ethanol intake of all groups (Hilakivi-Clarke et al. Citation1991, Jaworski et al. Citation2005). Especially notable is the finding of environmentally induced alterations in ethanol intake also in ethanol-preferring rats, indicating a gene-environment interaction. The MS15 procedure reduced ethanol intake while the MS360 procedure resulted in a greater intake in male ethanol-preferring AA rats (Roman et al. Citation2003, Citation2005) with an inherent high voluntary ethanol intake.
Sex differences in the effects of maternal separation on voluntary ethanol intake
It is well acknowledged that psychiatric disorders display differences between males and females in humans as well as experimental animals (Palanza Citation2001). Sex differences in various long-term effects after short and prolonged periods of maternal separation have been reported (Lehmann et al. Citation1999, McIntosh et al. Citation1999, Wigger and Neumann Citation1999, Kalinichev et al. Citation2001, Kalinichev et al. Citation2002, Papaioannou et al. Citation2002a, Citation2002b, Park et al. Citation2003). Review of the studies presented herein lead to the conclusion that maternal separation affects female and male rats similarly in four of the studies (Weinberg Citation1987, Lancaster Citation1998, Marmendal et al. Citation2004, Roman et al. Citation2005). However, in other studies distinct differences between female and male rats were found with no effects of maternal separation in female Wistar rats (Gustafsson et al. Citation2005, Roman et al. Citation2004). These results are in line with the findings in another study using female Wistar rats (Marmendal et al. Citation2004) and the findings in ethanol-avoiding ANA rats (Roman et al. Citation2005) where no effects of maternal separation on ethanol intake were detected. In female ethanol-preferring AA rats, both the MS15 and MS360 paradigms resulted in a reduced ethanol intake compared to AFR rats (Roman et al. Citation2005).
Sex differences in various aspects of intake of drugs of abuse have recently been summarized (Roth et al. Citation2004). The underlying mechanisms for the sex differences observed after maternal separation are at present unknown. Possible explanations may include different separation-induced neurochemical effects in males and females, hormone dependent effects, and/or sex dependent mother-infant behaviour during the postnatal period. An early study found that mother rats interacted differently with male and female offspring (Moore and Morelli Citation1979) while another study demonstrated no such differences (Champagne et al. Citation2003). It has also been reported that natural variations in maternal behaviour can result in sex-specific neurochemical alterations in adulthood (Francis et al. Citation2002). Furthermore, using the MS15 protocol and separating pups individually, in male and female Sprague-Dawley rats, it was demonstrated that the endogenous opioid peptide system was less affected in females than in male rats (Ploj et al. Citation1999, Ploj et al. Citation2001). Another factor of importance is of course the sex hormonal influences in female rats. The ethanol intake in females may vary across the oestrous cycle, although previous results presented a complex picture (Roberts et al. Citation1998, Ford et al. Citation2002). In the study using ethanol-preferring AA rats it was demonstrated that the rats continued their respective individual cycles throughout the experiment and the individual rats within as well as between each experimental group were at different, random stages of the oestrous cycle (Roman et al. Citation2005). This is in agreement with Lau et al. (Citation1996) and also shows that the maternal separation-induced effects on ethanol intake in female AA rats were not secondary to a female sex hormonal influence.
Summary and prospective
In the present review, data concerning the impact of postnatal environmental experiences on adult voluntary ethanol intake in rats have been summarized. The results originate from studies where the maternal separation paradigm has been utilized to create a safe environment (short separation) and an emotional stressful environment (prolonged separation), respectively. The model has then been used to evaluate the consequences on adult ethanol intake, with special emphasis on the propensity to initiate a high ethanol intake, in attempts to identify and further study protective and risk factors. At a first glance, these data tend to give a rather complex picture regarding the outcome of maternal separation and the question whether the model is robust should rightly be addressed. Looking closer, the complexity seems to a great extent relate to variations in the experimental paradigm. Recent reviews, focusing on other effects than ethanol intake, have pointed up a similar complex picture of the consequences of maternal separation (Lehmann and Feldon Citation2000, Pryce and Feldon Citation2003). One of the limitations of this model is that even though the experimental paradigm is identical with regard to experimental conditions, such as temperature and duration of separation, the handling of the rats is critical for the outcome. Another critical factor is the variability between rats, even within the same strain, so that different results can be observed within the same strain, most probably due to genetic diversity, different breeding conditions and also animal housing conditions in the respective laboratory (Crabbe et al. Citation1999). For instance, the Wistar rat strain is not identical across animal suppliers and the AA and ANA rats are selectively outbred, and maternal separation may, therefore, induce different effects depending on the genetic background in these animals. However, in those studies where the same experimental conditions have been used in a series of experiments in the same laboratory, valuable information has been provided concerning the impact of postnatal environmental influences on adult drug intake.
