Abstract
Corticotrophin-releasing factor (CRF) released during stress has been implicated in the suppression of the hypothalamo–pituitary–gonadal (HPG) axis, especially the gonadotrophin-releasing hormone (GnRH) pulse generator, the central neural regulator of pituitary LH and FSH secretion, resulting in reproductive dysfunction. The gonadal steroid 17β-oestradiol (E2) has been shown to enhance CRF- and stress-induced suppression of pulsatile LH secretion. In the present study, we investigated the potential direct action of CRF on GnRH neurones by using GT1-7 cells, an established GnRH cell line. Furthermore, we investigated the modulatory influence of E2 on the effects of CRF and expression of CRF type 2 receptors (CRF-R2). Expression of CRF-R2 in the GT1-7 cells was detected by reverse transcription-polymerase chain reaction (RT-PCR). CRF produced a dose-dependent suppression of GnRH mRNA expression, an effect reversed by the selective CRF-R2 antagonist, astressin2-B (Ast2-B). E2 combined with CRF resulted in a greater suppression of GnRH expression compared with either treatment alone. E2 also increased CRF-R2 expression. These results demonstrate for the first time expression of CRF-R2 in the GT1-7 cells and suggest that CRF may directly regulate GnRH gene expression, an effect mediated, at least in part, by CRF-R2. They also raise the possibility that up-regulation of CRF-R2 may contribute to the sensitising influence of E2 on CRF- and stress-induced suppression of the GnRH pulse generator.
Introduction
Corticotropin-releasing factor (CRF) is the principal hypophysiotrophic factor driving the hypothalamic–pituitary–adrenal (HPA) axis. CRF is a member of a related family of peptide ligands including urocortin (Ucn) I, UcnII and UcnIII. The physiological actions of these peptides are mediated by interactions with the two major CRF receptor subtypes, CRF type 1 receptors (CRF-R1) and CRF type 2 receptors (CRF-R2) (Perrin et al. Citation1999). Both CRF receptor subtypes are broadly distributed throughout the central nervous system, with heterogeneous anatomical distribution patterns observed (Chalmers et al. Citation1995). CRF-R1 expression is most abundant in the brainstem, cerebellum, amygdala and cortex, whereas CRF-R2 expression is more prevalent in sub-cortical structures, including the lateral septum and various hypothalamic nuclei (Chalmers et al. Citation1995). CRF-R1 has been shown to play a crucial role in stress-associated responses of the HPA axis and anxiety-related behaviour (Timpl et al. Citation1998). It has been reported that CRF-R2 may have a counteracting role in the HPA axis stress response (Bale et al. Citation2000; Coste et al. Citation2000). However, there is evidence for both anxiolytic (Bale et al. Citation2000; Kishimoto et al. Citation2000) and anxiogenic (Takahashi et al. Citation2001) roles for CRF-R2.
It is well established that CRF is a potent suppressor of the gonadotrophin-releasing hormone (GnRH) pulse generator, the central regulator of pituitary LH and FSH secretion (Knobil Citation1992). Central administration of CRF results in inhibition of LH pulses in rats (Rivier and Vale Citation1984; Cates et al. Citation2004) and monkeys (Gindoff and Ferin Citation1987; Williams et al. Citation1990). Moreover, central infusion of non-selective CRF receptor antagonists can reverse the LH pulse-suppressing effects of a variety of stressors including footshock (Rivier et al. Citation1986), fasting (Maeda et al. Citation1994), glucoprivation with 2-deoxyglucose (Tsukahara et al. Citation1999) or hypoglycaemia (Chen et al. Citation1996; Cates et al. Citation2004). However, study of the role played by the CRF receptor subtypes in mediating stress-induced suppression of the GnRH pulse generator has only recently been initiated. We have shown that the CRF-R2 antagonist, astressin2-B (Ast2-B), completely blocked restraint stress-induced suppression of pulsatile LH secretion and that central administration of the CRF-R2 specific ligand Ucn II induced a dose-dependent suppression in LH-pulse frequency in the rat (Li et al. Citation2005). Further, we have shown that Ast2-B markedly attenuated the LH pulse-suppressing effects of insulin-induced hypoglycaemia and innate immunological challenge with lipopolysaccharide (LPS), while the latter two responses were not blocked by selective CRF-R1 antagonists (Li et al. Citation2006). These data suggest a pivotal role of CRF-R2 in the control of the GnRH pulse generator.
