Abstract
Phenylethanolamine N-methyltransferase (PNMT) is the final enzyme in the catecholamine synthesizing cascade that converts noradrenaline (NA) to adrenaline (Adr). Both of these catecholamines are physiologically important hormones and neurotransmitters in mammals with profound influence on the activity of the cardiovascular system. Although PNMT activity and gene expression have been reported in the neonatal and also adult rat heart, little is known about the identity of the cells expressing PNMT mRNA. In this study, we have shown that besides PNMT in neuronal and intrinsic cardiac cells, this enzyme is expressed also in rat cardiomyocytes, as shown by immunofluorescence in isolated cardiomyocytes.
To determine which cells in the heart more sensitively show stress-induced changes in PNMT mRNA expression, we performed chemical sympathectomy by administration of 6-hydroxydopamine (6-OHDA), which destroys catecholaminergic terminals. We determined PNMT mRNA levels in the left atria and ventricles of control and stressed rats. In the rats treated with 6-OHDA, PNMT mRNA levels were not changed under normal, physiological conditions compared to vehicle treated rats. Similar results were observed on isolated cardiomyocytes from control and 6-OHDA treated rats. However, 6-OHDA treatment prevented immobilization-induced increase in PNMT mRNA expression. The results allow us to propose that in the heart, the immobilization-induced increase in PNMT gene expression is probably not in cardiomyocytes, but in neuronal cells.
Introduction
Adrenaline (Adr) and noradrenaline (NA) are physiologically important catecholamines in mammals with profound influence on the cardiovascular system. Under physiological conditions, the principal catecholamine that regulates cardiac function is NA, since it is a main mediator of the sympathetic neural influence on the heart (Goldstein Citation1995). Adr is involved in increased cardiac function. When exposed to stress, catecholamines are among the first compounds that are released as a response of the organism. Chronic Adr elevations result in cardiac hypertrophy (Johnson et al. Citation1983) and very high Adr levels induce cardiomyopathy (Kanemoto et al. Citation1988).
Phenylethanolamine N-methyltransferase (PNMT) is the final enzyme in the catecholamine synthesizing cascade that converts NA to Adr. Several authors have reported presence of the PNMT activity in the rat heart (Axelrod Citation1962; Torda et al. Citation1987; Kennedy and Ziegler Citation1991; Krizanova et al. Citation2001). The PNMT mRNA was detected for the first time in embryonic rat hearts (Ebert et al. Citation1996) and also in the adult rat heart (Krizanova et al. Citation2001). Moreover, recently we have shown that the PNMT mRNA exists in transplanted human hearts (Goncalvesova et al. Citation2004). Nevertheless, the precise localization of PNMT mRNA in the heart is still under investigation. As initially proposed, PNMT mRNA expression was detected in cardiac ganglionic cells (Slavikova et al. Citation2003). Huang et al. (Citation1996) identified distinct intrinsic cardiac adrenergic (ICA) cells in rodent and human heart that express PNMT. These cells do not have neuronal or chromaffin cell morphology, and they might provide an obligatory adrenergic supply to maintain cardiac function in early fetal development. ICA cells are transiently and progressively associated with regions of the heart that become pacemaking and conduction tissue (Ebert and Thompson Citation2001; Ebert et al. Citation2004; Pfeifer et al. Citation2004). Furthermore, the presence of ICA cells in adult rat and human hearts supports the concept that mammalian cells possess an ICA system throughout adult life (Huang et al. Citation2005).
Single and also repeated immobilization stress significantly increase the PNMT mRNA levels in both cardiac atria and ventricles (Krizanova et al. Citation2001; Kvetnansky et al. Citation2004). This effect is clearly modulated by glucocorticoids (at least in atria), because adrenalectomy and/or hypophysectomy prevents increase in the PNMT mRNA levels in response to the immobilization stimulus (Krizanova et al. Citation2001).
6-hydroxydopamine (6-OHDA) is a neurotoxin that destroys sympathetic nerve terminals. The toxic effect is dependent on uptake of 6-OHDA by the NA transporter, which is localized mainly to sympathetic nerve terminals rather than cell bodies or outer membranes of chromaffin cells. Administration of 6-OHDA to rats produces anatomical and functional noradrenergic denervation (Kostrzewa and Jacobowitz Citation1974). It is difficult to denervate the heart surgically, but chemical sympathectomy using 6-OHDA provides relatively a simple tool to study changes in the heart after lost of sympathetic input. Torda et al. (Citation1987) found increased PNMT activity and decreased NA and Adr content in all parts of the heart after chemical sympathectomy under basal conditions. They proposed an extraneuronal origin of PNMT in the heart.
