Abstract
The amygdala plays a crucial role in the orchestration and modulation of the organism response to aversive, stressful events. This response could be conceived as the result of two interdependent components. The first is represented by sets of visceral and motor responses aimed at helping the organism to cope with the present event. The second is the acquisition and modulation of memories relative to the stressful stimulus and its context. This latter component contributes to the instatement of conditioned stress responses that are essential to the capability of the organism to predict future exposures to similar stimuli in order to avoid them or counteract them effectively. In the amygdala, these two components become fully integrated. Massive networks link the amygdala to the hypothalamus, midbrain and brainstem. These networks convey visceral, humoral and nociceptive information to the amygdala and mediate its effects on the hypothalamic-pituitary-adrenal axis as well on autonomic and motor centers. On the other hand, interactions between the amygdala and interconnected cortical networks play a crucial role in acquisition, consolidation and extinction of learning relative to the stressful stimulus. Within the scope of this review, current evidence relative to the interaction between the amygdala and cortical networks will be considered in relationship to the integration of the conditioned response to stress.
Introduction
The amygdala, a set of nuclei located within the temporal lobe, plays an important role in the regulation of adaptive behavior. This regulation includes several interrelated domains such as the regulation of homeostasis, feeding, sexual and parenting behaviors. The amygdala accomplishes this regulation through networks of neural connections that include visceral centers as well as complex cortical circuits (Charney and Deutch Citation1996; McDonald Citation1998; Pitkanen Citation2000; Price Citation1999). By bringing these neural networks together, the amygdala mediates the integration of simple, phylogenetically old behaviors with more sophisticated ones, including the interpretation of complex information on the basis of learning relative to previous exposure to similar events. In this manuscript, the functional role of cortico-amygdalo-cortical circuits is considered in the context of the response to stress, understood as direct or perceived severe challenges to the organism's homeostasis.
Exposure to stressful stimuli elicits a complex set of physiological responses that is highly regulated at multiple levels of the central nervous system. The amygdala is at the core of this regulation (Charney Citation2003; Herman et al. Citation2003; McEwen Citation2003; Sapolsky Citation2003; Shekhar et al. Citation2003). Its role reflects the integration of temporally and hierarchically organized functions (Herman et al. Citation2003). The coordination of behavioral, neuroendocrine and neurochemical responses to a stressful stimulus is accompanied by adaptive mechanisms that include associative memory, memory consolidation and regulation of attention. The role of the amygdala in Pavlovian fear conditioning, a relatively simple instance of such integration, has been particularly well investigated (Davis and Whalen Citation2001; LeDoux Citation2000; LeDoux et al. Citation1990; Maren and Quirk Citation2004; Pape and Stork Citation2003; Pare et al. Citation2004). The most commonly used experimental design involves the use of a tone or light as an emotionally neutral conditioned stimulus (CS) and a footshock as an aversive unconditioned stimulus (US). When the CS is paired with US, the CS eventually becomes sufficient to elicit behavioral and visceral responses to danger similar to those triggered by the US. Growing evidence indicates that the amygdala receives nociceptive stimuli (US) as well as auditory stimuli from the thalamus and auditory cortex (CS) (LeDoux et al. Citation1990; Mascagni et al. Citation1993; McDonald Citation1998; Romanski and LeDoux Citation1993). Plastic events within the amygdala are thought to be necessary to enable the CS to acquire emotional valence and to trigger visceral and behavioral stress responses (for review see LeDoux Citation2003). Pavlovian fear conditioning could be thought of as an example of the co-existence and integration within the amygdala of the initial response, acquisition and modulation stages of the conditioned stress response. As such, it will be used in this review to discuss the multifaceted roles that amygdalar subregions and their cortical connections play in this response.
It is important to emphasize that cortico-amygdala networks are preferentially involved in the response to specific types of stressors, such as those that do not directly compromise homeostasis but induce stress on the basis of previous experiences (Figueiredo et al. Citation2003). It is also important to note that the characteristics of the response to stress change dynamically in relationship with the duration of exposure to the stressor. The effects of chronic stress are qualitatively different from those induced by an acute stressor. In the present review, we chose to focus predominantly on the responses to acute stressors, in order to highlight the role played by dynamic interactions among cortico-limbic regions in the conditioned response to stress. It should not be forgotten, however, that chronic stress induces profound morphological and neurochemical changes in brain regions such as the medial prefrontal cortex (mPFC), amygdala and hippocampus (Cook and Wellman Citation2004; Czeh et al. Citation2005; Joels et al. Citation2004; McEwen Citation2005; McEwen et al. Citation2002; Radley et al. Citation2004; Salm et al. Citation2004; Stewart et al. Citation2005; Trentani et al. Citation2002; Vyas et al. Citation2003, Citation2004; Wommack and Delville Citation2002). In turn, chronic stress-induced changes are likely to alter acute cortico-amygdalo-cortical stress response mechanisms (Correll et al. Citation2005).
Amygdalar networks and their role in the response to stress
The amygdala consists of a functionally integrated complex of cellular groups. Its thirteen nuclei and cortical areas evolved during different phylogenetical stages and present distinct cytoarchitectonic and neurochemical features as well as specific patterns of connections. Within the scope of this review, we will focus primarily on the central nucleus (CeA) and the basolateral complex (BLC), whose roles in conditioned stress, fear and anxiety have been investigated extensively (Bhatnagar et al. Citation2004; Cardinal et al. Citation2002; Charney Citation2003; Davis Citation1992a; Goldstein et al. Citation1996; Herman et al. Citation2003; LeDoux Citation2003; Shekhar et al. Citation2003; Shors and Mathew Citation1998). Importantly, other components of cortico-amygdalo-cortical networks, such as the medial nucleus of the amygdala, are also known to participate in responses to stress (Crane et al. Citation2005; Flugge et al. Citation1992; Ma and Morilak Citation2005; McDougall et al. Citation2004; Walker et al. Citation2005).
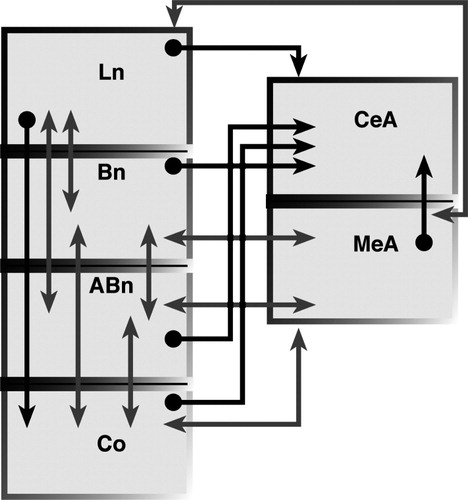
The CeA has cytoarchitectonic features that are typical of the striatum, with GABAergic medium spiny projection neurons as its prevalent cell type (McDonald Citation1982). The BLC includes the lateral (Ln), basal (Bn) and accessory basal (Abn) nuclei. The cytoarchitectonic profile of these cell groups strongly resembles that of the cortex, with a prevalence of glutamatergic pyramidal projection neurons, and subgroups of smaller GABAergic interneurons (McDonald Citation1985; Millhouse and DeOlmos Citation1983; Washburn and Moises Citation1992). Despite their differences, the BLC and CeA function as an integrated unit in processing and responses to emotionally relevant stimuli.
