Abstract
In populations of young and older adults, it has been shown that individuals may be categorized into one of three diurnal subgroups when salivary cortisol levels are assessed over a 2-day period and compared for their consistency across days: a typical subgroup, a flat subgroup, and an inconsistent subgroup. Interestingly, recent studies have reported that the typical subgroup represents the majority of the young and older adult population, a finding that is difficult to reconcile with previous studies showing increased cortisol levels in older adults with depression or cognitive impairments. In order to assess whether a typical diurnal cortisol profile is representative across different subgroups of older adults, we assessed diurnal cortisol cycle representation in a sample of older adults with subjective complaints of depression and/or memory problems. Furthermore, given the robust relationship between cortisol and cognitive function, the present study examined the association between the three diurnal subgroups and cognitive performance. Forty-two older individuals were recruited on the basis of reporting subjective complaints of either memory problems and/or depressive mood. Participants were asked to sample their saliva over a 2-day period and were then asked to undergo a neuropsychological evaluation that taps into short-term memory, declarative memory and language. The results showed that 69% of the sample presented a Flat cycle of salivary cortisol over a 2-day period while 19% presented an inconsistent pattern and 12% presented a typical pattern. Participants in the flat subgroup were significantly impaired on letter verbal fluency. Furthermore, a relationship was found between diurnal cortisol subgroup representation and subjective complaint profile. These findings show that older adults with complaints of memory problems and/or depressive symptoms do not present the typical profile of the diurnal cortisol cycle, and they provide a preliminary view of how diurnal cortisol profile relates to cognitive function during human aging.
Introduction
In the absence of significant external stimulation, the diurnal rhythm of cortisol is generally characterized by a peak in cortisol secretion after awakening and a steady decline throughout the day, with a trough in cortisol secretion at midnight (Edwards et al. Citation2001a,Citationb; Wust et al. Citation2000; Spath-Schwalbe et al. Citation1993). Recent studies have reported a possible profiling method for the diurnal cycle of cortisol secretion. Smyth et al. (Citation1997) examined patterns of diurnal salivary cortisol secretion in a group of young community-dwelling individuals over a two-day period. It was found that 51% of those tested showed a “Typical” strong decreasing cortisol pattern on both days, thus following previous characterizations of the diurnal cortisol cycle. On the other hand, 17% of the sample displayed a “Flat” cortisol cycle (or no cycle) on both days, and 31% of the sample exhibited an “Inconsistent” cycle, where one day was typical and the other day was flat.
The distribution of the three subgroups was recently examined by Ice et al. (Citation2004) in 48 community dwelling older adults. Using Smyth et al.'s calculation, it was found that 50% of the sample of older adults fell into the typical subgroup, 48% into the inconsistent subgroup, and 2% into the flat subgroup. A typical subgroup majority in an older adult population was somewhat surprising as previous studies have reported a flattening of the diurnal cortisol pattern during both normal and pathological aging (Van Cauter et al. Citation1996; Deuschle et al. Citation1997; Giordano et al. Citation2005).
Indeed, many researchers have shown that the aging process is characterized by a shift in the diurnal pattern, and a flattening of the cycle (Van Cauter et al. Citation1996; Deuschle et al. Citation1997; Giordano et al. 2005). Individuals with Alzheimer's disease display elevated afternoon cortisol secretion compared to controls (Weiner et al. Citation1997), and depressed individuals exhibit increased cortisol secretion in the afternoon hours compared to non-depressed individuals (Sachar et al. Citation1973; Citation1976; for a review see Burke et al. Citation2005). These two patterns of cortisol secretion would tend to suggest that these populations should present a higher rate of flat cycles when compared to normal older populations, but no study has yet measured the diurnal cycle of cortisol secretion in these populations. The reason for this may lie in the fact that populations of aged individuals suffering from dementia and/or clinically-diagnosed depression may present different diurnal patterns of cortisol secretion that may be explained by the underlying disease itself, or by other factors related to the disease (e.g. medication).
Another approach that can be taken is to assess populations that subjectively complain about memory deficits and/or depressive symptoms in old age, but do not meet the clinical criteria for depression or dementia. Many studies have reported that older adults with memory complaints are often depressed (Bolla et al. 1991; Heikkinen et al. Citation1995; Beck, Koenig Citation1996; Blazer et al. Citation1997; Dik et al. Citation2001), which sometimes culminates in a syndrome called “depressive pseudodementia” (Gron et al. Citation2002). Other population-based studies have reported that subjective memory complaints may predict future dementia (Schmand et al. Citation1996; Schofield et al. Citation1997a,Citationb; Geerlings et al. Citation1999; Dik et al. Citation2001; Citation1997), and a recent review of studies on the association between memory complaints and the risk to develop dementia shows that memory complaints predict dementia after a follow-up of at least 2 years, particularly in those with mild cognitive impairment (Jonker et al. Citation2000). Such predictive findings on cognitive decline have also been reported with regards to subjective reporting of depressive symptomatology (Paterniti et al. Citation2002; Wilson et al. Citation2002). In the community-based Amsterdam Study of the Elderly (AMSTEL), a sample of 3147 non-demented persons with normal cognition were tested at a 3-year interval. Results showed that for older adults with more than 8 years of education, depressed mood at baseline was strongly associated with incident Alzheimer's disease 3 years later (Geerlings et al. Citation2000a,Citationb).
Altogether, these results suggest that the presence of either subjective complaint of impaired memory and/or depression might have a significant impact on cortisol secretion and cognitive functioning. The goal of the present study was to evaluate the classification of the diurnal cortisol cycle in an older adult population with subjective memory complaints and/or reports of depressive symptoms, and to assess cognitive function in these older adults. Based on previous results obtained with older adults suffering from depression or Alzheimer's disease, we predicted that older adults with subjective complaints of memory deficits and/or depressive symptoms would present higher rates of the flat cycle when compared to the reported rates of the Flat diurnal pattern in previous studies assessing the diurnal cortisol profile.