The precise mechanism by which CRF affects the GnRH neural system remains to be fully elucidated. Synaptic contacts have been observed between CRF axon terminals and GnRH neurones in the medial preoptic area of rats (MacLusky et al. Citation1988). More recently, contacts have been observed between CRF fibres and GnRH perikarya in the medial preoptic area and infundibulum of humans (Dudas and Merchenthaler Citation2002; Dudas and Merchenthaler Citation2006), thus providing an anatomical substrate for a direct functional action of CRF on the GnRH neuronal system. Although the expression of CRF-R1 has been shown, both in vivo, by microarray analysis (Todman et al. Citation2005) and single-cell reverse transcription-polymerase chain reaction (RT-PCR) from GnRH neurones (Jasoni et al. Citation2005) and in vitro, by CRF-R1 immunoreactivity in the Gn11 cell, a GnRH cell line (Tellam et al. Citation1998), the presence of CRF-R2 in the GnRH neurone is yet to be identified.
The gonadal steroid, 17β-oestradiol (E2) has been shown to potentiate the suppression of pulsatile LH secretion in response to a variety of stressors in several species (Cagampang et al. Citation1991; Chen et al. Citation1992; Cagampang et al. Citation1997; Tsukahara et al. Citation1999; Li et al. Citation2003). Furthermore, E2 has been shown to enhance the inhibitory effects of CRF on pulsatile LH release (Cates et al. Citation2004). The mechanisms by which E2 exerts its sensitising effect on both stress- and CRF-induced suppression of LH pulses remains to be fully elucidated. However, E2 may mediate its sensitising effects directly on the GnRH neurones themselves, since the presence of oestrogen receptor (ER)-β immunoreactivity (Hrabovszky et al. Citation2001; Kallo et al. Citation2001) and the presence of ER-β but not ER-α mRNA (Hrabovszky et al. Citation2000) has been detected in GnRH neurones of rats. However, expression of both ER-α and ER-β has been detected in the GnRH neuronal GT1-7 cell line (Roy et al. Citation1999).
The aims of the present study were to identify expression of CRF-R2 in the established GnRH GT1-7 cell line (Mellon et al. Citation1990), to test the hypothesis that CRF can act directly on GnRH cells to suppress GnRH mRNA expression and to investigate the role of CRF-R2 in this response. Furthermore, we have investigated the modulatory influence of E2 on the effects of CRF and expression of CRF-R2 in the GT1-7 cell.
Materials and methods
Cell culture and reagents
The GT1-7 cells were cultured in DMEM supplemented with 10% FCS, 4.5 mg/ml glucose and penicillin/streptomycin and maintained at 37°C in an atmosphere of 5% CO2 as described previously (Bowe et al. Citation2003; Kinsey-Jones et al. Citation2005). Cells were sub-cultured into multi-well plates 1–2 days before experiments and grown to approximately 80% confluence. The growth medium was removed and replaced with phenol red-free DMEM supplemented with 2% charcoal-stripped FBS and penicillin/streptomycin, 12 h prior to cell treatments. Cell treatments were added to this medium to give the intended concentration and left for the appropriate time before cell harvesting. CRF, α-helical CRF9–41, Ast2-B (Sigma-Aldrich Ltd., Poole, UK) were dissolved in sterile H2O and made up to the final concentrations in medium. E2 (Sigma-Aldrich) was dissolved in alcohol and then diluted to the final concentration in medium.
Total Rna Extraction, Rt-pcr, And Quantitative Rt-pcr Analysis
GT1-7 cells were harvested on ice using TRI reagent (Sigma-Aldrich) and then homogenized. The homogenates were stored at room temperature for 5 min to allow total dissociation of nucleoprotein and total RNA was then extracted following the manufacturer's protocol. Reverse transcription was then carried out using the reverse transcriptase M-MLV RT (Promega, Madison, WI, USA) following the manufacturer's protocol and using a combination of oligo dT primers and random primers.
For the PCR to identify CRF-R2, the following primers were used: (sense) 5′-ATGTTTGTGGAGGGCTGCTA-3′ and (antisense) 5′-GTCCACCAAATCACCAGCTT-3′, which span an intron and do not differentiate between CRF-R2α and CRF-R2β. The CRF-R2 sense primer corresponds to nucleotides 792–811 of the Genbank sequence (NM009953.1). PCR reaction conditions were 5 min at 95°C for one cycle, then 30 s at 95°C, 30 s at 60°C and 30 s at 72°C for 35 cycles. Each reaction included 2 μl of sample cDNA, 1 μl of 25 μM antisense and sense primers, 5 μl of 2.5 μM dNTPs, 5 μl Taq DNA polymerase buffer containing 15 mM MgCl2, 0.5 μl Taq DNA polymerase (Promega). PCR product was sequenced and analysed using an ABI PRISM 310 (Applied Biosystems, Foster City, CA, USA).