In our hands, short daily maternal separation (15 min) seems to provide a protective environment in the sense that the individual animal shows a low propensity to start drinking ethanol in a two-bottle choice paradigm as an adult. This finding is robust in male rats, and a protective effect is observed also in several other studies, and both in ethanol non-preferring rats and in ethanol-preferring AA rats. This is in agreement with fairly consistent data from previous studies analyzing separation-induced behavioural effects and alterations of the HPA axis. In female rats, the protective effect may be present in AA rats, but in Wistar rats it is either absent or not as pronounced as in male rats and is merely recognized as a trend towards fewer rats with a high ethanol intake.
With respect to longer periods of separation, a more complex pattern emerges. This is also in agreement with other observations from maternal separation experiments. A prolonged daily maternal separation (360 min; MS360) might provide an emotionally disturbed environment, which is indicated by the finding that most male non-preferring rats and also AA rats respond to this environmental paradigm with an enhanced propensity to drink ethanol. However, certain individuals do not respond and the cause for this is at present unknown. The presence of MS360 “responder” and “non-responder” animals has been more readily observed when using male Wistar rats, B. This observation may constitute one of the obstacles in the interpretation of maternal separation outcome. It may be explained by the fact that Wistar rats are outbred and, therefore, have a heterogeneous genetic background. Genetic background may also be a cause for differential separation-induced effects since at present we have no control of the factor(s) that define a responder or a non-responder, respectively. However, considering the similar situation in humans, with a large individual variation in vulnerability to develop ethanol dependence, the finding of individual differences in the consequences of maternal separation (exemplified in and , B and D, respectively) is most of all interesting, and worth further investigation. Again, female rats respond differently from males and generally seem to be less susceptible to the environmental stressful experience caused by prolonged maternal separation.
At present, no conclusive data exist concerning the neurobiological consequences of an early environment providing protection or enhanced risk, respectively, for the propensity for excessive adult ethanol intake. The challenge for the future is to identify and characterize the neurobiological mechanisms underlying various risk factors as well as factors providing a safe environment early in life. It is important to establish robust experimental models to overcome the limitations of the rodent maternal separation paradigm. In particular, it is necessary to develop the experimental models to be able to individually follow the consequences of early experiences, i.e. to perform a combined evaluation of behaviour, neurochemistry and ethanol intake in one and the same individual. It is equally important to document and control for factors such as experimenter handling, animal housing and knowledge of genetic background, in the experiments. Regarding neurobiological mechanisms underlying the effects of maternal separation on adult ethanol intake, future studies should include further investigations of those neuronal circuits that have been indicated as target systems in previous studies, for instance, opioid, monoamine and HPA axis related systems, but also novel systems. Furthermore, more studies are needed to evaluate the effects of a stressful challenge in adult animals exposed to different early environmental factors. In a recent study in female rats it was shown that even though maternal separation had no effect on voluntary ethanol intake, the ethanol-induced effects on brain peptide levels were dependent on the early environmental setting (Gustafsson et al. Citation2005). This indicates the presence of individual differences in sensitivity to ethanol in animals exposed to different environmental factors. That is, early environmental stress may at times have minor influence on basal conditions and not until an additional challenge occurs later in life, induced by a stressful stimulus or drug exposure, differences may be revealed. A combination of the activation by drugs of abuse of the HPA axis and the alteration of HPA axis function by maternal separation, indicates that a stress-induced activation of the HPA axis may potentiate the rewarding effects of drugs and thereby facilitate the acquisition of drug intake. Thus, stress-induced effects on ethanol intake may differ in animals depending on environmental background, that is whether they have been subjected to short or prolonged periods of maternal separation. This in turn, could explain the differences in acquisition of voluntary ethanol intake and subsequent drug-taking behaviour summarized in the present review. Finally, gene-environment interactions, epigenetic effects and sex differences in relation to maternal separation are interesting future aspects to examine. In all these studies, the neurobiological basis for environmental influence on altered adult drug intake behaviour needs to be determined.