Method
Participants
The present study consisted of aged individuals who responded to the following newspaper's advertisement: “If you are more than 50 years old and feel depressed, or if you think that your memory is declining, you can participate in a project…” In all, 69 participants (52 women and 17 men) were recruited as part of a larger study. The study was approved by the Ethic Research Board of the Douglas Hospital and all participants signed a consent form prior to beginning the study.
All participants were interviewed by telephone at which time demographic information such as age, gender and education as well as a list of medications was acquired. During the same telephone interview, both memory complaints and depressive symptomatology were assessed.
Memory complaints were assessed using the Memory Assessment Clinics Questionnaire (MAC-Q) (Crook et al. Citation1992). This questionnaire requires participants to compare their memory at present to when they were in high school or college for five specific memory items. Participants answered on a Likert-type scale numbered from 1 (“much better”) to 5 (“much worse”), where a score of three is defined as no change (“about the same”). The sixth item of the questionnaire was a more general question about memory, whereby participants answered using the same subjective scale, however, now the scale ranged from 2 to 10 instead of 1–5 as with the more specific items. In this way, the general opinion of their memory capacity was weighted more heavily. Given this scale, scores could range between 7 and 35. A score of 25 out of 35 constitutes a significant memory complaint (Crook et al. Citation1992). Consequently, participants in this study were categorized as being high memory complainers if they scored >25 on the MAC-Q.
Presence of depressive symptomatology was evaluated using the Geriatric Depression Scale (GDS: Yesavage et al. Citation1983). This is a 30-item questionnaire where participants can answer “yes” or “no” to questions focused on issues related to geriatric depression. This dichotomous answer format is advantageous for older adults because of its simplicity and succinctness. The GDS is commonly used in geriatric populations and has been validated in these populations (Montorio and Izal Citation1996). A score of 12 out of 30 indicates significant symptoms of depression. Consequently, participants of this study were categorized as high depressive reporters if they scored >12 on the GDS. Of the 69 participants who were contacted, 47% scored above the threshold level for depressive symptomatology. Although slightly lower, this percentage coincides with that which was found in a study that screened the public for depression through the Internet (Houston et al. Citation2001). In this study, Houston and colleagues reported that 58% of those who completed the CES-D on-line screened positive for depression. Of those who scored high on the GDS, 25% were male and only 18% were taking medication for their depression. Thus, in parallel to that reported by Houston et al., a relatively large number of individuals were identified in this study as having depressive symptomatology, many of whom were not receiving treatment. In line with ethical procedures, participants scoring high on the GDS were referred to their doctor. Note, however, that these individuals remained in the study sample.
Cognitive and stress questionnaires
To assess global cognitive function, the Mini-Mental-Status-Examination (MMSE: Folstein et al. Citation1975), was administered to each participant. Although this test is not sensitive enough to isolate deficits in different domains of cognition in a normal older adult population, it was used in this study to allow for comparison with other geriatric research.
To provide a profile of subjective stress levels in each participant, the Psychological Stress Questionnaire (PSQ: Lemyre et al. Citation1990) was administered. The PSQ is a 27-item, Likert-type-scale questionnaire, asking participants to rate their feelings of fatigue, tension, and other factors relating to stress. The scale ranges from 1 (“Never”) to 7 (“Always”). All individual ratings were added to arrive at an overall PSQ score. The authors published t-scores corresponding to each raw score value ranging from 34.5 to 80. According to t-score values a score of 42.9 represents the 25th percentile, a score of 48 represents the 50th percentile and a score of 56 represents the 75th percentile.
Neuropsychological tests
All participants were administered a battery of cognitive tests which have been found in previous studies from our laboratory to load on frontal and hippocampal functioning and to be sensitive to high levels of cortisol (Lupien et al. Citation1994a,Citationb; Citation1999). In short, we assessed participants' performance on tests of verbal fluency, short-term memory and declarative memory of related and unrelated word pairs (for a complete description, see Lupien et al. Citation1994a,Citationb).
Short-term memory
The process of retaining information while it transfers to long-term memory was tested using the Digit Span test. The Digit Span test is a subtest of the Wechsler Memory Scale (Wechsler Citation1973). During this task, participants are asked to listen to a series of digits. After each digit series is read by the experimenter (ranging from two to eight digits), the participant is asked to repeat the digit series. Participants were asked to repeat each digit series until two errors were made in a row. The total number of digits correctly recalled on a previous row constituted the digit span of the participant.
Verbal fluency
The verbal fluency test used in the present study comprised two categories: one phonetic (letter) and one semantic (animals). Participants were asked to name as many words for each category within a one-minute time limit. The number of words generated by the participant served as the measure of verbal fluency.
Declarative memory
Declarative memory was assessed by a cued recall test previously shown by our group to be sensitive to high endogenous cortisol levels in older adults (Lupien et al. Citation1994a,Citationb; Citation1997). Participants were presented with twelve concrete word pairs following Lyons' (Citation1976) criteria, which were selected from Freibers' (Citation1968; Citation1970) free association word norms for Quebec French. The non-associative nature of the word-pairs is generally the primary determinant of declarative memory deficit, thus the list was comprised of six moderately related word pairs (related-pairs) and six unrelated word pairs (unrelated-pairs). The non-associative nature of the pairs was verified by asking 24 participants (12 young and 12 old) to give the first word that came to mind when presented with one of the words from a pair. None of the words from the unrelated pairs generated the expected words. The unrelated-pairs were constructed so as to match the related pairs in terms of word frequency, word length and grammatical category. Presentation of material and response recording were monitored by a Macintosh SE computer. The participant was presented with the list of paired words, which he/she had to read aloud. The list was presented twice in a different order. Following each presentation, the participant made a cued-recall where he/she had to recall a member of a pair when presented with the other.