The central nucleus and its autonomic network
The CeA is embedded in visceral circuits (reviewed in Pitkanen Citation2000) which enable it to play a pivotal role in the coordination of the autonomic, hormonal, neurochemical and motor aspects of the stress response. From this point of view, it has rightfully been described as the autonomic center of the amygdala (Swanson and Petrovich Citation1998). The CeA receives visceral, humoral and nociceptive information from a broad range of inputs, arising from the hypothalamus, brainstem, midline thalamus, and spinal cord (Cliffer et al. Citation1991; Ge et al. Citation2001; Menetrey and De Pommery Citation1991; Ottersen Citation1980, Citation1981; Ottersen and Ben-Ari Citation1979; Rinaman and Schwartz Citation2004; Saper and Loewy Citation1980; Veening Citation1978b). In turn, it provides substantial inputs to the lateral and medial hypothalamus, which mediate sympathetic and hypothalamo-pituitary-adrenocortical (HPA) axis activation, respectively (Gray et al. Citation1989; Prewitt and Herman Citation1998; Price and Amaral Citation1981; Rosen et al. Citation1991). The CeA also innervates several midbrain, pons and medulla nuclei, including the peduncolopontine tegmental and parabrachial nuclei, nucleus of the trigeminal nerve, nucleus reticularis pontis caudalis and the dorsal nucleus of the vagus (Fort et al. Citation1994; Krettek and Price Citation1978; Petrovich and Swanson Citation1997; Rosen et al. Citation1991; Semba and Fibiger Citation1992; Zhang et al. Citation2003). These projections mediate the CeA effects on several autonomic functions as well as reflex motor responses such as startle and freezing. The CeA is reciprocally connected to the bed nucleus of the stria terminalis (BNST) (Dong et al. Citation2001; Krettek and Price Citation1978; Veinante and Freund-Mercier Citation1998) and to neurochemically distinct regions such as the ventral tegmental area and substantia nigra pars compacta (dopamine), the raphe system (serotonin) and the locus coeruleus (norepinephrine) (Asan Citation1998; Commons et al. Citation2003; Li et al. Citation1990; Ottersen Citation1981; Petrov et al. Citation1994; Peyron et al. Citation1998; Wallace et al. Citation1992).
As shown in , the CeA receives projections from several amygdalar nuclei (Pare' et al. Citation1995; Petrovich et al. Citation1996; Pitkanen and Amaral Citation1998; Pitkanen et al. Citation1997). Contrary to the common pattern of intra-amygdalar connections, these projections are not reciprocal. This pattern strongly suggests that the CeA may integrate information from intra- as well as extra- amygdalar sources and indeed represent the gateway between the BLC and the visceral centers. Growing evidence, however, indicates that the CeA may in itself represent a crucial site of plasticity. Various forms of conditioned learning, including the acquisition of contextual conditioning, conditioned suppression and conditioned taste aversion have been shown to require the integrity of the CeA (Bahar et al. Citation2003; Goosens and Maren Citation2001; Killcross et al. Citation1997; Selden et al. Citation1991). Within this context, it is important to note that moderate projections from sensory-related cortical areas, entorhinal cortex and prefrontal cortex impinge directly on the CeA (Mascagni et al. Citation1993; McDonald Citation1998; McDonald and Mascagni Citation1997; McDonald et al. Citation1996; Veening Citation1978a). These direct inputs may provide processed sensory information necessary for specific forms of plasticity in this nucleus. Furthermore, the CeA also modulates the attentional aspects of stimulus processing (Holland and Gallagher Citation1993, Citation1999Holland and Gallagher 1993, 1999). This function may largely depend on the CeA projections to cholinergic neurons in the nucleus basalis magnocellularis, which in turn innervates several cortical areas including the posterior parietal cortex (Bucci et al. Citation1998; Chiba et al. Citation1995; Han et al. Citation1999; Holland Citation1997).
Figure 1 Simplified schematic diagram showing intrinsic connections within the BLC, cortical nucleus (CO), CeA and MeA. Note the intricate pattern of reciprocal connections among Ln, Bn and Abn and between these nuclei and CO and MeA (lighter gray). In contrast, connections of these nuclei with the CeA are generally not reciprocal (darker gray). Other amygdalar nuclei, including the ITC cell masses, were not included for the sake of simplicity. References are in the text.
The basolateral complex and its cortical network
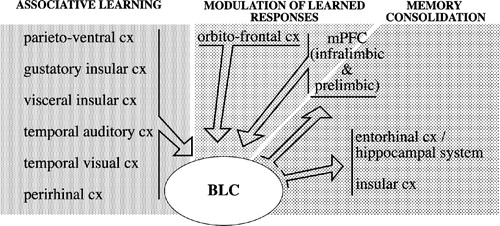
The BLC receives strong projections from a broad array of sensory-related associative cortical areas, including the gustatory dysgranular insular cortex, visceral dysgranular insular cortex, temporal auditory and visual cortical regions, parietal ventral and parietal rhinal cortices (somatosensory) (McDonald Citation1998) (). Massive reciprocal connections link the BLC to the mPFC (infralimbic and prelimbic), perirhinal cortex, hippocampus/entorhinal system and orbitofrontal cortex (Bacon et al. Citation1996; Barbas and De Olmos Citation1990; Berretta et al. Citation2001; Cassell and Wright Citation1986; Cunningham et al. Citation2002; Ghashghaei and Barbas Citation2002; Krettek and Price Citation1977; McDonald Citation1998; McDonald and Mascagni Citation1997; McDonald et al. Citation1996; Pitkanen et al. Citation2000; Porrino et al. Citation1981; Sesack et al. Citation1989; Shi and Cassell Citation1999; Vertes Citation2004; Verwer et al. Citation1996). As shown in , intricate connections link nuclei within the BLC to each other and to other amygdala cells groups (Pitkanen Citation2000; Pitkanen et al. Citation1997). Additional BLC inputs include projections originating from the midline and posterior thalamic nuclei, ventral globus pallidus, and from some hypothalamic and pontine nuclei (Haber et al. Citation1985; Kelley et al. Citation1982; Moga et al. Citation1995; OttersenCitation1980; Ottersen and Ben-Ari Citation1979; Turner and Herkenham Citation1991; Woolf and Butcher Citation1982). Neurochemically distinct ventral tegmental area, raphe system and locus coeruleus also project to the BLC (Asan Citation1998; Nitecka et al. Citation1980; Vertes Citation1991). The BLC sends substantial projections to the nucleus accumbens, caudoputamen, BNST, substantia innominata, and mediodorsal nucleus of the thalamus (Cornwall and Phillipson Citation1988; Groenewegen Citation1988; Grove Citation1988; Kelley et al. Citation1982; Kita and Kitai Citation1990; Krettek and Price Citation1978; McDonald Citation1987, Citation1991; Ray and Price Citation1992; Russchen and Price Citation1984; Weller and Smith Citation1982).
Figure 2 Simplified diagram depicting BLC-cortical interactions within the context of acquisition, modulation and consolidation of stress-related memories. Inputs from a broad range of associative sensory cortical areas, representing all sensory modalities, converge in the BLC. Multifaceted information relative to specific events can then be associated with its emotional valence. The BLC mediates long-term consolidation of stress-related memories through its projections to several cortical (and subcortical, not shown) regions. Finally, projections from prefrontal cortex to the amygdala are thought to modulate the expression of learned responses on the basis of the current valence of specific stimuli. For the sake of simplicity, the list of the cortical areas included is not intended to be comprehensive and the complexity of their connections with the amygdala is not fully represented. References are in the text.