In conclusion, despite some contradictory results presented herein, it is our general inference that short periods of maternal separation can be viewed as a protective environment with regard to long-term effects on voluntary ethanol intake in male rats. We further suggest prolonged periods of maternal separation to be a potential risk factor for increased alcohol intake in male rats. Young people today encounter increased ethanol availability, and in many cases in combination with an increasingly stressful environment. An increased knowledge about individual differences in vulnerability to excessive ethanol intake and ethanol dependence with special emphasis on the interaction between genetic programming and environmental influences is of vital importance to facilitate future ethanol dependence prevention programmes. The rodent maternal separation model reviewed herein may represent one of the experimental tools in this endeavour.
Acknowledgements
IN has grants from The Swedish Research Council, The Alcohol Research Council of The Swedish Alcohol Retailing Monopoly, Mobilisering mot narkotika and AFA. ER has a grant from The Facias Foundation.
References
- Anand KJS, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior?. Biol Neonate 2000; 77: 69–82
- Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity?. Neurosci Biobehav Rev 2003; 27: 3–18
- Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors?. Int J Dev Neurosci 1998; 16: 149–164
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 1999; 160: 1–12
- Barr CS, Newman TK, Becker ML, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Serotonin transporter gene variation is associated with alcohol sensitivity in rhesus macaques exposed to early-life stress. Alcohol Clin Exp Res 2003; 27: 812–817
- Barr CS, Newman TK, Lindell S, Shannon C, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch Gen Psychiatry 2004; 61: 1146–1152
- Boehm SL, II, Valenzuela CF, Harris RA. Alcohol: Neurobiology. Substance Abuse. A Comprehensive Textbook, JH Lowinson, P Ruiz, RB Millman, JG Langrod. Lippincott Williams & Wilkins, Philadelphia 2005; 121–151
- Bohman M, Sigvardsson S, von Knorring AL, Cloninger CR. Inheritance and environment in alcohol abuse–an overview of current Swedish research. Läkartidningen 1984; 81: 2509–2513
- Brady KT, Sonne SC. The role of stress in alcohol use, alcoholism treatment, and relapse. Alcohol Res Health 1999; 23: 263–271
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci 2004; 19: 1863–1874
- Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res Rev 2005, In press
- Busto UE. Pharmacogenetics of alcohol: Treatment implications. Alcohol Clin Exp Res 2000; 24: 1323–1326
- Calhoun JB. The ecology and sociology of the Norway Rat. Public Health Service, Bethesda 1962, Publication No. 1008
- Canetti L, Bachar E, Galili-Weisstub E, De-Nour AK, Shalev AY. Parental bonding and mental health in adolescence. Adolescence 1997; 32: 381–394
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science 2002; 297: 851–854
- Caspi A, Moffitt TE, Morgan J, Rutter M, Taylor A, Arseneault L, Tully L, Jacobs C, Kim-Cohen J, Polo-Tomas M. Maternal expressed emotion predicts children's antisocial behavior problems: Using monozygotic-twin differences to identify environmental effects on behavioral development. Dev Psychol 2004; 40: 149–161
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science 2003; 301: 386–389
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. Am J Psychiatry 2003; 160: 1041–1052
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav 2003; 79: 359–371
- Ciccocioppo R, Angeletti S, Panocka I, Massi M. Nociceptin/orphanin FQ and drugs of abuse. Peptides 2000; 21: 1071–1080
- Ciccocioppo R, Economidou D, Fedeli A, Massi M. The nociceptin/orphanin FQ/NOP receptor system as a target for treatment of alcohol abuse: A review of recent work in alcohol-preferring rats. Physiol Behav 2003; 79: 121–128
- Cirulli F, Berry A, Alleva E. Early disruption of the mother-infant relationship: Effects on brain plasticity and implications for psychopathology. Neurosci Biobehav Rev 2003; 27: 73–82
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: Interactions with laboratory environment. Science 1999; 284: 1670–1672
- De Bellis MD. Developmental traumatology: A contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology 2002; 27: 155–170