For the quantitative PCR, the following primers were used: GnRH (sense) 5′-CTACTGCTGACTGTGTGTTTG-3′ (antisense) 5′-CATCTTCTTCTGCCTGGCT-3′; 28S (sense) 5′-TTGAAAATCCGGGGGAGAG-3′ (antisense) 5′-ACATTGTTCCA-ACATGCCAG-3′, CRF-R2 primers as before. The GnRH sense primer corresponds to nucleotides 1617–1637 of the GenBank sequence (M14872); the antisense ends at position 4535 of the GenBank sequence. These primer pairs flank intron–exon boundaries. The LightCyclerTM (Roche Biochemicals, Lewes, UK) was used for real-time quantitative analysis of GnRH mRNA expression. The sample cDNA prepared as above was used as a template for the PCR. During PCR, the amplified GnRH product was detected after each annealing phase in real time using the Faststart DNA Master SYBR Green I kit (Roche Biochemicals). Each reaction included 2 μl of sample cDNA (optimised so that sample values of the PCR product were within the standard curve), 0.5 μl of 25 μM antisense and sense primers, 2 μl 15 mM MgCl2, 1 μl Faststart DNA master SYBR Green mix and 4 μl of water to give a total reaction volume of 10 μl. The GnRH reaction conditions were 10 min at 95°C for one cycle, then 10 s at 95°C, 10 s at 65°C and 10 s at 72°C for 35 cycles. The CRF-R2 reaction conditions were 10 min at 95°C for one cycle, then 10 s at 95°C, 10 s at 60°C and 10 s at 72°C for 35 cycles. The 28S reaction conditions were 10 min at 95°C for one cycle, then 15 s at 95°C, 10 s at 54°C and 5 s at 72°C for 28 cycles. Reaction conditions for GnRH, CRF-R2 and 28S were optimized separately to give the best results for each primer and for the different quantities of target in samples. Preliminary experiments were done to optimize the Mg2 + concentration, to confirm PCR specificity by agarose gel electrophoresis and melting curve analysis and to prepare the PCR products used to generate standard curves in real-time PCR. GnRH and CRF-R2 were quantified, against a standard curve of samples containing known GnRH/28S PCR product concentrations, using the LightCyclerTM program. 28S rRNA was quantified as a reference gene against a separate standard curve of samples containing known concentrations of 28S product. The melting curves for GnRH, CRF-R2 and 28S generated by the LightCyclerTM program demonstrated that single products were amplified.
Statistical analysis
The effects of treatment were assessed on raw data using one-way ANOVA followed by Dunnett's test to determine significance before being converted to percentage values. Experimental sample numbers represent results from different RNA extracts obtained from different wells of GT1-7 cells treated identically. Results are given as the mean ± SEM percentage difference from the control.
Results
Expression of CRF-R2 in the GT1-7 cell
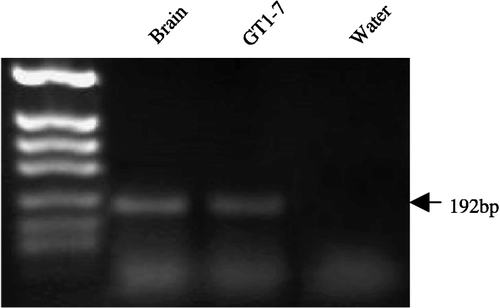
Expression of CRF-R2 mRNA was detected in the GT1-7 cell samples. shows expression of a single band at 192 bp; this band is identical to that observed in the mouse whole brain controls. DNA sequencing of these PCR products confirmed the identity as that of CRF-R2.
Figure 1 RT-PCR analysis of the CRF-R2 transcript present in GT1-7 cells. Expression of the expected 216-bp CRF-R2 product is present in the GT1-7 sample. Mouse brain tissue served as a positive control and water as a negative control. The experiment was repeated three times on independent samples. The amplified products were resolved in a 1% agarose gel and DNA fragments from a HpaII digest of pBluescriptII served as a molecular weight marker.