Cortisol sampling
Participants were provided with a home kit to collect saliva for measurement of basal cortisol levels using a methodology validated in previous research (Lupien et al. Citation1997). Research has shown that saliva sampling is a reliable measure of free, unbound, cortisol secretion, and is more convenient than blood sampling as participants can sample their saliva in the comfort of their own home (Kirschbaum and Hellhammer Citation1994). Each kit contained four sampling filters, a plastic Ziploc® bag for storage of the samples, and a log book to record the exact date and time of sampling. Participants were asked to sample their saliva over two consecutive days. On the chosen day of sampling, participants sampled their saliva four times: At awakening, 14:00, 16:00 h, and before they retired to bed, in order to obtain a good estimate of their circadian rhythm.
Three and a half by 5 cm filters were cut from Whatman #42 filter paper for the saliva samples (Whatman, Clifton, NJ, USA). The top centimetre of the length of the filter was utilized for data recording and was demarked with a small cutout notch (a small, v-shaped cutout on the side of filter used to delineate the limit at which saliva has to saturate the paper). Participants were required to place the filter paper in their mouth until the paper was completely saturated. Once saturated, the filter was air dried and stored in the provided Ziploc bag and was then sent back to the laboratory. On their arrival at the laboratory, all samples were stored at − 20°C until subsequent radioimmunoassay. This method has proven to yield reliable cortisol levels in previous studies (Lupien et al. Citation1997; Citation1998).
Cortisol was extracted from the filter paper using 2 ml of ethanol for 1 h at room temperature. A 300 μl aliquot of the cortisol extract was assayed using the radiotracer and cortisol-specific antibody [3H] labelled cortisol (B-63 antibody from Endocrine Sciences, Tarzana, CA, USA). The antibody cross-reacts less than 4% with deoxycorticosteroid or deoxycortisol. Interassay variability was 3.5%. Cortisol scores were represented in μg/dl. Further, because raw cortisol scores are positively skewed, all values were log-transformed before analysis.
Identification of the diurnal cortisol cycle subgroups
Evaluation of subgroups was done according to Smyth et al.'s (Citation1997) calculations, using the two consecutive sampling days. First, individual daily cortisol diurnal cycles were calculated as the slope of the regression line predicting cortisol level from time of day. Second, in order to differentiate a consistent from an inconsistent diurnal variation, coefficients for day 1 and day 2 cortisol cycles were generated for each participant. Participants' diurnal cycle was characterized as “inconsistent” if the difference in the absolute value of their coefficients was greater than the standard deviation of the mean difference between day 1 and day 2 slopes. For the remaining participants who showed a consistent cycle, further calculations were performed to differentiate those participants who displayed a typical diurnal cycle from those who displayed a flattened cycle. To do this, the average slope for cortisol cycle over both days was computed. Participants with an average value greater than the negative value of the standard deviation (i.e. having a more positive integer) were categorized as having a flat cycle and those with a slope less than the negative standard deviation (i.e. more negative integer) was categorized as having a typical cycle.
Exclusion criteria
Participants were excluded from the present analysis if they were taking medications that are known to interfere with cortisol secretion (i.e. anti-depressants and glucocorticoids). As well, since the effects of cortisol secretion on cognitive functioning was being considered in the present analysis, participants who scored below average on the MMSE (i.e. a score below 24, suggestive of early stages of dementia) were excluded from the present analysis. Finally, participants were excluded from the analysis if they failed to complete the five sampling time-points over the 2-day period. Following the exclusion criteria, 42 participants remained, consisting of 32 women and 10 men.
Statistical analysis
The first goal of this study was to assess the three diurnal subgroups in this sample of subjective complainers in order to compare them, as a whole, to that which has been reported in healthy samples (Smyth et al. Citation1997; Ice et al. Citation2004). Since the method for calculating the diurnal cycle subgroups is not dependent on raw cortisol values, but rather utilizes a formula that standardizes cortisol values, it was felt that our data could be compared to the distribution of participants in each diurnal subgroup reported in previous studies. In addition, due to this method of calculation, the absence of a “control” group in the present study does not necessarily preclude validity of the data. Consequently, calculations as described by Smyth et al. (Citation1997) were performed, thus dividing the total sample of participants into the typical, flat and inconsistent cortisol subgroups, based on their 2-days pattern of salivary cortisol. Analyses of variance controlling for age and gender (ANCOVA) were then performed to assess whether the three diurnal subgroups differed on psychological variables and cognitive performance.
In order to assess whether there was a relationship between diurnal cortisol subgroup and type of subjective complaint, participants were split into three groups based on their complaint scores: high memory complaints with low depressive complaints (“Memory Complaints” group); low memory complaints with high depressive complaints (“Depressive Complaints” group); and high memory complaints with high depressive complaints (“Memory-Depressive Complaints” group). A chi-square test for independence with Yates correction was performed on these data to assess whether a relationship exists between type of subjective complaint and diurnal cortisol subgroup status. All results were considered significant at the 0.05 alpha level.
Results
Sample demographics
The mean age of the group under study was 68.36 years (SEM = 1.14). Number of years of education averaged 14.09 years (SEM = 0.46), and the mean IQ of the group was 106.32 (SEM = 2.58). Administration of the MMSE revealed that the group under study was cognitively intact, with a mean score of 28.19 (SEM = 0.31).