- de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt M. Stress, genes and the mechanism of programming the brain for later life. Neurosci Biobehav Rev 2005; 29: 271–281
- Deas D, Thomas S. Comorbid psychiatric factors contributing to adolscent alcohol and other drug use. Alcohol Res Health 2002; 26: 116–121
- Denenberg VH, Smith SA. Effects of infantile stimulation and age upon behavior. J Comp Phys Psych 1963; 56: 307–312
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science 2004; 305: 1014–1017
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol 1999; 375: 13–30
- Di Chiara G, Acquas E, Tanda G. Ethanol as a neurochemical surrogate of conventional reinforcers: The dopamine-opioid link. Alcohol 1996; 13: 13–17
- Dick DM, Foroud T. Candidate genes for alcohol dependence: A review of genetic evidence from human studies. Alcohol Clin Exp Res 2003; 27: 868–879
- DSM. American Psychiatric Association, Diagnostic and statistical manual of mental disorders. American Psychiatric Press, Washington 2000
- Enoch MA, Goldman D. The genetics of alcoholism and alcohol abuse. Curr Psychiatry Rep 2001; 3: 144–151
- Eriksson CJP. Ethanol and acetaldehyde metabolism in rat strains genetically selected for their ethanol preference. Biochem Pharmacol 1973; 22: 2283–2292
- Fadda F, Rossetti ZL. Chronic ethanol consumption: From neuroadaptation to neurodegeneration. Prog Neurobiol 1998; 56: 385–431
- Fahlke C, Hansen S. Effect of local intracerebral corticosterone implants on alcohol intake in the rat. Alcohol Alcohol 1999; 34: 851–861
- Fleming AS, Rosenblatt JS. Maternal behavior in the virgin and lactating rat. J Comp Physiol Psychol 1974; 86: 957–972
- Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, Riley B. Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Arch Gen Psychiatry 2004; 61: 738–744
- Ford MM, Eldridge JC, Samson HH. Microanalysis of ethanol self-administration: Estrous cycle phase-related changes in consumption patterns. Alcohol Clin Exp Res 2002; 26: 635–643
- Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: Gender differences. J Neuroendocrinol 2002; 14: 349–353
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: News from mouse molecular genetics. Nat Rev Neurosci 2003; 4: 1002–1012
- Gianoulakis C. Implications of endogenous opioids and dopamine in alcoholism: Human and basic science studies. Alcohol Alcohol Suppl 1996; 1: 33–42
- Gianoulakis C. Influence of the endogenous opioid system on high alcohol consumption and genetic predisposition to alcoholism. J Psychiatry Neurosci 2001; 26: 304–318
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem 2004; 4: 39–50
- Gilmer WS, McKinney WT. Early experience and depressive disorders: Human and non-human primate studies. J Affect Disord 2003; 75: 97–113
- Gordon HW. Early environmental stress and biological vulnerability to drug abuse. Psychoneuroendocrinology 2002; 27: 115–126
- Grota LJ, Ader R. Continuous recording of maternal behaviour in Rattus Norvegicus. Anim Behav 1969; 17: 722–729
- Gunzerath L, Goldman D. G × E: A NIAAA workshop on gene-environment interactions. Alcohol Clin Exp Res 2003; 27: 540–562
- Gustafsson L, Ploj K, Nylander I. Effects of maternal separation on voluntary ethanol intake and brain peptide systems in female Wistar rats. Pharmacol Biochem Behav 2005, In Press
- Haberny KA, Paule MG, Scallet AC, Sistare FD, Lester DS, Hanig JP, Slikker W, Jr. Ontogeny of the N-methyl-D-aspartate (NMDA) receptor system and susceptibility to neurotoxicity. Toxicol Sci 2002; 68: 9–17
- Hall FS, Wilkinson LS, Humby T, Robbins TW. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse 1999; 32: 37–43
- Heath AC, Madden PA, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Rohrbaugh JW, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med 1999; 29: 1069–1081
- Heilig M, Thorsell A. Brain neuropeptide Y (NPY) in stress and alcohol dependence. Rev Neurosci 2002; 13: 85–94
- Helzer JE, Pryzbeck TR. The co-occurrence of alcoholism with other psychiatric disorders in the general population and its impact on treatment. J Stud Alcohol 1988; 49: 219–224
- Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exp Neurol 2004; 190(Suppl 1)S8–21
- Hibell B, Andersson B, Bjarnasson T, Ahlström S, Balakireva O, Kokkevi A, Morgan M. The ESPAD report 2003. Alcohol and other drug use among students in 35 European countries. CAN, Stockholm 2004