The aim of this study was to determine whether cardiomyocytes might also express the PNMT mRNA and whether chemical sympathectomy affects expression of the PNMT gene in normal conditions and after a single exposure to immobilization stress.
Materials and methods
Animals
For our experiments we used 4 months old male Sprague-Dawley rats (ca 350 g, Charles River, Suzfeld, Germany). Prior to experiments, the rats were housed for at least 1 week, four animals per cage in a controlled environment (22 ± 2°C, 12 h light/dark cycle, lights on at 6 a.m.). Food and water were available ad libitum. The Ethical Committee of the Institute of Experimental Endocrinology (SAS, Bratislava, Slovakia) approved all of the experiments described. Rats were divided into two groups. A control group of rats was injected i.p. twice with 1 ml of vehicle (0.9% NaCl and 0.1% ascorbic acid). The other group of rats was treated twice (24 h interval between first and second i.p. injection) with 6-OHDA (100 mg/kg body weight), dissolved in the vehicle. Two weeks after the 6-OHDA treatment, half of the 6-OHDA-treated rats and half of the control rats were subjected to immobilization stress as described previously (Kvetnansky and Mikulaj Citation1970). Briefly, rats were bound in the prone position to a wooden board by taping the rat's limbs to specially prepared metal mounts attached to a board. Head motion was limited by two metal loops fixed over the neck area. The rats remained in this position for 2 h and were killed 3 h after the immobilization. All four groups of rats (controls, 6-OHDA treated, immobilized controls and immobilized 6-OHDA treated rats) were killed by decapitation. Hearts were rapidly removed, left atria (LA) and left ventricles (LV) excised and immediately frozen in liquid nitrogen and stored at − 70°C until assay.
Preparation of cardiomyocytes
Cardiomyocytes were prepared from the LV of non-stressed vehicle- and 6-OHDA-treated rats. For these experiments, male Wistar rats (220–270 g) were divided into two groups, control and 6-OHDA treated (as described above). Rats were anaesthetized with pentobarbital (i.p., 10–20 mg/100 g body weight; SPOFA, United Pharmaceutical Works, Czech Republic); heparin was given i.p. at the same time (LECIVA, Czech Republic, 0.2 ml). The heart was rapidly excised and placed into cold KREBS solution (5°C). The aorta was then cannulated and the heart perfused with KREBS solution (37°C, 1 mM CaCl2) to washout the blood and to stabilize the heart. After 3–5 min, the perfusion was switched to calcium-free KREBS solution for 5 min. Afterwards, heart was perfused with 0.11% collagenase A (Roche Diagnostics, Germany) and 0.5% albumin in calcium-free KREBS solution. When the heart became swollen and turned slightly pale, the enzymatic digestion was stopped, atria were removed and the heart was placed in a Petri dish containing HEPES solution with 2% albumin, minced with scissors and individual cardiomyocytes were obtained by repeated gentle sucking with a pipette. The cell suspension was filtered through cotton gauze, placed in a conical tube and centrifuged (Jouan BBVV) at 125 mg for 5 min. The supernatant was removed, the myocytes were resuspended in albumin-free HEPES buffer then frozen and stored at − 70°C until used.
Immunofluorescence
Isolated cardiomyocytes were fixed with cold methanol at − 20°C for 15 min. After washing with PBS containing 1% fetal calf serum FCS three times, the cells were incubated with a PNMT-specific rabbit antibody (CA-401 bMTrab, Protos Biotech Corporation, New York, USA) diluted 1:1000 in PBS with 10% FCS at 37°C for 1 h. A parallel sample was incubated with a goat PNMT-specific antibody (sc-16456, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) diluted 1:100 and a negative control sample was incubated with PBS plus 10% FCS without the primary antibody. Cells were then washed three times with PBS containing 1% FCS and exposed either to anti-rabbit Alexa 488-conjugated secondary antibody (Advanced Targeting Systems, San Diego, CA, USA) or to Alexa Fluor 594 anti-goat antibody (Invitrogen, Molecular Probes, Eugene, Oregon, USA), each diluted 1:1000 in PBS with 10% FCS for 1 h at 37°C in the dark. After washing three times with PBS, the cells were placed onto microscope slides, examined with a Nikon E400 microscope and photographed with a Nikon Coolpix 990 camera.