Psychological factors
Mean score on the PSQ was 51.26 (SEM = 1.65), which as a group is a considerably high level of reported stress, representing slightly more than the 50th percentile. Mean depression score on the GDS was 11.52 (SEM = 0.95) and mean memory complaints on the MAC-Q was 27.67 (SEM = 0.53). Thus, overall, the sample under investigation appeared to be relatively “distressed”. Correlation analyses revealed a significant relationship between GDS and PSQ, r = 0.582, p < 0.05, but this association became non-significant when applying a Bonferroni correction for multiple analyses. Finally, MAC-Q was not found to correlate with either GDS or PSQ.
Global impact of subjective complaints
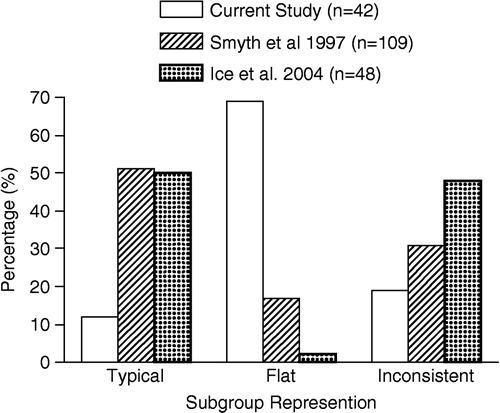
To assess global impact of subjective complaints on diurnal cortisol profile, we first assessed diurnal subgroups in the entire sample, regardless of complaint profile. Based on the diurnal cortisol permutation devised by Smyth et al. within a population of subjective complainers, we found that 69% (29/42) of the sample exhibited a flat cycle, 12% (5/42) showed a typical cycle, and 19% (8/42) of the sample displayed an inconsistent cycle. These percentages differ greatly from those found in a healthy young population by Smyth et al. (Citation1997), who reported 17% exhibiting a flat cycle, 31% exhibiting an inconsistent cycle, and 51% classified as typical. These percentages further differ from those reported by Ice et al. (Citation2004), who reported 2% of elderly individuals exhibiting a flat cycle, 48% exhibiting an inconsistent cycle, and 50% exhibiting a typical cycle. Chi-square analysis indicates that the differences in subgroups between the current study and that of Smyth et al. (Citation1997) and Ice et al. (Citation2004) are indeed significantly different, χ2(4) = 63.95, p < 0.05. Specifically, the current study found a greater representation of the flat subgroup and a smaller representation of the typical subgroup compared to both the Smyth et al. (Citation1997) and Ice et al. (Citation2004) studies (ps < 0.05). In terms of the Inconsistent profile, the current study only differed significantly from Ice et al. (Citation2004). presents the percentage of young and older adults falling into the typical, flat and inconsistent cortisol subgroups in the three aforementioned studies.
Figure 1 Percentage representation of the three diurnal cortisol subgroups in the present study (elderly with memory problems/depressed mood) and that found by Smyth et al. (Citation1997; healthy young population) and Ice et al. (Citation2004; healthy elderly). Chi-square analysis for independence found overall significant relationship between study and subgroup representation (p = 0.001).
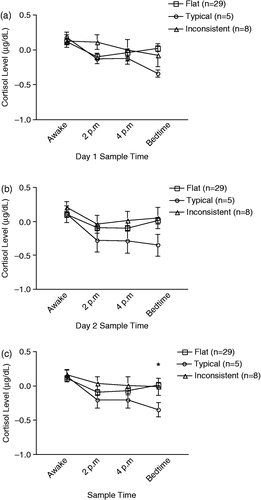
In order to assess whether the flat cortisol profile observed in 69% of our population was due to low morning salivary cortisol or to high afternoon or evening salivary cortisol, we compared the three groups for mean morning (awakening), afternoon (averaged 2 and 4 pm), and evening (bedtime) cortisol levels, controlling for age and gender (–c). As presented in , while mean morning and afternoon cortisol levels did not differ between the three diurnal subgroups on the two-day sampling period, the ANCOVA revealed a significant group difference for mean evening (bedtime) cortisol levels averaged across the two sampling days, F (1, 39) = 4.34, p = 0.02. Specifically, Tukey HSD post-hoc comparisons showed that the Flat subgroup displayed higher evening levels compared to the Typical subgroup, with a 95% confidence interval between 0.07 and 0.65 (p = 0.01; see ). A trend was found for the Inconsistent subgroup to secrete higher evening cortisol levels compared to the Typical subgroup, however, this was not significant due to high inter-group deviations within the Inconsistent subgroup (CI = − 0.67–0.01, p = 0.056). depicts the cortisol secretion for each of the individual sampling days ( and b) and the mean cortisol levels for the two sampling days combined ().
Figure 2 Mean ( ± SEM) cortisol levels (nonlog, μg/dl) across the four sampling periods for (a) day 1, (b) day 2, and (c) overall (i.e. day 1 and 2 combined) mean cortisol levels across sampling periods. Significant differences were found between the typical and flat cortisol subgroups for mean bedtime cortisol levels (p = 0.02). Trend was found between typical and inconsistent subgroups (p = 0.08). *,.
The relationship between diurnal subgroup and demographic/psychological variables was then assessed. As presented in , significant differences were solely found for the MAC-Q score, F (2, 35) = 6.05, p = 0.01. Tukey HSD post hoc comparisons revealed that the Inconsistent subgroup reported fewer memory complaints when compared to the Flat subgroup with a 95% confidence interval between 1.09 and 7.12, p = 0.01. This difference was also observed between the Inconsistent subgroup and the Typical subgroup, however, this difference failed to reach significance (p = 0.09, see ).
Table I. Means (SEM) score on demographic variables as a function of diurnal cortisol subgroup.