Effects of CRF on GnRH expression
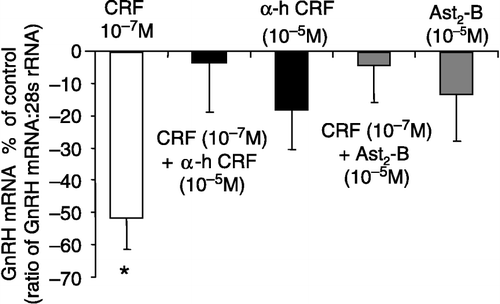
GT1-7 cells were exposed to 10− 6 M CRF treatments for 6, 12, 24 and 48 h initially to determine the optimum exposure time ((A)). A significant suppression (61.1 ± 6.8%) of GnRH mRNA expression was observed at the 6 h time point (P < 0.05, one-way ANOVA; (A)). Although a tendency to suppress GnRH mRNA expression was observed at the other time points, significance was not reached. Consequently, all subsequent experiments were carried out at the 6 h time point. GT1-7 cells were treated with different concentrations of CRF ranging from 10− 6 to 10− 9 M ((B)). CRF treatments induced a dose-dependent decrease in GnRH mRNA expression, with significant suppressions of 63.7 ± 6.9 and 51.5 ± 10.7% observed at 10− 6 and 10− 7 M, respectively (P < 0.05, one-way ANOVA; (B)). The suppressive effects of CRF (10− 7 M) on GnRH mRNA expression were completely reversed by co-treatment with the non-selective CRF receptor antagonist α-helical CRF9–41 (10− 5 M), which alone had no effect on GnRH expression (P>0.05, one-way ANOVA; ). To investigate the role of CRF-R2 in mediating the inhibitory effects of CRF on GnRH gene expression, the CRF-R2 selective antagonist Ast2-B was used. The suppressive effects of CRF (10− 7 M) on GnRH expression were completely reversed by co-treatment with Ast2-B (10− 5 M), which alone had no effect on GnRH mRNA expression (P>0.05, one-way ANOVA; ).
Figure 2 Effects of CRF on GnRH mRNA expression in GT1-7 cells at different time points and with different doses. (A) A significant suppression in GnRH mRNA expression was observed with 6 h CRF (10− 7 M) treatment. (B) The effect of 6 h treatment with different concentrations of CRF on GnRH mRNA expression in the GT1-7 cell line. CRF induced a dose-dependant inhibition of GnRH mRNA expression with the maximum response at 10− 6 M. Quantification of GnRH mRNA and 28S rRNA was carried out on all samples and the values expressed as a ratio of GnRH mRNA to 28S rRNA (mean ± SEM). The results are presented as the percentage of control. *P < 0.05 vs. control: One-way ANOVA, followed by Dunnett's test. All treatments were performed in triplicate and experiments were repeated three times.
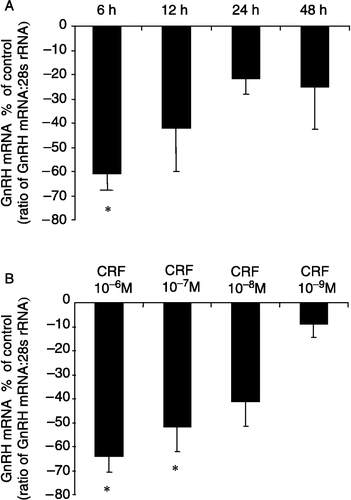
Figure 3 Effect of CRF alone or in combination with the CRF receptor antagonists: α-helical CRF9–41 (α-h CRF) or Ast2-B, on GnRH mRNA expression in the GT1-7 cell line. The inhibitory effect of CRF (10− 7 M) on GnRH mRNA expression was reversed by both α-h CRF and Ast2-B. Quantification of mRNAs for GnRH and 28S rRNA was carried out on all samples and the values expressed as a ratio of GnRH mRNA to 28S rRNA (mean ± SEM). The results are presented as the percentage of control. *P < 0.05 vs. control, CRF+α-hCRF, CRF + Ast2-B, α-hCRF alone or Ast2-B alone: One-way ANOVA, followed by Dunnett's test. All treatments were performed in triplicate and experiments were repeated 3–4 times.
Effect of CRF and E2 co-administration on GnRH expression
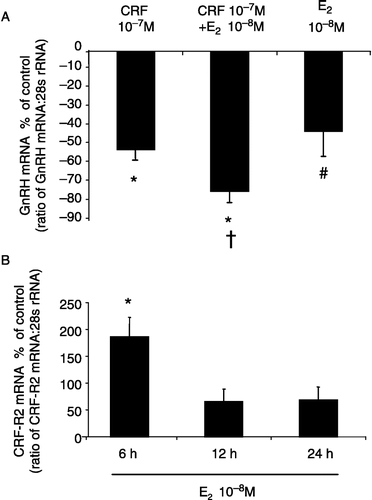
Co-treatment with CRF and E2 for 6 h resulted in a significant decrease (73.6 ± 6.8%) in GnRH mRNA expression in the GT1-7 cells (P < 0.05, one-way ANOVA; (A)). This decrease in GnRH expression was significantly greater than that observed with 6 h CRF or E2 treatments alone ((A)).