Preparation of primary cardiomyocyte culture
Adult ventricular cardiac cells prepared by enzymatic digestion (as described above) were used. The incubation flasks were pre-coated for two days with gelatin (0.02%)/fibronectin (sigma) in an incubator in 5%CO2 at 37°C. Coating is necessary to enable the cardiomyocytes to attach to the bottom of the flask and to grow in a monolayer. The isolated cells were placed into the pre-treated flasks and kept in a specific medium designed for cardiomyocytes only: EX-CELL 320 with HEPES and L-glutamine (JRH Biosciences, USA). insulin (15 ug/ml), penstrephten (100 U/ml), non-essential amino acids (0.1 mM), retinoic acid (1 μM) and 10% fetal calf serum were added (all from Gibco). The cells were allowed to settle to the flask bottom for two days and then the medium was changed repeatedly, usually every two days. The cells were then left to stabilize and grow continually for about two weeks.
Experiments were performed on cultures growing for at least two weeks in strictly controlled conditions. At this time only cardiomyocytes are found in the culture. Viability of the cells in culture was regularly tested by immunohistochemical methods.
RNA isolation and relative quantification of mRNA levels by reverse transcription with subsequent polymerase chain reaction (RT-PCR)
Total RNA from frozen heart tissue and cardiomyocytes was isolated by TRI Reagent method (MRC Ltd). The purity and integrity of isolated RNAs was checked on a GeneQuant Pro spectrophotometer (Amersham Biosciences). Reverse transcription was performed using 2 μg of total RNAs and Ready-To-Go You-Prime First-Strand Beads (Amersham Biosciences) with pd (N6) primer (Amersham Biosciences). PCR specific for PNMT mRNA was carried out as described previously (Krizanova et al. Citation2001) using primers PT1: 5′-TAC CTC CGC AAC AAC TAC GC-3′ (position 1,171–1,190) and PT2: 5′-AAG GCT CCT GGT TCC TCT CG-3′ (position 1,904–1,923), yielding a 260-bp fragment. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA was used as a housekeeper gene control for semi-quantitative evaluation of PCR: primers GA1: 5′-AGA TCC ACA ACG GAT ACA TT-3′ and GA2: 5′-TCC CTC AAG ATT GTC AGC AGC AA-3′ were used to amplify a 309-bp fragment from each first strand sample. PCR products were analyzed on 2% agarose gels.
Tissue catecholamine (noradrenaline and adrenaline) determination
Catecholamine concentration was determined by the modified method of Peuler and Johnson (Citation1977). Frozen tissue was weighed and then immediately homogenized in 0.1 M HClO4. Homogenate was centrifuged at 10,000 rpm for 15 min. An aliquot of supernatant was taken and diluted with water to a final concentration of 1 mg tissue in 50 μl of solution (pH 8.4). The rest of the method was the same as for plasma catecholamine quantification (Peuler and Johnson Citation1977).
Statistical analysis
Results are presented as a mean ± SEM and each group represents an average of 6–10 animals. Statistical significance between groups was determined by one-way analysis of variance (ANOVA), and for multiple comparisons an adjusted t-test modified by Bonferroni's correction was used. p < 0.05 was considered to be significant.
Results
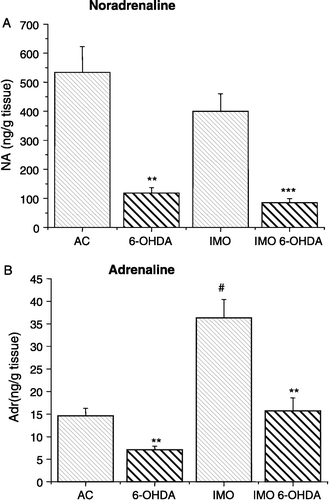
Administration of 6-OHDA significantly decreased levels of NA in the LV in control unstressed rats and also in rats immobilized once ((A)). Levels of Adr were also decreased after 6-OHDA treatment in the LV of both control and immobilized rats ((B)). As expected, a significant increase in Adr, but not NA levels was observed after exposure to a single immobilization stress ().
Figure 1 NA and Adr content of the LV wall. Significantly decreased NA content was observed in the 6-hydroxydopamine-treated groups (6-OHDA, IMO 6-OHDA) compared to the vehicle-treated groups (AC, IMO) of rats. NA content was not altered by immobilization stress. Adr level was significantly increased 1 h after a single 2 h immobilization stress in the vehicle treated (IMO) and 6-hydroxydopamine treated (IMO 6-OHDA) groups of rats, compared with the respective unstressed groups (B). The increase in Adr in the IMO 6-OHDA group was less than in the IMO group. Results are presented as group mean ± SEM, n = 7–10 rats per group. Statistical significance of the effects of 6-OHDA are **p < 0.01 and ***p < 0.001; significance of AC vs. IMO, #p < 0.01.