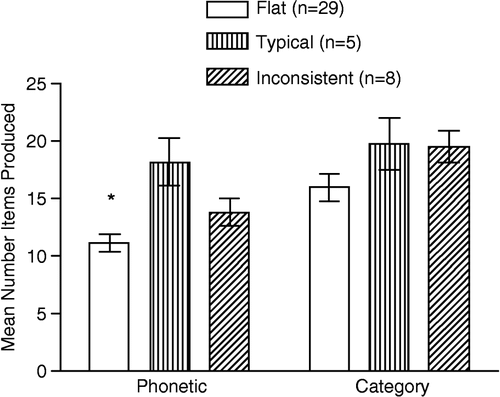
Diurnal subgroup did not significantly differ in performance on the digit span test and the declarative memory task (). However, controlling for gender and age, we found a significant group difference on the phonetic verbal fluency subtest, F (1, 37) = 4.65, p = 0.02. As shown in , we found that the Flat cortisol subgroup named fewer words than the Typical subgroup, with a 95% confidence interval between 2.17 and 11.95. Although not significant (p = 0.21), the Inconsistent subgroup tended to perform more poorly than the Typical group as well (see ).
Table II. Means (SEM) score on neurocognitive tests as a function of diurnal cortisol subgroup.
Impact of the type of subjective complaints
In assessing the relationship between diurnal cortisol (Typical, Flat, Inconsistent) and type of subjective complaint (memory, depression, memory–depression), a chi-square test for independence revealed that subjective complaint profile and diurnal cortisol subgroup representation were related, χ2(4) = 9.69, p < 0.05.Calculation of the contingency coefficient showed that the strength of the relationship was 0.51 out of 0.82 or 62% of the total, which is a good degree of association between subjective complaint profile and diurnal cortisol subgroup. As presented in , when assessing where the differences lay, it was found that participants in the Flat diurnal subgroup were more likely to display complaints of memory or a combination of memory and depression than depression alone. Furthermore, participants in the Inconsistent diurnal subgroup were more likely to display complaints regarding depression than memory, or a combination of the two (ps < 0.05). Finally, no one in the Typical subgroup reported symptoms of depression alone.
Table III. Relationship between cortisol subgroup representation and subjective complaints.
Discussion
In the present study, it was found that within a population of older adults who complain of memory deficits and/or depressive symptoms, the majority of individuals displayed a flat diurnal cortisol cycle, regardless of the nature of the complaint. This is in contrast to what has previously been reported in both healthy young (Smyth et al. Citation1997) and older populations (Ice et al. Citation2004), in which a typical diurnal cortisol pattern was observed to be the norm. This suggests that a typical pattern of diurnal cortisol secretion in older adults may be more representative of a successful aging process (for a review, see Lupien and Wan Citation2004), than of a typical aging process.
The salivary cortisol cycle found in the Flat subgroup was characterized by a normal morning peak in cortisol levels, with evening cortisol levels remaining high and not reaching the typical low nadir phase. Such high cortisol levels in the evening have been previously reported in depressed patients and have also been reported in older individuals with dementia (Sachar et al. Citation1973; Sachar et al. Citation1976; Dori et al. Citation1994; Weiner et al. Citation1997). Further, other findings show that high cortisol levels correlate with poorer cognitive performance (for a review, see Lupien and Lepage Citation2001), a symptom not only of dementia, but depression as well (Ganguli et al. Citation2006). However, whether the older adults presenting a flat pattern of diurnal cortisol will go on to develop a full, clinical picture of either depressive or cognitive impairments can only be assessed through a longitudinal study, and it is still possible that the pattern of cortisol secretion observed in this population is related to personality factors that could be associated with both types of complaints and increased cortisol secretion in older populations.
Indeed, various studies have reported significant associations between certain personality traits, depression, complaints of memory and/or stress, and cortisol secretion (Folkman et al. Citation1986; Bohnen et al. Citation1991; Kirschbaum et al. Citation1995; van Eck et al. Citation1996; Roy et al. Citation1998; Cappeliez and O'Rourke Citation2002; Wilson et al. Citation2003; Cappeliez et al. Citation2005; Pruessner et al. Citation2005). These results suggest that a particular type of personality could lead to both high complaints of memory problems and depression, and increased cortisol secretion as observed in the present study. In this study, we found that scores on the GDS were not associated with scores on the PSQ or MAC-Q, although the two latter were correlated. This lack of association between depression and memory complaint scores seems to be at odds with previous studies reporting such an association (Geerlings et al. Citation1999; Geerlings et al. Citation2000a,Citationb; Jonker et al. Citation2000; Schoevers et al. Citation2000). However, it is important to note that previous studies that have found associations between self-reported scales of depression and memory problems did so in samples of people that presented large variations in each scale (i.e. in people with very low to very high scores on depression and/or memory scales). In our case, and since we recruited people on the basis of subjective complaints of memory deficits and/or depressive symptoms, we decreased the variability of scores of our participants, which might explain the lack of association.
Interestingly, when we assessed the association of the type of complaints with the diurnal cortisol profile, it was found that the Flat diurnal cycle was more frequent in those with memory complaints only, or a combination of both memory and depressive complaints. In contrast, those with unique complaints of depression were more likely to fall within the Inconsistent diurnal subgroup. Overall, it appeared that participants were more likely to fall in either the Flat subgroup or the Inconsistent subgroup, depending on the subjective complaint that they reported. Interestingly, the link between the Flat and Inconsistent subgroups was the presence of a flat diurnal pattern on at least one of the two sampling days. Future studies should assess the full range of variations in subjective complaints of memory deficits and/or depressive symptoms in order to determine whether certain traits could correlate with both complaints and the diurnal cortisol profile in humans.