Thus the BLC is embedded within a vast network of cortical regions and is strongly connected with the basal ganglia and limbic thalamus. These circuits are crucial to the regulation of the stress response. Growing evidence indicates that the main contributions of the BLC to this response are the formation and retrieval of associative memories and the modulation of memory consolidation relative to stressful events.
Projections from sensory association cortical areas to the BLC—associative memory
Associative learning could be defined as neural plastic events resulting from the convergence of sensory stimuli and biologically relevant information (Fanselow and Poulos Citation2005). The idea that the amygdala plays a crucial role in associative learning is a relatively old one (Jones and Mishkin Citation1972). The involvement of this nuclear complex in multiple forms of associative learning, including cue-, place- and object- reward associations, conditioned taste aversion, appetitive conditioning and conditioned fear and anxiety, is now well recognized (for reviews see Baxter and Murray Citation2000; Davis Citation2000; Easton and Gaffan Citation2000; Gallagher Citation2000; Holland et al. Citation2002; Holland and Gallagher Citation1999; for reviews see McGaugh Citation2002; White and McDonald Citation2002). Pavlovian fear conditioning has been used extensively to investigate the role of the BLC/CeA in associative learning. In the simple form of this paradigm illustrated above, thalamic inputs relative to CS and US converge in the Ln (LeDoux Citation2003). While information originating from the thalamus is likely to support conditioning of simple sensory stimuli, sensory association and mesiotemporal cortical areas provide the BLC with a remarkable array of highly processed information related to complex sensory stimuli (). The BLC is thus in an ideal position to integrate sensory information both within and across modalities and to use it to attach emotional value to newly acquired experiences in order to guide adaptive behavior. During a stressful event, the convergence in the BLC of complex information relative to explicit sensory stimuli and environmental context (conditioned) and visceral (unconditioned) information relayed by hypothalamic and midline thalamic nuclei, may generate multiple orders of conditioned learning. This newly learned information will be used in the future to predict and react appropriately to future presentation of similar events.
BLC projections to the cortex—modulation of memory consolidation
Newly acquired short-term memories can be transformed, over time, into more stable long-term memories (Davis and Squire Citation1984; McGaugh Citation2000). The capability of turning the experience of aversive events into long lasting memories is of obvious importance for the survival of the organism. It is thus not surprising that stress hormones and neuromodulators, namely glucocorticoids and norepinephrine, have powerful effects on memory consolidation (Gold and Van Buskirk Citation1975; Hui et al. Citation2004; Izquierdo and Dias Citation1983; Lupien and McEwen Citation1997; McEwen and Sapolsky Citation1995; McGaugh Citation2004; Okuda et al. Citation2004; Roozendaal Citation2002; Roozendaal et al. Citation2004). Overwhelming evidence indicates that the BLC plays a crucial role in mediating the effects of stress hormones on memory consolidation (Barros et al. Citation2002; Bianchin Citation1999; Lalumiere et al. Citation2004; McGaugh Citation2002; McGaugh Citation2004; Schafe and LeDoux Citation2000). Exposure to stress is associated with release of norepinephrine in the BLC, mediated by the nucleus of the solitary tract directly or via its projections to the locus coeruleus, which in turn innervates the BLC (Herman et al. Citation2003; Myers and Rinaman Citation2002; Williams et al. Citation1998). Stress also results in increased release of glucocorticoids from the adrenal cortex. Although centrally-released norepinephrine and glucocorticoids reach, and profoundly affect, several brain regions, it is the concurrent activation and interaction of beta-adrenergic and glucocorticoid receptors within the BLC that mediates their effects on memory consolidation (for review see McGaugh Citation2004). Within BLC intrinsic circuits, this interaction is also affected by other neurotransmitters such as acetylcholine, GABA and glutamate (McGaugh Citation2004; Pare Citation2003; Power et al. Citation2000, Citation2003).
A growing body of evidence supports the idea that these neuromodulatory interactions affect the output of the BLC to several of its target regions and that it is in these regions that memory consolidation ultimately occurs (for review see McGaugh Citation2004; Pare Citation2003) (). BLC effects on memory consolidation in the nucleus accumbens, hippocampus, entorhinal and insular cortices have been described (Liang and McGaugh Citation1983; Miranda and McGaugh Citation2004; Roesler et al. Citation2002; Roozendaal and McGaugh Citation1997; Roozendaal et al. Citation1999; Setlow et al. Citation2000). Emerging from these studies is the notion that the BLC modulates long-term memory consolidation relative to different forms of learning occurring in distinct brain regions. Interestingly, the BLC has also been found to mediate other forms of stress hormone-dependent effects on memory, such as impairment of memory retrieval and working memory (Nathan et al. Citation2004), functions typically attributed to the hippocampus and prefrontal cortex, respectively. Such highly diversified effects suggest that the BLC may play a far-reaching role in the modulation of stress-related learning in several cortical regions.
The mechanisms underlying the modulation of stress-related memories are not well understood at present. In some cortical regions, such as the hippocampus, effects of the amygdala on specific plastic events have been demonstrated (Akirav and Richter-Levin Citation1999; Frey et al. Citation2001; Ikegaya et al. Citation1995; Pelletier and Pare Citation2004). The hippocampus receives both direct and indirect inputs from the BLC (Berretta et al. Citation2001; Pitkanen et al. Citation2000). Activation of β-adrenoceptors in the BLC affects memory consolidation in the hippocampus (Roozendaal et al. Citation1999). For example, such activation has been recently shown to be associated with the induction of the immediate early gene Arc in the hippocampus as well as with changes in performance of an inhibitory avoidance task (McIntyre et al. Citation2005). Electrophysiological evidence indicates that stimulation of the BLC facilitates long-term potentiation in the dentate gyrus, while intra-amygdala infusion of β-adrenergic or muscarinic receptor antagonists has the opposite effect (Akirav and Richter-Levin Citation1999; Frey et al. Citation2001; Ikegaya et al. Citation1997, Citation1995, Citation1996). Modulation of synaptic plasticity may in part account for BLC effects on memory consolidation in the hippocampus. In addition, synchronized oscillatory activity in the BLC and hippocampus has also been proposed as a possible mechanism for enhancing memory consolidation (Pape and Stork Citation2003; Pelletier and Pare Citation2004). Morphological and neurochemical changes may also accompany such effects. Infusion of a GABAA receptor antagonist in the BLC profoundly alters hippocampal inhibitory intrinsic circuits (Berretta et al. Citation2004, Citation2001). These effects are remarkably long lasting as substantial changes affecting specific hippocampal interneuronal subpopulations are detectable 96 h following an acute infusion (Berretta et al. Citation2004). Interestingly, these intrinsic circuits are known to control oscillatory patterns in the hippocampus (for review see Freund and Buzsaki Citation1996). It is also important to note that GABA has been shown to inhibit norepinephrine release within the BLC (Hatfield et al. Citation1999), suggesting that blockade of GABAA receptors may instead increase norepinephrine release and thus mimic a state of emotional arousal. This possibility is supported by evidence showing that infusion of GABA receptor antagonists in the amygdala also induce physiologic and behavioral changes associated with a defense reaction (Sajdyk and Shekhar Citation1997; Sanders et al. Citation1995; Sanders and Shekhar Citation1995; Thielen and Shekhar Citation2002). It is possible that long-lasting changes relative to specific hippocampal intrinsic GABAergic networks may represent a component of the stress-induced enhancement of memory consolidation mediated by the amygdala.