- Hilakivi L, Eriksson CJP, Sarviharju M, Sinclair JD. Revitalization of the AA and ANA rat lines: Effects on some line characteristics. Alcohol 1984; 1: 71–75
- Hilakivi-Clarke LA, Turkka J, Lister RG, Linnoila M. Effects of early postnatal handling on brain beta-adrenoceptors and behavior in tests related to stress. Brain Res 1991; 542: 286–292
- Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Paediatr Suppl 1994; 397: 9–18
- Huot RL, Ladd CO, Plotsky PM. Maternal deprivation. Encyclopedia of stress, G Fink. Academic Press, London/San Diego 2000; Vol. 2: 699–707
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001; 158: 366–373
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev 1992; 72: 165–229
- Jamensky NT, Gianoulakis C. Content of dynorphins and kappa-opioid receptors in distinct brain regions of C57BL/6 and DBA/2 mice. Alcohol Clin Exp Res 1997; 21: 1455–1464
- Jamensky NT, Gianoulakis C. Comparison of the proopiomelanocortin and proenkephalin opioid peptide systems in brain regions of the alcohol-preferring C57BL/6 and alcohol-avoiding DBA/2 mice. Alcohol 1999; 18: 177–187
- Jaworski JN, Francis DD, Brommer CL, Morgan ET, Kuhar MJ. Effects of early maternal separation on ethanol intake GABA receptors and metabolizing enzymes in adult rats. Psychopharmacology (Berl) 2005, In Press
- Kalinichev M, Easterling KW, Holtzman SG. Repeated neonatal maternal separation alters morphine-induced antinociception in male rats. Brain Res Bull 2001; 54: 649–654
- Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav 2002; 73: 131–140
- Katner SN, Weiss F. Neurochemical characteristics associated with ethanol preference in selected alcohol-preferring and -nonpreferring rats: A quantitative microdialysis study. Alcohol Clin Exp Res 2001; 25: 198–205
- Kaufmann W. Developmental neurotoxicity. The Laboratory Rat, GJ Krinke. Academic Press, London/San Diego 2000; 227–250
- Kehoe P, Shoemaker WJ, Arons C, Triano L, Suresh G. Repeated isolation stress in the neonatal rat: Relation to brain dopamine systems in the 10-day-old rat. Behav Neurosci 1998; 112: 1466–1474
- Kehoe P, Shoemaker WJ, Triano L, Hoffman J, Arons C. Repeated isolation in the neonatal rat produces alterations in behavior and ventral striatal dopamine release in the juvenile after amphetamine challenge. Behav Neurosci 1996; 110: 1435–1444
- Kelai S, Aissi F, Lesch KP, Cohen-Salmon C, Hamon M, Lanfumey L. Alcohol intake after serotonin transporter inactivation in mice. Alcohol Alcohol 2003; 38: 386–389
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry 2003; 60: 929–937
- Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol 2002; 66: 285–306
- Koob GF. Stress, corticotropin-releasing factor, and drug addiction. Ann NY Acad Sci 1999; 897: 27–45
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001; 24: 97–129
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytiä P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res 1998; 22: 3–9
- Kosten TA, Sanchez H, Zhang XY, Kehoe P. Neonatal isolation enhances acquisition of cocaine self-administration and food responding in female rats. Behav Brain Res 2004; 151: 137–149
- Kreek MJ. Drug addictions. Molecular and cellular endpoints. Ann NY Acad Sci 2001; 937: 27–49
- Kreek MJ, Koob GF. Drug dependence: Stress and dysregulation of brain reward pathways. Drug Alcohol Depend 1998; 51: 23–47
- Kreek MJ, Nielsen DA, LaForge KS. Genes associated with addiction: Alcoholism, opiate, and cocaine addiction. Neuromol Med 2004; 5: 85–108
- Kuhn CM, Schanberg SM. Responses to maternal separation: Mechanisms and mediators. Int J Dev Neurosci 1998; 16: 261–270
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res 2000; 122: 81–103
- Lancaster FE. Sex differences in voluntary drinking by Long Evans rats following early stress. Alcohol Clin Exp Res 1998; 22: 830–836
- Langbehn DR, Cadoret RJ, Caspers K, Troughton EP, Yucuis R. Genetic and environmental risk factors for the onset of drug use and problems in adoptees. Drug Alcohol Depend 2003; 69: 151–167
- Langeland W, Draijer N, van den Brink W. Psychiatric comorbidity in treatment-seeking alcoholics: The role of childhood trauma and perceived parental dysfunction. Alcohol Clin Exp Res 2004; 28: 441–447
- Langeland W, Hartgers C. Child sexual and physical abuse and alcoholism: A review. J Stud Alcohol 1998; 59: 336–348
- Lau C, Klinefelter G, Cameron AM. Reproductive development and functions in the rat after repeated maternal deprivation stress. Fundam Appl Toxicol 1996; 30: 298–301