Since cortisol has been found to impact on cognitive function (Lupien et al. Citation2002; Karlamangla et al. Citation2005), neuropsychological data for verbal fluency, digit span and declarative memory were analysed. Of the three cognitive tests, significant group differences were found only for the phonetic verbal fluency subtest. Here, it was found that the Inconsistent and Flat Subgroups named fewer words than the Typical subgroup. No differences were found on the semantic fluency subtest, which, unlike phonetic fluency, has been reported as a useful tool in diagnosing dementia (Barr and Brandt Citation1996; Crossley et al. Citation1997; Tierney et al. Citation2005). Interestingly, however, Ravdin et al. (Citation2003) evaluated the influence of mild depressive symptoms on verbal fluency in healthy older adults and found that participants with mild depressive symptoms performed worse than controls on letter fluency while performing at the same level on the semantic fluency task. This is of interest considering that the flat and inconsistent subgroups reported higher levels of depressive symptomatology compared to the typical subgroup. Regarding the declarative memory task, the lack of significant findings may be attributed to the acute testing method of cortisol secretion. A previous study by Lupien et al. (Citation1994a,Citationb) reported a significant effect of cortisol secretion on declarative memory only when assessing chronic cortisol exposure over a four-year period and were unable to find this effect when assessing the effects of acute 24-h cortisol levels.
While the present investigation provides a very interesting description regarding diurnal variation and cognitive performance in subjective complainers, it has several limitations. First, no conclusive statements can be made in terms of how they compare to a psychologically healthy, or non-distressed, population. Indeed, in order to make such robust statements, a non-subjective-complaint group is required as a control. Another limitation regarding the present sample was the sample size itself. It is understandable that a larger sample would have been more accommodating in drawing stronger conclusions regarding rhythmic cortisol variations. A third potential limitation of the present investigation was the number of samples taken throughout the day and the home-based method that was employed. While it has been suggested that four salivary samples is the minimal number that should be used to draw an accurate picture of the diurnal cycle (Stewart and Seeman Citation2000), it has also been suggested that diurnal cortisol patterns do not show spurious fluctuations and are instead quite stable (Linkowski et al. Citation1993), thus allowing four samples to sufficiently estimate the diurnal cortisol pattern (Smyth et al. Citation1997). Further, in terms of home-based saliva sampling, while scepticism remains in terms of compliance issues (Kudielka et al. Citation2003), it is believed that home-based sampling is superior to sampling taken in the laboratory as it has been shown that basal cortisol levels are increased up to five-fold in hospital settings (Scheer et al. Citation2002). Indeed, laboratory measures are likely to overestimate the levels of cortisol secreted at particular times of the day, thus leading to inaccurate diurnal patterns. Furthermore, significant correlations between home-based cortisol samples and laboratory-based cortisol samples in older adults have been previously reported by our group (Lupien et al. Citation1998), and higher compliance has been reported in young-elderly compared to young populations (Dori et al. Citation1994; Becker et al. Citation2002). Thus, it is believed that the confound of compliance was minimized. Finally, future studies should take into account quality of sleep as this may influence the diurnal pattern of cortisol secretion (Kudielka et al. Citation2006).
Although preliminary, and in need of additional studies, the results of our study provide a thorough psychoneuroendocrine description of older adults with subjective complaints. Since depression and subjective memory complaints are both indicative of future cognitive decline (Geerlings et al. Citation2000a,Citationb; Jonker et al. Citation2000), and increased secretion of stress hormones has been shown to be associated with both memory impairments (Lupien et al. Citation1994a,Citationb) and hippocampal atrophy (Lupien et al. Citation1998), it will be important in future studies to assess the longitudinal changes of cognitive function, depressive symptoms, and cortisol profiles in these populations in order to delineate the predictive values of subjective complaints of memory deficits and/or depressive symptoms on both mental and physical health in older adults.
Acknowledgements
This study was supported by an Operating Grant from the Canadian Institute of Health Research (CIHR: grant #15000) to SJL. The work of AJF is supported by a PhD Studentship from CIHR, and the work of SJL is supported by an Investigator Award from the CIHR Institute of Aging.
References
- Barr A, Brandt J. Word-list generation deficits in dementia. J Clin Exp Neuropsychol 1996; 18: 810–822
- Beck DA, Koenig MD. Minor depression: A review of the literature. Int J Psychiatry Med 1996; 26: 177–209
- Becker SL, Dezil CM, Burtcel B, Kawabata H, Hodder S. Young HIV-infected adults are at greater risk for medication nonadherence. MedGenMed 2002; 4: 21
- Blazer DG, Hays JC, Fillenbaum GC, Gold DT. Memory complaint as a predictor of cognitive decline: A comparison of African American and White elders. J Aging Health 1997; 9: 171–184
- Bohnen N, Nicolson N, Sulon J, Jolles J. Coping style, trait anxiety and cortisol reactivity during mental stress. J Psychosom Res 1991; 35: 141–147
- Bolla KI, Lindgren KN, Bonaccorsy C, Bleecker ML. Memory complaints in older adults. Fact or fiction? Arch Neurol 1991; 48: 61–64
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology 2005; 30: 846–856
- Cappeliez P, O'Rourke N. Profiles of reminiscence among older adults: Perceived stress, life attitudes and personality variables. Int J Aging Hum Dev 2002; 54: 255–266
- Cappeliez P, O'Rourke N, Chaudhury H. Functions of reminiscence and mental health in later life. Aging Ment Health 2005; 9: 295–301
- Crook TH, Feher EP, Larrabee GJ. Assessment of memory complaint in age-associated memory impairment: The MAC-Q. Int Psychogeriatr 1992; 4: 165–176
- Crossley M, D'Arcy C, Rawson NS. Letter and category fluency in community-dwelling Canadian seniors: A comparison of normal participant to those with dementia of the Alzheimer or vascular type. J Clin Exp Neuropsychol 1997; 19: 52–62
- Deuschle M, Gotthardt U, Schweiger U, Weber B, Korner A, Schmider J, Standhardt H, Lammers CH, Heuser I. With aging in humans the activity of the hypothalamus–pituitary–adrenal system increases and its circadian rhythm flattens. Life Sci 1997; 61: 2239–2246
- Dik MG, Jonker C, Comijs HC, Bouter LM, Twisk JWR, van Kamp GJ, Deeg DJ. Memory complaints and APOE-E4 accelerate cognitive decline in cognitive normal elderly. Neurology 2001; 57: 2217–2222
- Dori D, Casale G, Solerte SB, Fioravanti M, Migliorati G, Cuzzoni G, Ferrari E. Chrono-neuroendocrinological aspects of physiological aging and senile dementia. Chronobiologia 1994; 21: 121–126
- Edwards S, Clow A, Evans P, Hucklebridge F. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci 2001a; 68: 2093–2103
- Edwards S, Evans P, Hucklebridge F, Clow A. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology 2001b; 26: 613–622
- Folkman S, Lazarus RS, Gruen RJ, DeLongis A. Appraisal, coping, health status and psychological symptoms. J Pers Soc Psychol 1986; 50: 571–579
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-Mental State’ a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198
- Freibers VV. 1968. Normes d'Association Libre aux 100 mots de Kent-Rosanoff, étude des réseaux associatifs, Rapport 1. Université de Montréal.