Medial prefrontal cortex modulates the amygdala response to stress
The mPFC has been shown to play an important modulatory role in the response to stress (Amat et al. Citation2005; Diorio et al. Citation1993; McDougall et al. Citation2004; Spencer et al. Citation2005; Sullivan and Gratton Citation1999). Growing evidence supports the idea that the amygdala is one of the main targets of this modulation (Correll et al. Citation2005; Figueiredo et al. Citation2003). We bring here as an example the role that the mPFC and amygdala play in the phenomenon of extinction of fear conditioning.
As discussed above, associative memory of aversive stimuli is crucial to the organism's capability of predicting danger and responding appropriately. Once instated, conditioned fear associations are especially long lasting, or possibly indelible (LeDoux et al. Citation1989), even in the absence of further exposure to coincident occurrence of the conditioned and unconditioned stimuli. A new learning event, defined as extinction of fear conditioning, has to take place in order to inhibit the stress response to a CS that no longer predicts danger (Davis et al. Citation2003; Rescorla Citation2001). This mechanism is particularly important in light of the fact that responses to aversive stimuli, with their associated behavioral, visceral and hormonal components, imply high energetic costs as well as risks and, thus, have to be weighed against the actual likelihood of the occurrence of a stressful event.
Circuits reciprocally linking the amygdala to the mPFC have been shown to be critically involved in the modulation of fear responses during extinction (for reviews see Maren and Quirk Citation2004; Pare et al. Citation2004) (). The functional integrity of the amygdala is crucial, as demonstrated by experiments using local pharmacological manipulations (Falls et al. Citation1992; Lu et al. Citation2001). Growing experimental evidence shows that the mPFC plays an essential role in long-term extinction memory (Morgan et al. Citation1993; Quirk et al. Citation2000; Santini et al. Citation2004). In rats not exposed to extinction learning, electrical stimulation of the infralimbic cortex during exposure to a CS reduces conditioned responses (Milad and Quirk Citation2002). These researchers have also demonstrated that electrical stimulation of the infralimbic cortex produces an inhibition of brainstem-projecting CeA neurons (Quirk et al. Citation2003). Following extinction training, infralimbic neurons respond to the CS with a strong increase of their activity (Milad and Quirk Citation2002).
Together, these results indicate that the mechanisms mediating extinction of conditioned fear may involve activation of the mPFC and consequent inhibition of the CeA leading to a failure to trigger a stress response through activation of the hypothalamus and brainstem. It has been argued recently that intercalated (ITC) cells of the amygdala are ideal candidates to mediate the infralimbic-driven inhibition of CeA neurons (Pare et al. Citation2004; Quirk et al. Citation2003; Royer and Pare Citation2002). ITC cell masses are clusters of GABAergic neurons that form a net throughout the rostral half of the lateral-basolateral nuclear complex (McDonald and Augustine Citation1993; Millhouse Citation1986; Nitecka and Ben-Ari Citation1987; Pare and Smith Citation1993a; Pitkanen and Amaral Citation1994). These neurons are known to receive projections from the BLC and to send inhibitory inputs to the CeA (Delaney and Sah Citation2001; Millhouse Citation1986; Pare and Smith Citation1993a,CitationbPare and Smith 1993a, b; Royer et al. Citation1999). The infralimbic cortex also sends strong projections to ITC cell masses (McDonald et al. Citation1996) (Berretta et al. unpublished observation). Experiments from our laboratory, using induction of the immediate early gene Fos as an index of neuronal activation, indicate that infralimbic inputs increase the activity of ITC cells (Berretta et al. Citation2005). This effect might explain how infralimbic stimulation inhibits CeA neurons (Quirk et al. Citation2003) and the expression of conditioned fear responses (Milad and Quirk Citation2002). Thus, infralimbic activation of ITC neurons may play a major role in modulating the response of brainstem projecting CeA neurons to CS-related inputs arising in the BLC.
Involvement of cortico-amygdalo-cortical circuits in psychiatric disorders
The cortico-amygdalar networks described above have been found to be involved in a remarkable array of psychiatric diseases, including anxiety disorders, major depression, schizophrenia, and bipolar disorder. Importantly, stress and anxiety appear to be important components of many of these diseases. Recent views on post traumatic stress disorder (PTSD) envision this condition in terms of fear conditioning and postulate that an impairment of extinction of conditioned responses may play an important role in this disease (Gorman et al. Citation2000; Pitman et al. Citation2001). Imaging studies in PTSD patients have shown altered activation of the amygdala and of several cortical regions such as the anterior cingulate gyrus and lateral anterior prefrontal cortex as well as occipital and parietal regions that support visual and spatial aspects of stimuli representations (Bremner Citation2002; Lanius et al. Citation2002; Lanius et al. Citation2003; Liberzon and Phan Citation2003; Pissiota et al. Citation2002; Rauch et al. Citation1996; Shin et al. Citation1997; Shin et al. Citation2004). Although these results are still controversial, they are generally consistent with current knowledge on conditioned fear brain circuits and support the hypothesis that persistent, intrusive memory of an intensely traumatic event may be mediated by increased amygdalar activity (Grillon et al. Citation1996; Rauch et al. Citation2000). An important component of this hypothesis is the failure of cortical inhibitory mechanisms to reduce amygdala activity, and thus mediate extinction learning (Armony and LeDoux Citation1997; Charney Citation2003). Recently, increased activation of the visual cortex induced by amygdala hyperactivity has also been suggested to play an important role by mediating emotion-driven retrieval of visual images relative to the traumatic event (Gilboa et al. Citation2004). In summary, it is reasonable to propose that altered cortico-amygdalo-cortical interactions may represent an important factor in the pathogenesis of PTSD.
Similar mechanisms could be involved in the pathogenesis of panic and other anxiety disorders (Gorman et al. Citation2000). As an example, imaging investigations suggest the involvement of the amygdala and frontal and temporal cortical regions in panic disorder (Brambilla et al. Citation2002; Eren et al. Citation2003; Massana et al. Citation2002, Citation2003; Thomas et al. Citation2001; Wik et al. Citation1997). The role played by the amygdala in this disorder, and more generally in anxiety, has been investigated extensively using animal models (Davis Citation1992a,Citationb; Shekhar et al. Citation2003, Citation1999). These studies indicate that altered neuroregulatory mechanisms in the amygdala, including disruption of GABAergic inhibition and stimulation of corticotropin releasing factor receptors result in anxiety-like behavior (Sajdyk et al. Citation1999; Sanders and Shekhar Citation1995). Interestingly, this latter effect depends on NMDA receptors (Rainnie et al. Citation2004), suggesting that excitatory inputs to the amygdala may play an important role in these responses.
Conclusions
The amygdala and the massive cortical networks in which it is embedded, regulate multiple stages and aspects of the stress response (). Connections with the hypothalamus and brainstem, mediated primarily by the CeA, provide a broad array of visceral, hormonal and humoral information and allow the amygdala to orchestrate comprehensive, multifaceted responses to a stressful event. Within cortico-amygdalo-cortical circuits, such responses are strongly associated with plastic events, from associative learning triggered by initial exposure to an aversive event to long term memory consolidation and, in some cases, to extinction of conditioned responses according to the current predictive value of the CS. Given the importance and complexity of these mechanisms, it is not surprising that cortico-amygdala-cortical circuits have been found to play an important role in a broadening number of psychiatric conditions involving anxiety and vulnerability to stress.