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: Consistent or confusing?. Rev Neurosci 2000; 11: 383–408
- Lehmann J, Pryce CR, Bettschen D, Feldon J. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacol Biochem Behav 1999; 64: 705–715
- Lende DH, Smith EO. Evolution meets biopsychosociality: An analysis of addictive behavior. Addiction 2002; 97: 447–458
- Levine S. Infantile experience and resistance to physiological stress. Science 1957; 126: 405
- Levine S. Enduring effects of early experience on adult behavior. Hormones, brain and behavior, Vol. 4, DW Pfaff, AP Arnold, SE Fahrbach, AM Etgen, RT Rubin. Academic Press, London/San Diego 2002a; 535–542
- Levine S. Regulation of the hypothalamic-pituitary-adrenal axis in the neonatal rat: The role of maternal behavior. Neurotox Res 2002b; 4: 557–564
- Lin Z, Walther D, Yu XY, Li S, Drgon T, Uhl GR. SLC18A2 promoter haplotypes and identification of a novel protective factor against alcoholism. Hum Mol Genet 2005; 14: 1393–1404
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic- pituitary–adrenal responses to stress. Science 1997; 277: 1659–1662
- Lu L, Shepard JD, Scott Hall F, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: A review. Neurosci Biobehav Rev 2003; 27: 457–491
- Lynch WJ, Mangini LD, Taylor JR. Neonatal isolation stress potentiates cocaine seeking behavior in adult male and female rats. Neuropsychopharmacology 2005; 30: 322–329
- Macri S, Mason GJ, Wurbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring's HPA and fear responses in rats. Eur J Neurosci 2004; 20: 1017–1024
- Marmendal M, Roman E, Eriksson CJP, Nylander I, Fahlke C. Maternal separation alters maternal care, but has minor effects on behavior and brain opioid peptides in adult offspring. Dev Psychobiol 2004; 45: 140–152
- Marquardt AR, Ortiz-Lemos L, Lucion AB, Barros HM. Influence of handling or aversive stimulation during rats' neonatal or adolescence periods on oral cocaine self-administration and cocaine withdrawal. Behav Pharmacol 2004; 15: 403–412
- Matthews K, Dalley JW, Matthews C, Tsai TH, Robbins TW. Periodic maternal separation of neonatal rats produces region- and gender-specific effects on biogenic amine content in postmortem adult brain. Synapse 2001; 40: 1–10
- Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: The effects of repeated maternal separation in the rat. Neurosci Biobehav Rev 2003; 27: 45–55
- Matthews K, Robbins TW, Everitt BJ, Caine SB. Repeated neonatal maternal separation alters intravenous cocaine self-administration in adult rats. Psychopharmacology (Berl) 1999; 141: 123–134
- McBride WJ, Lovinger DM, Machu T, Thielen RJ, Rodd ZA, Murphy JM, Roache JD, Johnson BA. Serotonin-3 receptors in the actions of alcohol, alcohol reinforcement, and alcoholism. Alcohol Clin Exp Res 2004; 28: 257–267
- McDowell J, Kitchen I. Development of opioid systems: Peptides, receptors and pharmacology. Brain Res 1987; 434: 397–421
- McIntosh J, Anisman H, Merali Z. Short- and long-periods of neonatal maternal separation differentially affect anxiety and feeding in adult rats: Gender-dependent effects. Brain Res Dev Brain Res 1999; 113: 97–106
- Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: A neurobiological mechanism for vulnerability to drug abuse?. Psychoneuroendocrinology 2002; 27: 127–138
- Moore CL, Morelli GA. Mother rats interact differently with male and female offspring. J Comput Physiol Psychol 1979; 93: 677–684
- Möller C, Wiklund L, Thorsell A, Hyytiä P, Heilig M. Decreased measures of experimental anxiety in rats bred for high alcohol preference. Alcohol Clin Exp Res 1997; 21: 656–660
- Naranjo CA, Chu AY, Tremblay LK. Neurodevelopmental liabilities in alcohol dependence: Central serotonin and dopamine dysfunction. Neurotox Res 2002; 4: 343–361
- Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry 2004; 65(Suppl 1)18–28
- Newport DJ, Stowe ZN, Nemeroff CB. Parental depression: Animal models of an adverse life event. Am J Psychiatr 2002; 159: 1265–1283
- Nilsson KW, Sjöberg RL, Damberg M, Alm PO, Öhrvik J, Leppert J, Lindström L, Oreland L. Role of the serotonin transporter gene and family function in adolescent alcohol consumption. Alcohol Clin Exp Res 2005; 29: 564–570
- Nylander I, Hyytiä P, Forsander O, Terenius L. Differences between alcohol-preferring (AA) and alcohol-avoiding (ANA) rats in the prodynorphin and proenkephalin systems. Alcohol Clin Exp Res 1994; 18: 1272–1279