- Freibers VV. 1970. Normes d'Association Libre aux Cinq premières réponses aux 100 mots de Kent-Rosanoff, études des réseaux associatifs, Rapport 2. Université de Montréal.
- Ganguli M, Du Y, Dodge HH, Ratcliff GG, Chang CC. Depressive symptoms and cognitive decline in late life: A prospective epidemiological study. Arch Gen Psychiatry 2006; 63: 153–160
- Geerlings MI, Jonker C, Bouter LM, Ader HJ, Schmand B. Association between memory complaints and incident Alzheimer's disease in elderly people with normal baseline cognition. Am J Psychiatry 1999; 156: 531–537
- Geerlings MI, Schmand B, Braam AW, Jonker C, Bouter LM, van Tilburg W. Depressive symptoms and risk of Alzheimer's disease in more highly educated older people. J Am Geriatr Soc 2000a; 48: 1092–1097
- Geerlings MI, Schoevers RA, Beekman AT, Jonker C, Deeg DJ, Schmand B, Ader HJ, Bouter LM, van Tilburg W. Depression and risk of cognitive decline and Alzheimer's disease. Results of two prospective community-based studies in The Netherlands. Br J Psychiatry 2000b; 176: 568–575
- Gron G, Bittner D, Schmitz B, Wunderlich AP, Riepe MW. Subjective memory complaints: Objective neural markers in patients with Alzheimer's disease and major depressive disorder. Ann Neurol 2002; 51: 491–498
- Giordano R, Bo M, Pellegrino M, Vezzari M, Baldi M, Picu A, Balbo M, Bonelli L, Migliaretti G, Ghigo E, Arvat E. Hypothalamus-pituitary-adrenal hyperactivity in human aging is partially refractory to stimulation by mineralcorticoid receptor blockade. J Clin Endocrinol Metab 2005; 90: 5656–5662
- Heikkinen RL, Berg S, Avlund K. Depressive symptoms in late life, results from a study in three Nordic Urban localities. J Cross Cult Gerontol 1995; 10: 315–330
- Houston TK, Cooper LA, Vu HT, Kahn J, Toser J, Ford DE. Screening the public for depression through the internet. Psychiatr Serv 2001; 52: 362–367
- Ice GH, Katz-Stein A, Himes J, Kane RL. Diurnal cycles of salivary cortisol in older adults. Psychoneuroendocrinology 2004; 29: 355–370
- Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry 2000; 15: 983–991
- Kirschbaum C, Wust S, Faig HG, Hellhammer DH. Heritability of cortisol responses to human corticotropin-releasing hormone, ergometry and psychological stress. J Clin Endocrinol Metab 1992; 75: 1526–1530
- Karlamangla AS, Singer BH, Chodosh J, McEwen BS, Seeman TE. Urinary cortisol excretion as a predictor of incident cognitive impairment. Neurobiol Aging 2005; 26(Suppl 1)8–84
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuro-endocrine research: Recent developments and applications. Psychoneuroendocrinology 1994; 19: 313–333
- Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D, Schommer N, Hellhammer DH. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med 1995; 57: 468–474
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med 2003; 65: 313–319
- Kudielka BM, Federenko IS, Hellhammer DH, Wust S. Morningness and eveningnesss: The free cortisol rise after awakening in early birds and night owls. Biol Psychol 2006; 72: 141–146
- Lemyre L, Tessier R, Fillion L. La mesure du stress psychologique. Videorom K.M.M. Inc, Sillery (Quebec) 1990
- Linkowski P, van Onderbergen A, Kerkhofs M, Bosson D, Mendlewicz J, van Cauter E. Twin study of the 24-hour cortisol profile: Evidence for genetic control of the human circadian clock. Am J Physiol 1993; 264: E173–E181
- Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NPV, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. J Neurosci 1994a; 14: 2893–2903
- Lupien SJ, Lecours AR, Lussier I, Schwartz G, Nair NP, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. J Neurosci 1994b; 14: 2893–2903
- Lupien S, Gaudreau S, Tchiteya BM, Mahue F, Sharma S, Nair NPV, Hauger RL, McEwen BS, Meany MJ. Stress-induced declarative memory impairment in healthy elderly subjects: Relationship to cortisol reactivity. J Clin Endocrinol Metab 1997; 82: 2070–2075
- Lupien S, de Leon M, de Santi S, Convit A, Tarshish C, Nair NVP, Thakur M, McEwen BS, Hauger RL, Meany MJ. Cortisol levels during aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1998; 1: 69–79
- Lupien S, Nair NPV, Briere S, Maheu F, Tu MT, Lemay M, McEwen BS, Meany M. Increased cortisol levels and impaired cognition in human aging: Implications for depression and dementia in later life. Rev Neurosci 1999; 10: 117–139
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: Can't live with it, can't live without it. Behav Brain Res 2001; 127: 137–158
- Lupien S, Wilkinson CW, Briere S, Menard C, Ng Ying Kin NMK, Nair NVP. The modulatory effects of corticosteroids on cognition: Studies in young human populations. Psychoneuroendocrinology 2002; 27: 401–416