References
- Akirav I, Richter-Levin G. Biphasic modulation of hippocampal plasticity by behavioral stress and basolateral amygdala stimulation in the rat. J Neurosci 1999; 19(23)10530–10535
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci 2005; 8(3)365–371
- Armony JL, LeDoux JE. How the brain processes emotional information. Ann N Y Acad Sci 1997; 821: 259–270
- Asan E. The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol 1998; 142: 1–118
- Bacon SJ, Headlam AJ, Gabbott PL, Smith AD. Amygdala input to medial prefrontal cortex (mPFC) in the rat: A light and electron microscope study. Brain Res 1996; 720(1-2)211–219
- Bahar A, Samuel A, Hazvi S, Dudai Y. The amygdalar circuit that acquires taste aversion memory differs from the circuit that extinguishes it. Eur J Neurosci 2003; 17(7)1527–1530
- Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol 1990; 300(4)549–571
- Barros DM, Pereira P, Medina JH, Izquierdo I. Modulation of working memory and of long- but not short-term memory by cholinergic mechanisms in the basolateral amygdala. Behav Pharmacol 2002; 13(2)163–167
- Baxter MG, Murray EA. Reinterpreting the behavioral effects of the amygdala in non-human primates. The amygdala—a functional analysis, JP Aggleton. Oxford University Press, New York 2000
- Berretta S, Lange N, Bhattacharyya S, Sebro R, Garces J, Benes FM. Long term effects of amygdala GABA receptor blockade on specific subpopulations of hippocampal interneurons. Hippocampus 2004; 14: 876–894
- Berretta S, Munno DW, Benes FM. Amygdalar activation alters the hippocampal GABA system: ‘Partial’ modelling for postmortem changes in schizophrenia. J Comp Neurol 2001; 431: 129–138
- Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience 2005; 132: 943–953
- Bhatnagar S, Vining C, Denski K. Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann N Y Acad Sci 2004; 1032: 315–319
- Bianchin M, Mello e Souza T, Medina JH, Izquierdo I. The amygdala is involved in the modulation of long-term memory, but not in working or short-term memory. Neurobiol Learn Mem 1999; 71(2)127–131
- Brambilla P, Barale F, Caverzasi E, Soares JC. Anatomical MRI findings in mood and anxiety disorders. Epidemiol Psichiatr Soc 2002; 11(2)88–99
- Bremner JD. Neuroimaging studies in post-traumatic stress disorder. Curr Psychiatry Rep 2002; 4(4)254–263
- Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J Neurosci 1998; 18(19)8038–8046
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 2002; 26(3)321–352
- Cassell MD, Wright DJ. Topography of projections from the medial prefrontal cortex to the amygdala in the rat. Brain Res Bull 1986; 17(3)321–333
- Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand Suppl 2003, 417: 38–50
- Charney DS, Deutch AA. Functional neuroanatomy of anxiety and fear: Implications for the pathophysiology and treatment of anxiety disorders. Crit Rev Neurobiol 1996; 10(3-4)419–446
- Chiba AA, Bucci DJ, Holland PC, Gallagher M. Basal forebrain cholinergic lesions disrupt increments but not decrements in conditioned stimulus processing. J Neurosci 1995; 15(11)7315–7322
- Cliffer K, Burstein R, Giesler G, Jr. Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J Neurosci 1991; 11(3)852–868
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology 2003; 28(2)206–215
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol 2004; 60(2)236–248
- Cornwall J, Phillipson OT. Afferent projections to the dorsal thalamus of the rat as shown by retrograde lectin transport—I. The mediodorsal nucleus. Neuroscience 1988; 24(3)1035–1049
- Correll CM, Rosenkranz JA, Grace AA. Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biol Psychiatry 2005; 58(5)382–391
- Crane JW, French KR, Buller KM. Patterns of neuronal activation in the rat brain and spinal cord in response to increasing durations of restraint stress. Stress 2005; 8(3)199–211
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: Implications for the development of normal and abnormal function during adolescence. J Comp Neurol 2002; 453(2)116–130
- Czeh B, Simon M, van der Hart MG, Schmelting B, Hesselink MB, Fuchs E. Chronic stress decreases the number of parvalbumin-immunoreactive interneurons in the hippocampus: Prevention by treatment with a substance P receptor (NK1) antagonist. Neuropsychopharmacology 2005; 30(1)67–79
- Davis HP, Squire LR. Protein synthesis and memory: A review. Psychol Bull 1984; 96(3)518–559
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci 1992a; 15: 353–375
- Davis M. The role of the amygdala in fear-potentiated startle: Implications for animal models of anxiety. Trends Pharmacol Sci 1992b; 13(1)35–41
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. The amygdala: A functional analysis, JP Aggleton. Oxford University Press, Oxford 2000; 213–287
- Davis M, Walker DL, Myers KM. Role of the amygdala in fear extinction measured with potentiated startle. Ann N Y Acad Sci 2003; 985: 218–232
- Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Mol Psychiatry 2001; 6(1)13–34
- Delaney AJ, Sah P. Pathway-specific targeting of GABA(A) receptor subtypes to somatic and dendritic synapses in the central amygdala. J Neurophysiol 2001; 86(2)717–723
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (Cingulate Gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci 1993; 13: 3839–3847
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol 2001; 436(4)430–455
- Easton A, Gaffan D. The amygdala and the memory of reward: The importance of fibres of passage from the basal forebrain. The amygdala—a functional anlysis, JP Aggleton. Oxford University Press, New York 2000
- Eren I, Tukel R, Polat A, Karaman R, Unal S. Evaluation of regional cerebral blood flow changes in panic disorder with Tc99m-HMPAO SPECT. Psychiatry Res 2003; 123(2)135–143
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci 1992; 12(3)854–863
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol 2005; 56: 207–234
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci 2003; 18(8)2357–2364
- Flugge G, Johren O, Fuchs E. [3H]Rauwolscine binding sites in the brains of male tree shrews are related to social status. Brain Res 1992; 597(1)131–137
- Fort P, Luppi PH, Jouvet M. Afferents to the nucleus reticularis parvicellularis of the cat medulla oblongata: A tract-tracing study with cholera toxin B subunit. J Comp Neurol 1994; 342(4)603–618
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus 1996; 6(4)347–470
- Frey S, Bergado-Rosado J, Seidenbecher T, Pape HC, Frey JU. Reinforcement of early long-term potentiation (Early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: Heterosynaptic induction mechanisms of late-LTP. J Neurosci 2001; 21(10)3697–3703
- Gallagher M. The amygdala and associative learning. The amygdala—a functional analysis, JP Aggleton. Oxford University Press Inc, New York 2000
- Ge X, Yang Z, Duan L, Rao Z. Evidence for involvement of the neural pathway containing the peripheral vagus nerve, medullary visceral zone and central amygdaloid nucleus in neuroimmunomodulation. Brain Res 2001; 914(1-2)149–158
- Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience 2002; 115(4)1261–1279
- Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, Bonne O. Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry 2004; 55(3)263–272
- Gold PE, Van Buskirk RB. Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behav Biol 1975; 13(2)145–153
- Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci 1996; 16(15)4787–4798
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem 2001; 8(3)148–155
- Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry 2000; 157(4)493–505
- Gray TS, Carney ME, Magnuson DJ. Direct projections from the central amygdaloid nucleus to the hypothalamic paraventricular nucleus: Possible role in stress-induced adrenocorticotropin release. Neuroendocrinology 1989; 50(4)433–446
- Grillon C, Southwick SM, Charney DS. The psychobiological basis of posttraumatic stress disorder. Mol Psychiatry 1996; 1(4)278–297
- Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal—prefrontal topography. Neuroscience 1988; 24(2)379–431
- Grove EA. Neural associations of the substantia innominata in the rat: Afferent connections. J Comp Neurol 1988; 277(3)315–346
- Haber SN, Groenewegen HJ, Grove EA, Nauta WJH. Efferent connections of the ventral pallidum: Evidence of a dual striato pallidofugal pathway. J Comp Neurol 1985; 235: 322–335
- Han JS, Holland PC, Gallagher M. Disconnection of the amygdala central nucleus and substantia innominata/nucleus basalis disrupts increments in conditioned stimulus processing in rats. Behav Neurosci 1999; 113(1)143–151
- Hatfield T, Spanis C, McGaugh JL. Response of amygdalar norepinephrine to footshock and GABAergic drugs using in vivo microdialysis and HPLC. Brain Res 1999; 835(2)340–345
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 2003; 24(3)151–180
- Holland P, Petrovich G, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol Behav 2002; 76(1)117
- Holland PC. Brain mechanisms for changes in processing of conditioned stimuli in Pavlovian conditioning: Implication for behavior theory. Anim Learn Behav 1997; 25: 373–399
- Holland PC, Gallagher M. Amygdala central nucleus lesions disrupt increments, but not decrements, in conditioned stimulus processing. Behav Neurosci 1993; 107(2)246–253
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci 1999; 3(2)65–73
- Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiol Learn Mem 2004; 81(1)67–74
- Ikegaya Y, Nakanishi K, Saito H, Abe K. Amygdala beta-noradrenergic influence on hippocampal long-term potentiation in vivo. Neuroreport 1997; 8(14)3143–3146
- Ikegaya Y, Saito H, Abe K. High-frequency stimulation of the basolateral amygdala facilitates the induction of long-term potentiation in the dentate gyrus in vivo. Neurosci Res 1995; 22(2)203–207
- Ikegaya Y, Saito H, Abe K. The basomedial and basolateral amygdaloid nuclei contribute to the induction of long-term potentiation in the dentate gyrus in vivo. Eur J Neurosci 1996; 8(9)1833–1839
- Izquierdo I, Dias RD. Effect of ACTH, epinephrine, beta-endorphin, naloxone, and of the combination of naloxone or beta-endorphin with ACTH or epinephrine on memory consolidation. Psychoneuroendocrinology 1983; 8(1)81–87
- Joels M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, Verkuyl M, Lucassen PJ, Krugers HJ. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress 2004; 7(4)221–231
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus—reinforcement associations. Exp Neurol 1972; 36(2)362–377
- Kelley AE, Domesick VB, Nauta WJH. The amygdalostriatal projection in the rat—an anatomical study by anterograde and retrograde tracing methods. Neuroscience 1982; 7: 615–630
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature 1997; 388: 377–380
- Kita H, Kitai ST. Amygdaloid projections to the frontal cortex and the striatum in the rat. J Comp Neurol 1990; 298: 40–49
- Krettek JE, Price JL. Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and to the subiculum in the rat and cat. J Comp Neurol 1977; 172: 723–752
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol 1978; 178: 225–254
- Lalumiere RT, Nguyen LT, McGaugh JL. Post-training intrabasolateral amygdala infusions of dopamine modulate consolidation of inhibitory avoidance memory: Involvement of noradrenergic and cholinergic systems. Eur J Neurosci 2004; 20(10)2804–2810
- Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, Gati JS, Menon RS. Brain activation during script-driven imagery induced dissociative responses in PTSD: A functional magnetic resonance imaging investigation. Biol Psychiatry 2002; 52(4)305–311
- Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Recall of emotional states in posttraumatic stress disorder: An fMRI investigation. Biol Psychiatry 2003; 53(3)204–210
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 2003; 23(4-5)727–738
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci 2000; 23: 155–184
- LeDoux JE, Xagoraris A, Romanski L. Indelebility of subcortical emotional memories. J Cognit Neurosci 1989; 1: 238–243
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: Sensory interface of the amygdala in fear conditioning. J Neurosci 1990; 10(4)1062–1069
- Li YQ, Jia HG, Rao ZR, Shi JW. Serotonin-, substance P- or leucine-enkephalin-containing neurons in the midbrain periaqueductal gray and nucleus raphe dorsalis send projection fibers to the central amygdaloid nucleus in the rat. Neurosci Lett 1990; 120(1)124–127
- Liang KC, McGaugh JL. Lesions of the stria terminalis attenuate the enhancing effect of post-training epinephrine on retention of an inhibitory avoidance response. Behav Brain Res 1983; 9(1)49–58
- Liberzon I, Phan KL. Brain-imaging studies of posttraumatic stress disorder. CNS Spectr 2003; 8(9)641–650
- Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J Neurosci 2001; 21(16)RC162
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Res Brain Res Rev 1997; 24(1)1–27
- Ma S, Morilak DA. Norepinephrine release in medial amygdala facilitates activation of the hypothalamic-pituitary-adrenal axis in response to acute immobilisation stress. J Neuroendocrinol 2005; 17(1)22–28
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci 2004; 5(11)844–852
- Mascagni F, McDonald AJ, Coleman JR. Corticoamygdaloid and corticocortical projections of the rat temporal cortex—a phaseolus-vulgaris leucoagglutinin Study. Neuroscience 1993; 57: 697–715
- Massana G, Gasto C, Junque C, Mercader J, Gomez B, Massana J, Torres X, Salamero M. Reduced levels of creatine in the right medial temporal lobe region of panic disorder patients detected with (1)h magnetic resonance spectroscopy. Neuroimage 2002; 16(3 Pt 1)836
- Massana G, Serra-Grabulosa JM, Salgado-Pineda P, Gasto C, Junque C, Massana J, Mercader JM, Gomez B, Tobena A, Salamero M. Amygdalar atrophy in panic disorder patients detected by volumetric magnetic resonance imaging. Neuroimage 2003; 19(1)80–90
- McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol 1982; 208(4)401–418
- McDonald AJ. Immunohistochemical identification of gamma-aminobutyric acid-containing neurons in the basolateral amygdala. Neurosci Lett 1985; 53: 203–207
- McDonald AJ. Organization of amygdaloid projections to the mediodorsal thalamus and prefrontal cortex: A fluorescence retrograde transport study in the rat. J Comp Neurol 1987; 262(1)46–58
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience 1991; 44: 15–33
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol 1998; 55(3)257–332
- McDonald AJ, Augustine JR. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience 1993; 52(2)281–294
- McDonald AJ, Mascagni F. Projections of the lateral entorhinal cortex to the amygdala: A Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 1997; 77(2)445–459
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: A Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 1996; 71(1)55–75
- McDougall SJ, Widdop RE, Lawrence AJ. Medial prefrontal cortical integration of psychological stress in rats. Eur J Neurosci 2004; 20(9)2430–2440