- Nylander I, Silberring J. Opioid peptides in drug dependence and neurological diseases. Progress in HPLC-HPCE, GA Qureshi, H Parvez, P Caudy, S Parvez. VSP BV, Utrecht 1998; Vol. 7: 171–192
- Nylander I, Vlaskovska M, Terenius L. Brain dynorphin and enkephalin systems in Fischer and Lewis rats: Effects of morphine tolerance and withdrawal. Brain Res 1995; 683: 25–35
- O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatr 1992; 49: 881–887
- Oreland L. Platelet monoamine oxidase, personality and alcoholism: The rise, fall and resurrection. Neurotoxicology 2004; 25: 79–89
- Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav 2004; 81: 339–358
- Overstreet DH, Halikas JA, Seredenin SB, Kampov-Polevoy AB, Viglinskaya IV, Kashevskaya O, Badishtov BA, Knapp DJ, Mormede P, Kiianmaa K, Li TK, Rezvani AH. Behavioral similarities and differences among alcohol-preferring and-nonpreferring rats: Confirmation by factor analysis and extension to additional groups. Alcohol Clin Exp Res 1997; 21: 840–848
- Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neurosci Biobehav Rev 2005; 29: 209–226
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition?. Nat Rev Neurosci 2002; 3: 715–727
- Palanza P. Animal models of anxiety and depression: How are females different?. Neurosci Biobehav Rev 2001; 25: 219–233
- Papaioannou A, Dafni U, Alikaridis F, Bolaris S, Stylianopoulou F. Effects of neonatal handling on basal and stress-induced monoamine levels in the male and female rat brain. Neuroscience 2002a; 114: 195–206
- Papaioannou A, Gerozissis K, Prokopiou A, Bolaris S, Stylianopoulou F. Sex differences in the effects of neonatal handling on the animal's response to stress and the vulnerability for depressive behaviour. Behav Brain Res 2002b; 129: 131–139
- Park MK, Hoang TA, Belluzzi JD, Leslie FM. Gender specific effect of neonatal handling on stress reactivity of adolescent rats. J Neuroendocrinol 2003; 15: 289–295
- Petrakis IL, Gonzalez G, Rosenheck R, Krystal JH. Comorbidity of alcoholism and psychiatric disorders. An overview. Alcohol Res Health 2002; 26: 81–89
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: Role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol 1996; 36: 359–378
- Ploj K, Nylander I. Long-term effects on brain opioid and opioid receptor like-1 receptors after short periods of maternal separation in rats. Neurosci Lett 2003; 345: 195–197
- Ploj K, Pham TM, Bergström L, Mohammed AH, Henriksson BG, Nylander I. Neonatal handling in rats induces long-term effects on dynorphin peptides. Neuropeptides 1999; 33: 468–474
- Ploj K, Roman E, Bergström L, Nylander I. Effects of neonatal handling on nociceptin/orphanin FQ and opioid peptide levels in female rats. Pharmacol Biochem Behav 2001; 69: 173–179
- Ploj K, Roman E, Gustavsson L, Nylander I. Basal levels and alcohol-induced changes in nociceptin/orphanin FQ, dynorphin, and enkephalin levels in C57BL/6J mice. Brain Res Bull 2000; 53: 219–226
- Ploj K, Roman E, Nylander I. Effects of maternal separation on brain nociceptin/orphanin FQ peptide levels in male Wistar rats. Pharmacol Biochem Behav 2002; 73: 123–129
- Ploj K, Roman E, Nylander I. Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience 2003a; 121: 787–799
- Ploj K, Roman E, Nylander I. Long-term effects of short and long periods of maternal separation on brain opioid peptide levels in male Wistar rats. Neuropeptides 2003b; 37: 149–156
- Pohorecky LA. Interaction of ethanol and stress: Research with experimental animals—an update. Alcohol Alcohol 1990; 25: 263–276
- Pohorecky LA. Stress and alcohol interaction: An update of human research. Alcohol Clin Exp Res 1991; 15: 438–459
- Pryce CR, Bettschen D, Feldon J. Comparison of the effects of early handling and early deprivation on maternal care in the rat. Dev Psychobiol 2001; 38: 239–251
- Pryce CR, Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: Manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev 2003; 27: 57–71
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF. Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res 1998; 22: 1564–1569
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction 2001; 96: 103–114
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav 2004; 79: 439–450