- Lupien SJ, Wan N. Successful ageing: From cell to self. Philos Trans R Soc Lond B, Biol Sci 2004; 359: 1413–1426
- Lyons J. Semantics. Cambridge UP, Cambridge 1976; 12
- Montorio I, Izal M. The geriatric depression scale: A review of its development and utility. Int Psychogeriatr 1996; 8: 103–112
- Morrell RW, Park DC, Kidder DP, Martin M. Adherence to antihypertensive medication across the life span. Gerontologist 1997; 37: 609–619
- Paterniti S, Verdier-Taillefer MH, Dufouil C, Alperovitch A. Depressive symptoms and cognitive decline in elderly people. Br J Psychiatry 2002; 181: 406–410
- Pruessner JC, Baldwin MW, Dedovic K, Renwick R, Mahani NK, Lord C, Meaney M, Lupien S. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. Neuroimage 2005; 28: 815–826
- Ravdin LD, Katzen HL, Agrawal P, Relkin NR. Letter and semantic fluency in older adults: Effects of mild depressive symptoms and age-stratified normative data. Clin Neuropsychol 2003; 17: 195–202
- Roy MP, Steptoe A, Kirschbaum C. Life events and social support as moderators of individual differences in cardiovascular and cortisol reactivity. J Pers Soc Psychol 1998; 75: 1273–1281
- Sachar EJ, Hellman L, Roffwarg HP, Halpern FS, Fukushima DK, Gallagher TF. Disrupted 24-hour patterns of cortisol secretion in psychotic depression. Arch Gen Psychiatry 1973; 28: 19–24
- Sachar EJ, Roffwarg HP, Gruen PH, Altman N, Sassin J. Neuroendocrine studies of depressive illness. Pharmakopsychiatr Neuropsychopharmkol 1976; 9: 11–17
- Scheer FAJL, Paassen BV, van Montfrans GAV, Fliers E, van Someren EJS, van Heerikhuize JJ, Buijs RM. Human basal cortisol levels are increased in hospital compared to home setting. Neurosci Lett 2002; 333: 79–82
- Schmand B, Jonker C, Geerlings MI, Lindeboom J. Subjective memory complaints in the elderly: Depressive symptoms and future dementia. Br J Psychiatry 1997; 171: 373–376
- Schmand B, Jonker C, Hooijer C, Lindeboom J. Subjective memory complaints may announce dementia. Neurology 1996; 46: 121–125
- Schoevers RA, Beekman AT, Deeg DJ, Geerlings MI, Jonker C, van Tilburg W. Risk factors for depression in later life; results of a prospective community based study (AMSTEL). J Affect Disord 2000; 59: 127–137
- Schofield PW, Jacobs D, Marder K, Sano M, Stern Y. The validity of new memory complaints in the elderly. Arch Neurol 1997a; 54: 756–759
- Schofield PW, Marder K, Dooneief G, Jacobs DM, Sano M, Stern Y. Association of subjective memory complaints with subsequent cognitive decline in community-dwelling elderly individuals with baseline cognitive impairment. Am J Psychiatry 1997b; 154: 609–615
- Smyth JM, Ockenfels MC, Gorin AA, Catley D, Porter LS, Kirschbaum C, Hellhammer DH, Stone AA. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology 1997; 22: 89–105
- Spath-Schwalbe E, Uthgenannt D, Voget G, Kern W, Born J, Fehm HL. Corticotropin-releasing hormone-induced adrenocorticotropin and cortisol secretion depends on sleep and wakefulness. J Clin Endocrinol Metab 1993; 77: 1170–1173
- Stewart J, Seeman T. 2000. John, D. and Catherine, T. MacArthur Research Network on Socioeconomic Status and Health [On-line]. Available: http://www.macses.ucsf.edu/Research/Allostatic/notebook/salivarycort.html Salivary cortisol measurement.
- Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology 2005; 64: 1853–1859
- Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab 1996; 81: 2468–2473
- van Eck MM, Nicolson NA, Berkhof H, Sulon J. Individual differences in cortisol responses to a laboratory speech task and their relationship to responses to stressful daily events. Biol Psychol 1996; 43: 69–84
- Van der Flier WM, van Buchem MA, Weverling-Rijnsburger AWE, Mutsaers AR, Bollen ELEM, Admiraal-Behloul F, Westendorp RGJ, Middelkoop HAM. Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol 2004; 251: 671–675
- Weiner MF, Vobach S, Olsson K, Svetlik D, Risser RC. Cortisol secretion and Alzheimer's disease progression. Biol Psychiatry 1997; 42: 1030–1038
- Wechsler D. Wechsler adult intelligence scale-revised. The Psychological Corporation, San Antonio, TX 1973
- Wilson BS, Barnes LL, Mendes de Leon CF, Aggarwal NT, Schneider JS, Bach J, Pilat J, Beckett LA, Arnold SE, Evans DA, Bennett DA. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology 2002; 59: 364–370
- Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer's disease. Neurology 2003; 61: 1479–1485
- Wust S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology 2000; 25: 707–720
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 1983; 17: 37–49