- McEwen BS. Mood disorders and allostatic load. Biol Psychiatry 2003; 54(3)200–207
- McEwen BS. Glucocorticoids, depression, and mood disorders: Structural remodeling in the brain. Metabolism 2005; 54(5 Suppl 1)20–23
- McEwen BS, Magarinos AM, Reagan LP. Structural plasticity and tianeptine: Cellular and molecular targets. Eur Psychiatry 2002, 17 Suppl 3: 318–330
- McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol 1995; 5(2)205–216
- McGaugh JL. Memory—a century of consolidation. Science 2000; 287(5451)248–251
- McGaugh JL. Memory consolidation and the amygdala: A systems perspective. Trends Neurosci 2002; 25(9)456
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 2004; 27: 1–28
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc Natl Acad Sci USA 2005; 102(30)10718–10723
- Menetrey D, De Pommery J. Origins of spinal ascending pathways that reach central areas involved in visceroception and visceronociception in the rat. Eur J Neurosci 1991; 3(3)249–259
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 2002; 420(6911)70–74
- Millhouse OE. The intercalated cells of the amygdala. J Comp Neurol 1986; 247(2)246–271
- Millhouse OE, DeOlmos J. Neuronal configurations in lateral and basolateral amygdala. Neuroscience 1983; 10(4)1269–1300
- Miranda MI, McGaugh JL. Enhancement of inhibitory avoidance and conditioned taste aversion memory with insular cortex infusions of 8-Br-cAMP: Involvement of the basolateral amygdala. Learn Mem 2004; 11(3)312–317
- Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol 1995; 359(2)221–238
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning—contribution of medial prefrontal cortex. Neurosci Lett 1993; 163: 109–113
- Myers EA, Rinaman L. Viscerosensory activation of noradrenergic inputs to the amygdala in rats. Physiol Behav 2002; 77(4-5)723–729
- Nathan SV, Griffith QK, McReynolds JR, Hahn EL, Roozendaal B. Basolateral amygdala interacts with other brain regions in regulating glucocorticoid effects on different memory functions. Ann N Y Acad Sci 2004; 1032: 179–182
- Nitecka L, Amerski L, Panek-Mikula J, Narkiewicz O. Tegmental afferents of the amygdaloid body in the rat. Acta Neurobiol Exp (Wars) 1980; 40(3)609–624
- Nitecka L, Ben-Ari Y. Distribution of GABA-like immunoreactivity in the rat amygdaloid complex. J Comp Neurol 1987; 266(1)45–55
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci USA 2004; 101(3)853–858
- Ottersen OP. Afferent connections to the amygdaloid complex of the rat and cat: II. Afferents from the hypothalamus and the basal telencephalon. J Comp Neurol 1980; 194(1)267–289
- Ottersen OP. Afferent connections to the amygdaloid complex of the rat with some observations in the cat. III. Afferents from the lower brain stem. J Comp Neurol 1981; 202(3)335–356
- Ottersen OP, Ben-Ari Y. Afferent connections to the amygdaloid complex of the rat and cat. I. Projections from the thalamus. J Comp Neurol 1979; 187: 401–424
- Pape HC, Stork O. Genes and mechanisms in the amygdala involved in the formation of fear memory. Ann N Y Acad Sci 2003; 985: 92–105
- Pare D. Role of the basolateral amygdala in memory consolidation. Prog Neurobiol 2003; 70(5)409–420
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol 2004; 92(1)1–9
- Pare D, Smith Y. Distribution of GABA immunoreactivity in the amygdaloid complex of the cat. Neuroscience 1993a; 57(4)1061–1076
- Pare D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience 1993b; 57(4)1077–1090
- Pare' D, Smith Y, Pare' JF. Intra-amygdaloid projections of the basolateral and basomedial nuclei in the cat: Phaseolus vulgaris-leucoagglutinin anterograde tracing at the light and electron microscopic level. Neuroscience 1995; 69(2)567–583
- Pelletier JG, Pare D. Role of amygdala oscillations in the consolidation of emotional memories. Biol Psychiatry 2004; 55(6)559–562
- Petrov T, Krukoff TL, Jhamandas JH. Chemically defined collateral projections from the pons to the central nucleus of the amygdala and hypothalamic paraventricular nucleus in the rat. Cell Tissue Res 1994; 277(2)289–295
- Petrovich GD, Risold PY, Swanson LW. Organization of projections from the basomedial nucleus of the amygdala: A PHAL study in the rat. J Comp Neurol 1996; 374(3)387–420
- Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res 1997; 763(2)247–254
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience 1998; 82(2)443–468
- Pissiota A, Frans O, Fernandez M, von Knorring L, Fischer H, Fredrikson M. Neurofunctional correlates of posttraumatic stress disorder: A PET symptom provocation study. Eur Arch Psychiatry Clin Neurosci 2002; 252(2)68–75
- Pitkanen A. Connectivity of the rat amygdaloid complex. The amygdala—a functional analysis, JP Aggleton. Oxford University Press Inc., New York 2000
- Pitkanen A, Amaral DG. The distribution of GABAergic cells, fibers, and terminals in the monkey amygdaloid complex: An immunohistochemical and in situ hybridization study. J Neurosci 1994; 14(4)2200–2224
- Pitkanen A, Amaral DG. Organization of the intrinsic connections of the monkey amygdaloid complex: Projections originating in the lateral nucleus. J Comp Neurol 1998; 398(3)431–458
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci 2000; 911: 369–391
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: An emerging framework for understanding functions of the amygdala. Trends Neurosci 1997; 20(11)517–523
- Pitman RK, Shin LM, Rauch SL. Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. J Clin Psychiatry 2001, 62 Suppl 17: 47–54
- Porrino LJ, Crane AM, Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J Comp Neurol 1981; 198: 121–136
- Power AE, Roozendaal B, McGaugh JL. Glucocorticoid enhancement of memory consolidation in the rat is blocked by muscarinic receptor antagonism in the basolateral amygdala. Eur J Neurosci 2000; 12(10)3481–3487
- Power AE, Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiol Learn Mem 2003; 80(3)178–193
- Prewitt CM, Herman JP. Anatomical interactions between the central amygdaloid nucleus and the hypothalamic paraventricular nucleus of the rat: A dual tract-tracing analysis. J Chem Neuroanat 1998; 15(3)173–185
- Price JL. Prefrontal cortical networks related to visceral function and mood. Ann N Y Acad Sci 1999; 877: 383–396
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci 1981; 1(11)1242–1259
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 2003; 23(25)8800–8807
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci 2000; 20(16)6225–6231
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 2004; 125(1)1–6
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci 2004; 24(14)3471–3479
- Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry 1996; 53(5)380–387
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biol Psychiatry 2000; 47(9)769–776
- Ray JP, Price JL. The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain-prefrontal cortex topography. J Comp Neurol 1992; 323: 167–197
- Rescorla RA. Experimental extinction. Handbook of contemporaries learning theories, RR Mowrer, S Klein. Erlbaum, Mahwah, NJ 2001; 119–157
- Rinaman L, Schwartz G. Anterograde Transneuronal Viral Tracing of Central Viscerosensory Pathways in Rats. J Neurosci 2004; 24(11)2782–2786
- Roesler R, Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of 8-Br-cAMP infused into the entorhinal cortex of rats after training. Eur J Neurosci 2002; 15(5)905–910
- Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala—corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex 1993; 3: 515–532