Figure 4 (A) Effect of CRF and E2 co-administration on GnRH mRNA expression in GT1-7 cells. The suppression of GnRH mRNA expression observed with co-treatment of E2 and CRF was significantly greater than with CRF or E2 alone. Quantification of GnRH mRNA and 28S rRNA was carried out on all samples and the values expressed as a ratio of GnRH mRNA to 28S rRNA (mean ± SEM). The results are presented as the percentage of control. *P < 0.05 vs. control, †P < 0.05 vs. CRF 10− 7 M, #P < 0.05 vs. CRF 10− 7 M + E2 10− 8 M: One-way ANOVA, followed by Dunnett's test. (B) The effects of E2 on CRF-R2 mRNA expression in GT1-7 cells at different time points. A significant increase in CRF-R2 expression was observed with 6 h E2 (10− 8 M) treatment. Quantification of CRF-R2 mRNA and 28S rRNA was carried out on all samples and the values expressed as a ratio of CRF-R2 mRNA to 28S rRNA (mean ± SEM). The results are presented as the percentage of control. *P < 0.05 vs. control: One-way ANOVA, followed by Dunnett's test. All treatments were performed in triplicate and experiments were repeated 3–4 times.
Effects of E2 on CRF-R2 expression in the GT1-7 cell
To investigate the possible modulation of CRF-R2 expression by E2, GT1-7 cells were exposed to 10− 8 M E2 treatments for 6, 12 and 24 h. A significant increase (186.3 ± 36.2%) in CRF-R2 mRNA expression was observed at the 6 h time point (P < 0.05, one-way ANOVA; (B)). Although a tendency to increase CRF-R2 mRNA expression was observed at the other time points, significance was not achieved.
Discussion
In the present study, we have shown for the first time that CRF induced a dose-dependent decrease in GnRH mRNA expression in GT1-7 cells, an effect which was reversed by the non-selective CRF receptor antagonist, α-helical CRF9–41 and the CRF-R2 selective antagonist, Ast2-B, indicating that this effect was CRF receptor-mediated and more specifically, may involve activation of the type 2 receptor. These data support previous in vivo studies showing that infusion of CRF induced a potent suppressive effect on the hypothalamic–pituitary–gonadal (HPG) axis, specifically on the GnRH pulse generator, thus decreasing pulsatile LH secretion in rats (Rivier and Vale Citation1984; Cates et al. Citation2004), monkeys (Gindoff and Ferin Citation1987; Williams et al. Citation1990) and humans (Barbarino et al. Citation1989). Indeed, CRF antagonists can block the suppression of the GnRH pulse generator by a variety of stressful stimuli. However, little is known about the mechanisms that underlie the pivotal role of CRF in stress-induced suppression of the GnRH neural system. The results from the present study raise the possibility that CRF may act at the level of the GnRH neurone. This is supported by evidence showing that CRF infusion into the GnRH neurone-rich medial preoptic area induced a decrease in both plasma LH levels (Rivest et al. Citation1993) and LH pulse frequency (personal observation). Further, an in vitro study using Gn11 cells, transfected with a GnRH promoter/luciferase reporter construct, demonstrated a decrease in GnRH promoter activity with CRF treatment (Tellam et al. Citation1998). However, future studies describing the release of GnRH from GT1-7 cells in response to CRF are required.
Expression of CRF-R1 has been shown in GnRH neurones, using green fluorescent protein promoter transgenic mice and oligonucleotide microarrays (Todman et al. Citation2005) and immunocytochemistry (Jasoni et al. Citation2005). We have detected CRF-R1 mRNA expression in GT1-7 cells (personal observation) and CRF-R1 immunoreactivity has also been detected in the Gn11 cell (Tellam et al. Citation1998). In the present study we have identified, for the first time, expression of CRF-R2 mRNA in the GT1-7 cell. Furthermore, our results indicate that this receptor is functionally active, since co-administration of Ast2-B and CRF completely reversed the suppressive effects of CRF on GnRH mRNA expression. This suggests that the inhibitory action of CRF on the GT1-7 cells may be mediated, at least in part, by activation of CRF-R2. However, further work is required to determine protein levels for the receptor in the GT1-7 cells. We have recently shown that CRF-R2 plays a pivotal role in restraint (Li et al. Citation2005), LPS or hypoglycaemic (Li et al. Citation2006) stress-induced suppression of LH pulses in the rat. Furthermore, central administration of UcnII, a highly selective ligand for CRF-R2, induced a dose-dependent suppression of pulsatile LH secretion (Li et al. Citation2005). In light of the results from the present study, the crucial role played by CRF-R2 in mediating suppression of the GnRH pulse generator by a variety of stressful stimuli may possibly involve an action at the level of the GnRH neurone. The presence of synaptic connection between CRF axon terminals and GnRH neurones in rats (MacLusky et al. Citation1988) and humans (Dudas and Merchenthaler Citation2002, Citation2006), provides the anatomical substrate for a direct interaction between the CRF and the GnRH neural systems in situ. However, further work is required to examine whether CRF-R2 is expressed in the GnRH neurones in vivo, since definitive evidence for absence of the receptor is lacking (Todman et al. Citation2005). Furthermore, although GT1-7 cells have a neural phenotype, synthesise GnRH, release GnRH in a pulsatile fashion, and are widely used as an in vitro model for the GnRH neuronal system, it is important to appreciate the limitations of extrapolating findings in the present study to the physiological mechanisms underlying the operation of the GnRH pulse generator in vivo.