- Roman E. Maternal separation in rats. An experimental model for long-term effects of early life experiences on neurochemistry, voluntary ethanol intake and exploration and risk assessment behavior. Acta Universitatis Upsaliensis Comprehensive Summaries of Uppsala Dissertations from the Faculty of Pharmacy 2004; 313: 1–81
- Roman E, Gustafsson L, Hyytiä P, Nylander I. Short and prolonged periods of maternal separation and voluntary ethanol intake in male and female ethanol-preferring AA and ethanol-avoiding ANA rats. Alcohol Clin Exp Res 2005; 29: 591–601
- Roman E, Hyytiä P, Nylander I. Maternal separation alters acquisition of ethanol intake in male ethanol-preferring AA rats. Alcohol Clin Exp Res 2003; 27: 31–37
- Roman E, Ploj K, Nylander I. Maternal separation has no effect on voluntary ethanol intake in female Wistar rats. Alcohol 2004; 33: 31–39
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: A review of preclinical studies. Neurosci Biobehav Rev 2004; 28: 533–546
- Ruedi-Bettschen D, Feldon J, Pryce CR. Circadian- and temperature-specific effects of early deprivation on rat maternal care and pup development: Short-term markers for long-term effects?. Dev Psychobiol 2004; 45: 59–71
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res Rev 1986; 11: 65–76
- Savelieva KV, Caudle WM, Findlay GS, Caron MG, Miller GW. Decreased ethanol preference and consumption in dopamine transporter female knock-out mice. Alcohol Clin Exp Res 2002; 26: 758–764
- Schuckit MA, Smith TL, Kalmijn J. The search for genes contributing to the low level of response to alcohol: Patterns of findings across studies. Alcohol Clin Exp Res 2004; 28: 1449–1458
- Schumann G, Spanagel R, Mann K. Candidate genes for alcohol dependence: Animal studies. Alcohol Clin Exp Res 2003; 27: 880–888
- Sinclair JD, Le A D, Kiianmaa K. The AA and ANA rat lines, selected for differences in voluntary alcohol consumption. Experientia 1989; 45: 798–805
- Spak F, Allebeck P, Spak L, Thundal KL. Gothenburg study of women and alcohol: Problems during childhood and youth an important risk factor. Läkartidningen 2001; 98: 1109–1114
- Spanagel R. Alcohol addiction research: From animal models to clinics. Best Pract Res Clin Gastroenterol 2003; 17: 507–518
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev 2003; 27: 33–44
- Thadani PV. The intersection of stress, drug abuse and development. Psychoneuroendocrinology 2002; 27: 221–230
- Tuominen K, Hilakivi LA, Päivärinta P, Korpi ER. Behavior of alcohol-preferring AA and alcohol-avoiding ANA rat lines in tests of anxiety and aggression. Alcohol 1990; 7: 349–353
- Valdez GR, Koob GF. Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: Implications for the development of alcoholism. Pharmacol Biochem Behav 2004; 79: 671–689
- Van Ree JM, Niesink RJM, Van Wolfswinkel L, Ramsey NF, Kornet MLMW, Van Furth WR, Vanderschuren LJMJ, Gerrits MAFM, Van den Berg CL. Endogenous opioids and reward. Eur J Pharmacol 2000; 405: 89–101
- Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 1. Common mechanisms and manifestations. Neurosci Biobehav Rev 2003; 27: 507–515
- Vazquez DM, Eskandari R, Zimmer A, Levine S, Lopez JF. Brain 5-HT receptor system in the stressed infant rat: Implications for vulnerability to substance abuse. Psychoneuroendocrinology 2002; 27: 245–272
- Vazquez V, Penit-Soria J, Durand C, Besson MJ, Giros B, Dauge V. Maternal deprivation increases vulnerability to morphine dependence and disturbs the enkephalinergic system in adulthood. J Neurosci 2005; 25: 4453–4462
- Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatr 1992; 49: 876–880
- Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci 2004; 7: 847–854
- Weinberg J. Effects of early experience on responsiveness to ethanol: A preliminary report. Physiol Behav 1987; 40: 401–406
- Weininger O. Physiological damage under emotional stress as a function of early experience. Science 1954; 119: 285–286
- Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav 1999; 66: 293–302
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci 2004; 5: 483–494
- Zhang XY, Sanchez H, Kehoe P, Kosten TA. Neonatal isolation enhances maintenance but not reinstatement of cocaine self-administration in adult male rats. Psychopharmacology (Berl) 2005; 177: 391–399
- Zimmerberg B, Shartrand AM. Temperature-dependent effects of maternal separation on growth, activity, and amphetamine sensitivity in the rat. Dev Psychobiol 1992; 25: 213–226