- Roozendaal B. Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem 2002; 78(3)578–595
- Roozendaal B, de Quervain DJ, Schelling G, McGaugh JL. A systemically administered beta-adrenoceptor antagonist blocks corticosterone-induced impairment of contextual memory retrieval in rats. Neurobiol Learn Mem 2004; 81(2)150–154
- Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur J Neurosci 1997; 9(1)76–83
- Roozendaal B, Nguyen BT, Power AE, McGaugh JL. Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proc Natl Acad Sci USA 1999; 96(20)11642–11647
- Rosen JB, Hitchcock JM, Sananes CB, Miserendino MJ, Davis M. A direct projection from the central nucleus of the amygdala to the acoustic startle pathway: Anterograde and retrograde tracing studies. Behav Neurosci 1991; 105(6)817–825
- Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci 1999; 19(23)10575–10583
- Royer S, Pare D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience 2002; 115(2)455–462
- Russchen FT, Price JL. Amygdalostriatal projections in the rat. Topographical organization and fiber morphology shown using the lectin PHA-L as an anterograde tracer. Neurosci Letters 1984; 47: 15–22
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res 1999; 100(1-2)207–215
- Sajdyk TJ, Shekhar A. Excitatory amino acid receptor antagonists block the cardiovascular and anxiety responses elicited by gamma-aminobutyric acid. A receptor blockade in the basolateral amygdala of rats. J Pharmacol Exp Ther 1997; 283(2)969–977
- Salm AK, Pavelko M, Krouse EM, Webster W, Kraszpulski M, Birkle DL. Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Brain Res Dev Brain Res 2004; 148(2)159–167
- Sanders SK, Morzorati SL, Shekhar A. Priming of experimental anxiety by repeated subthreshold GABA blockade in the rat amygdala. Brain Res 1995; 699(2)250–259
- Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav 1995; 52(4)701–706
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci 2004; 24(25)5704–5710
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res 1980; 197(2)291–317
- Sapolsky RM. Stress and plasticity in the limbic system. Neurochem Res 2003; 28(11)1735–1742
- Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci 2000; 20(18)RC96
- Selden NR, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience 1991; 42(2)335–350
- Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: A retro- and antero-grade transport and immunohistochemical study. J Comp Neurol 1992; 323(3)387–410
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract-tracing study with phaseolus vulgaris leucoagglutinin. J Comp Neurol 1989; 290: 213–242
- Setlow B, Roozendaal B, McGaugh JL. Involvement of a basolateral amygdala complex-nucleus accumbens pathway in glucocorticoid-induced modulation of memory consolidation. Eur J Neurosci 2000; 12(1)367–375
- Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. The amygdala, panic disorder, and cardiovascular responses. Ann N Y Acad Sci 2003; 985: 308–325
- Shekhar A, Sajdyk TS, Keim SR, Yoder KK, Sanders SK. Role of the basolateral amygdala in panic disorder. Ann N Y Acad Sci 1999; 877: 747–750
- Shi CJ, Cassell MD. Perirhinal cortex projections to the amygdaloid complex and hippocampal formation in the rat. J Comp Neurol 1999; 406(3)299–328
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. A positron emission tomographic study of symptom provocation in PTSD. Ann N Y Acad Sci 1997; 821: 521–523
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry 2004; 61(2)168–176
- Shors TJ, Mathew PR. NMDA receptor antagonism in the lateral/basolateral but not central nucleus of the amygdala prevents the induction of facilitated learning in response to stress. Learn Mem 1998; 5(3)220–230
- Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: Possible role of the bed nucleus of the stria terminalis. J Comp Neurol 2005; 481(4)363–376
- Stewart MG, Davies HA, Sandi C, Kraev Rog, IV, achevsky VV, Peddie CJ, Rodriguez JJ, Cordero MI, Donohue HS, Gabbott PL. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: A three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience 2005; 131(1)43–54
- Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci 1999; 19(7)2834–2840
- Swanson LW, Petrovich GD. What is the amygdala?. Trends Neurosci 1998; 21(8)323–331
- Thielen SK, Shekhar A. Amygdala priming results in conditioned place avoidance. Pharmacol Biochem Behav 2002; 71(3)401–406
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry 2001; 58(11)1057–1063
- Trentani A, Kuipers SD, Ter Horst GJ, Den Boer JA. Selective chronic stress-induced in vivo ERK1/2 hyperphosphorylation in medial prefrontocortical dendrites: Implications for stress-related cortical pathology?. Eur J Neurosci 2002; 15(10)1681–1691
- Turner BH, Herkenham M. Thalamoamygdaloid projections in the rat: A test of the amygdala's role in sensory processing. J Comp Neurol 1991; 313(2)295–325
- Veening JG. Cortical afferents of the amygdaloid complex in the rat: An HRP study. Neurosci Lett 1978a; 8: 191–195
- Veening JG. Subcortical afferents of the amygdaloid complex in the rat: An HRP study. Neurosci Lett 1978b; 8: 196–202
- Veinante P, Freund-Mercier MJ. Intrinsic and extrinsic connections of the rat central extended amygdala: An in vivo electrophysiological study of the central amygdaloid nucleus. Brain Res 1998; 794(2)188–198
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol 1991; 313(4)643–668
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 2004; 51(1)32–58
- Verwer RW, Van Vulpen EH, Van Uum JF. Postnatal development of amygdaloid projections to the prefrontal cortex in the rat studied with retrograde and anterograde tracers. J Comp Neurol 1996; 376(1)75–96
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res 2003; 965(1-2)290–294
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience 2004; 128(4)667–673
- Walker DL, Paschall GY, Davis M. Glutamate receptor antagonist infusions into the basolateral and medial amygdala reveal differential contributions to olfactory vs context fear conditioning and expression. Learn Mem 2005; 12(2)120–129
- Wallace DM, Magnuson DJ, Gray TS. Organization of amygdaloid projections to brainstem dopaminergic, noradrenergic, and adrenergic cell groups in the rat. Brain Res Bull 1992; 28(3)447–454
- Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci 1992; 12(10)4066–4079
- Weller KL, Smith DA. Afferent connections to the bed nucleus of the stria terminalis. Brain Res 1982; 232(2)255–270
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem 2002; 77(2)125–184
- Wik G, Fredrikson M, Fischer H. Evidence of altered cerebral blood-flow relationships in acute phobia. Int J Neurosci 1997; 91(3-4)253–263
- Williams CL, Men D, Clayton EC, Gold PE. Norepinephrine release in the amygdala after systemic injection of epinephrine or escapable footshock: Contribution of the nucleus of the solitary tract. Behav Neurosci 1998; 112(6)1414–1422
- Wommack JC, Delville Y. Chronic social stress during puberty enhances tyrosine hydroxylase immunoreactivity within the limbic system in golden hamsters. Brain Res 2002; 933(2)139–143
- Woolf NJ, Butcher LL. Cholinergic projections to the basolateral amygdala: A combined Evans Blue and acetylcholinesterase analysis. Brain Res Bull 1982; 8: 751–763
- Zhang X, Cui J, Tan Z, Jiang C, Fogel R. The central nucleus of the amygdala modulates gut-related neurons in the dorsal vagal complex in rats. J Physiol 2003; 553(Pt 3)1005–1018