While we have only investigated the role of CRF-R2 in the present study, the potential role of CRF-R1 cannot be ignored and requires further study. Although expression of CRF-R1 mRNA was recently detected in mouse GnRH neurones (Jasoni et al. Citation2005; Todman et al. Citation2005), double-labeled in situ hybridisation of mRNAs for GnRH and CRF-R1 failed to localize CRF-R1 mRNA in GnRH neurones in the rat (Hahn et al. Citation2003). Nevertheless, we have recently shown that administration of a selective CRF-R1 antagonist reversed the inhibitory effects of restraint on LH pulses in the rat (Li et al. Citation2006).
Stress-induced suppression of pulsatile LH secretion is potentiated by the presence of E2. This effect is observed in response to hypoglycaemia in rats (Cagampang et al. Citation1997; Li et al. Citation2003), monkeys (Chen et al. Citation1992) and sheep (Adam and Findlay Citation1997) and in response to fasting (Cagampang et al. Citation1991) and glucoprivation with 2-deoxyglucose (Tsukahara et al. Citation1999; Nagatani et al. Citation2001) in rats. Furthermore, we have recently shown that E2 augments CRF-induced suppression of LH pulses in the rat (Cates et al. Citation2004). The results from the present study show that E2 combined with CRF has a greater suppressive effect on GnRH mRNA expression in the GT1-7 cell compared with CRF or E2 treatments alone. These results suggest that one possible site of action of E2 to potentiate CRF- or, indeed, stress-induced disruption of the GnRH neural network, could be at the level of the GnRH neurone per se. GnRH neurones, including GT1-7 cells contain the necessary ERs (Roy et al. Citation1999; Hrabovszky et al. Citation2000; Hrabovszky et al. Citation2001; Kallo et al. Citation2001). In addition, there is considerable evidence that gonadal steroids increase expression of CRF mRNA in the hypothalamic paraventricular nucleus (PVN) (Bohler et al. Citation1990; Van Vugt et al. Citation1997; Li et al. Citation2003), potentially resulting in an increased readily releasable pool of CRF. However, the role of the PVN in mediating stress-induced suppression of the HPG axis is unclear, since complete electrolytic lesion of this nucleus does not abolish the suppression of LH release in response to either foot shock or interleukin-1 stress (Rivest and Rivier Citation1991). In the present study, we have shown that E2 is able to increase expression of CRF-R2 mRNA in the GT1-7 cell and may therefore increase the sensitivity of the cell to CRF signalling, thus providing a potential mechanism through which E2 sensitisation occurs. It is of note that the maximum increase in CRF-R2 mRNA expression at 6 h in response to E2 coincided with the time point for maximum suppression of GnRH mRNA examined in response to CRF. It has previously been shown that the stress-induced increase in hypothalamic CRF receptor expression is further enhanced in proestrus rats, suggesting a facilitatory action of E2 (Nappi and Rivest Citation1995). Furthermore, an oestrogen response element is present in the promoter region of CRF-R2 (Catalano et al. Citation2003), thus providing a potential mechanism of E2 modulation of CRF-R2 expression in the GT1-7 cell.
In summary, the results of the present study indicate that CRF acts directly on the GT1-7 cells to suppress GnRH gene expression and therefore raise the possibility of a direct action of CRF on the GnRH neural system in vivo. Furthermore, these effects appear to be mediated by CRF-R2, and may therefore, underlie the pivotal role played by CRF-R2 in stress-induced suppression of the GnRH pulse generator. The action of E2 to increase CRF-R2 expression may also contribute to the sensitising influence of this gonadal steroid on CRF- and stress-induced suppression of the GnRH neural system.
Acknowledgements
The authors thank Dr A. F. Parlow (NIDDK) for providing the LH RIA kit. The authors are grateful to Professor P.L. Mellon for providing the GT1-7 cells. This work was supported by the Wellcome Trust. J.E.B. is a recipient of the Guy's and St Thomas' Charitable Foundation (UK) PhD Studentship.
References
- Adam CL, Findlay PA. Effect of nutrition on testicular growth and plasma concentrations of gonadotrophins, testosterone and insulin-like growth factor I (IGF-I) in pubertal male Soay sheep. J Reprod Fertil 1997; 111: 121–125
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet 2000; 24: 410–414
- Barbarino A, De ML, Folli G, Tofani A, Della CS, D'Amico C, Mancini A, Corsello SM, Sambo P, Barini A. Corticotropin-releasing hormone inhibition of gonadotropin secretion during the menstrual cycle. Metabolism 1989; 38: 504–506
- Bohler HC, Jr, Zoeller RT, King JC, Rubin BS, Weber R, Merriam GR. Corticotropin releasing hormone mRNA is elevated on the afternoon of proestrus in the parvocellular paraventricular nuclei of the female rat. Brain Res Mol Brain Res 1990; 8: 259–262
- Bowe J, Li XF, Sugden D, Katzenellenbogen JA, Katzenellenbogen BS, O'Byrne KT. The effects of the phytoestrogen, coumestrol, on gonadotropin-releasing hormone (GnRH) mRNA expression in GT1-7 GnRH neurones. J Neuroendocrinol 2003; 15: 105–108
- Cagampang FR, Maeda KI, Tsukamura H, Ohkura S, Ota K. Involvement of ovarian steroids and endogenous opioids in the fasting-induced suppression of pulsatile LH release in ovariectomized rats. J Endocrinol 1991; 129: 321–328
- Cagampang FR, Cates PS, Sandhu S, Strutton PH, McGarvey C, Coen CW, O'Byrne KT. Hypoglycaemia-induced inhibition of pulsatile luteinizing hormone secretion in female rats: Role of oestradiol, endogenous opioids and the adrenal medulla. J Neuroendocrinol 1997; 9: 867–872
- Catalano RD, Kyriakou T, Chen J, Easton A, Hillhouse EW. Regulation of corticotropin-releasing hormone type 2 receptors by multiple promoters and alternative splicing: Identification of multiple splice variants. Mol Endocrinol 2003; 17: 395–410
- Cates PS, Li XF, O'Byrne KT. The influence of 17beta-oestradiol on corticotrophin-releasing hormone induced suppression of luteinising hormone pulses and the role of CRH in hypoglycaemic stress-induced suppression of pulsatile LH secretion in the female rat. Stress 2004; 7: 113–118
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: Comparison with CRF1 receptor mRNA expression. J Neurosci 1995; 15: 6340–6350
- Chen MD, O'Byrne KT, Chiappini SE, Hotchkiss J, Knobil E. Hypoglycemic ’stress’ and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: Role of the ovary. Neuroendocrinology 1992; 56: 666–673
- Chen MD, Ordog T, O'Byrne KT, Goldsmith JR, Connaughton MA, Knobil E. The insulin hypoglycemia-induced inhibition of gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: Roles of vasopressin and corticotropin-releasing factor. Endocrinology 1996; 137: 2012–2021
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet 2000; 24: 403–409
- Dudas B, Merchenthaler I. Close juxtapositions between luteinizing hormone-releasing hormone–immunoreactive neurons and corticotropin-releasing factor–immunoreactive axons in the human diencephalon. J Clin Endocrinol Metab 2002; 87: 5778–5784
- Dudas B, Merchenthaler I. Three-dimensional representation of the neurotransmitter systems of the human hypothalamus: Inputs of the gonadotrophin hormone-releasing hormone neuronal system. J Neuroendocrinol 2006; 18: 79–95
- Gindoff PR, Ferin M. Endogenous opioid peptides modulate the effect of corticotropin-releasing factor on gonadotropin release in the primate. Endocrinology 1987; 121: 837–842
- Hahn JD, Kalamatianos T, Coen CW. Studies on the neuroanatomical basis for stress-induced oestrogen-potentiated suppression of reproductive function: Evidence against direct corticotropin-releasing hormone projections to the vicinity of luteinizing hormone-releasing hormone cell bodies in female rats. J Neuroendocrinol 2003; 15: 732–742
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 2000; 141: 3506–3509
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 2001; 142: 3261–3264
- Jasoni CL, Todman MG, Han SK, Herbison AE. Expression of mRNAs encoding receptors that mediate stress signals in gonadotropin-releasing hormone neurons of the mouse. Neuroendocrinology 2005; 82: 320–328
- Kallo I, Butler JA, Barkovics-Kallo M, Goubillon ML, Coen CW. Oestrogen receptor beta-immunoreactivity in gonadotropin releasing hormone-expressing neurones: Regulation by oestrogen. J Neuroendocrinol 2001; 13: 741–748
- Kinsey-Jones JS, Li XF, Bowe JE, Brain SD, Lightman SL, O'Byrne KT. Effect of calcitonin gene-related peptide on gonadotrophin-releasing hormone mRNA expression in GT1-7 cells. J Neuroendocrinol 2005; 17: 541–544
- Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet 2000; 24: 415–419
- Knobil E. Remembrance: The discovery of the hypothalamic gonadotropin-releasing hormone pulse generator and of its physiological significance. Endocrinology 1992; 131: 1005–1006
- Li XF, Mitchell JC, Wood S, Coen CW, Lightman SL, O'Byrne KT. The effect of oestradiol and progesterone on hypoglycaemic stress-induced suppression of pulsatile luteinizing hormone release and on corticotropin-releasing hormone mRNA expression in the rat. J Neuroendocrinol 2003; 15: 468–476
- Li XF, Bowe JE, Lightman SL, O'Byrne KT. Role of corticotropin-releasing factor receptor-2 in stress-induced suppression of pulsatile luteinizing hormone secretion in the rat. Endocrinology 2005; 146: 318–322
- Li XF, Bowe JE, Kinsey-Jones JS, Brain SD, Lightman SF, O'Byrne KT. Differential role of corticotrophin-releasing hormone receptor types 1 and 2 in stress-induced suppression of pulsatile luteinising hormone secretion in the female rat. J Neuroendocrinol 2006; 18: 602–610
- MacLusky NJ, Naftolin F, Leranth C. Immunocytochemical evidence for direct synaptic connections between corticotrophin-releasing factor (CRF) and gonadotrophin-releasing hormone (GnRH)-containing neurons in the preoptic area of the rat. Brain Res 1988; 439: 391–395
- Maeda K, Cagampang FR, Coen CW, Tsukamura H. Involvement of the catecholaminergic input to the paraventricular nucleus and of corticotropin-releasing hormone in the fasting-induced suppression of luteinizing hormone release in female rats. Endocrinology 1994; 134: 1718–1722
- Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 1990; 5: 1–10
- Nagatani S, Thompson RC, Foster DL. Prevention of glucoprivic stimulation of corticosterone secretion by leptin does not restore high frequency luteinizing hormone pulses in rats. J Neuroendocrinol 2001; 13: 371–377
- Nappi RE, Rivest S. Ovulatory cycle influences the stimulatory effect of stress on the expression of corticotropin-releasing factor receptor messenger ribonucleic acid in the paraventricular nucleus of the female rat hypothalamus. Endocrinology 1995; 136: 4073–4083
- Perrin MH, Sutton SW, Cervini LA, Rivier JE, Vale WW. Comparison of an agonist, urocortin, and an antagonist, astressin, as radioligands for characterization of corticotropin-releasing factor receptors. J Pharmacol Exp Ther 1999; 288: 729–734
- Rivest S, Rivier C. Influence of the paraventricular nucleus of the hypothalamus in the alteration of neuroendocrine functions induced by intermittent footshock or interleukin. Endocrinology 1991; 129: 2049–2057
- Rivest S, Plotsky PM, Rivier C. CRF alters the infundibular LHRH secretory system from the medial preoptic area of female rats: Possible involvement of opioid receptors. Neuroendocrinology 1993; 57: 236–246
- Rivier C, Vale W. Influence of corticotropin-releasing factor on reproductive functions in the rat. Endocrinology 1984; 114: 914–921
- Rivier C, Rivier J, Vale W. Stress-induced inhibition of reproductive functions: Role of endogenous corticotropin-releasing factor. Science 1986; 231: 607–609
- Roy D, Angelini NL, Belsham DD. Estrogen directly respresses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-alpha (ERalpha)- and ERbeta-expressing GT1-7 GnRH neurons. Endocrinology 1999; 140: 5045–5053
- Takahashi LK, Ho SP, Livanov V, Graciani N, Arneric SP. Antagonism of CRF(2) receptors produces anxiolytic behavior in animal models of anxiety. Brain Res 2001; 902: 135–142
- Tellam DJ, Perone MJ, Dunn IC, Radovick S, Brennand J, Rivier JE, Castro MG, Lovejoy DA. Direct regulation of GnRH transcription by CRF-like peptides in an immortalized neuronal cell line. Neuroreport 1998; 9: 3135–3140
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet 1998; 19: 162–166
- Todman MG, Han SK, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience 2005; 132: 703–712
- Tsukahara S, Tsukamura H, Foster DL, Maeda KI. Effect of corticotropin-releasing hormone antagonist on oestrogen-dependent glucoprivic suppression of luteinizing hormone secretion in female rats. J Neuroendocrinol 1999; 11: 101–105
- Van Vugt DA, Washburn DL, Farley AE, Reid RL. Hypoglycemia-induced inhibition of LH and stimulation of ACTH secretion in the rhesus monkey is blocked by alprazolam. Neuroendocrinology 1997; 65: 344–352
- Williams CL, Nishihara M, Thalabard JC, Grosser PM, Hotchkiss J, Knobil E. Corticotropin-releasing factor and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey. Electrophysiological studies. Neuroendocrinology 1990; 52: 133–137