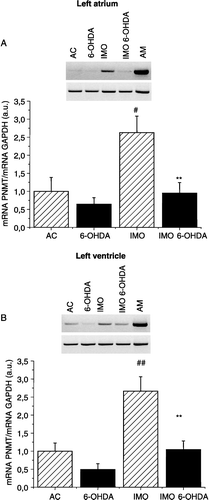
Next, we measured PNMT mRNA levels in the left atrium (as the major source of the PNMT mRNA in the heart; Krizanova et al. Citation2001) and in the LV (as the most powerful functional chamber of the heart). Using semi-quantitative RT-PCR, we determined a significant increase in PNMT mRNA levels in the cardiac atria and/or ventricles of the vehicle-treated group of rats after a single immobilization stress exposure (). Treatment with 6-OHDA did not change the PNMT mRNA levels in the left atrium and/or in the LV of rat hearts in normal, physiological conditions, but prevented the immobilization-induced increase in the left atrium ((A)) and also in the LV ((B)).
Figure 2 PNMT mRNA expression measured by RT-PCR in myocardium from the left atrium (A) and left ventricle (B). Typical gels for PNMT and GAPDH mRNAs are shown above the corresponding histograms. Significantly greater PNMT mRNA expression was observed in the vehicle-treated immobilized group of rats (IMO) compared to control (AC). After treatment with 6-OHDA, no immobilization-induced increase (IMO 6-OHDA) was observed. Results are presented as group mean ± SEM, n = 6–7 rats per group. Statistical significance for AC vs. IMO: #p < 0.05 and ##p < 0.01; significance between IMO and IMO 6-OHDA: **p < 0.01.
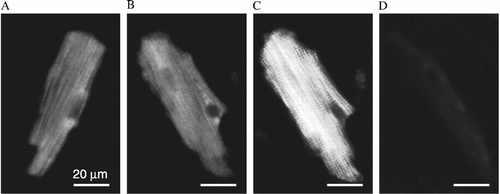
Since the heart is a heterogenous tissue composed of a variety of different cells types, we aimed to determine the potential for PNMT gene expression in cardiomyocytes. Using RT PCR, we found a clear signal for PNMT mRNA in isolated cardiomyocytes ((A)). When we isolated cardiomyocytes from the LV of control and 6-OHDA treated rats, we did not observe any significant difference in PNMT gene expression between these two groups ((B)). In order to show that the PNMT mRNA signal originates from cardiomyocytes and not from other cell types, which might be still present among the separated cardiomyocytes, we purified cardiomyocytes and prepared primary cultures free of contamination with other cell types ((C)). Comparison of the PNMT mRNA levels in a LV, isolated cardiomyocytes (M) and primary cardiomyocyte culture (PM) is shown in (C). We detected PNMT mRNA in cells of the primary cardiomyocyte culture, although the signal was weaker compared to unpurified cardiomyocytes ((C)). To verify presence of the PNMT in cardiomyocytes, we performed immunofluorescence on isolated cardiomyocytes using independently two specific PNMT antibodies. We detected a clear specific signal with rabbit PNMT antibody () and also with goat PNMT antibody (not shown).
Figure 3 Identification of PNMT mRNA by RT-PCR in cardiomyocytes (A), effect of 6-OHDA pre-treatment (B) and expression in primary culture of cardiomyocytes (C). In isolated cardiomyocytes (M) a clear signal for PNMT mRNA was observed. For comparison, adrenal medulla (AM), LA and LV were also used. A synthetic DNA ladder (L) was included on each gel. PNMT mRNA was measured also in cardiomyocytes of rats treated with 6-OHDA (M6-OHDA; B). No difference was observed in PNMT mRNA levels between cardiomyocytes from control (M) and from 6-OHDA-treated rats (M6-OHDA). To exclude the possibility that PNMT mRNA originates from non-cardiomyocyte cells, primary culture from purified cardiomyocytes was prepared (PM; part C). Cardiomyocytes were cultured on fibronectin in Ex-Cell 320 medium. In PM, a PNMT mRNA signal was also observed, although significantly weakerer compared to unpurified cardiomyocytes. Each column is the group mean ± SEM, from an average of at least three preparations.
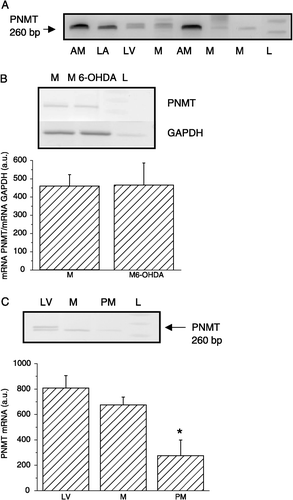
Figure 4 Identification of PNMT in cardiomyocytes by immunofluorescence. (A), (B), incubated with primary anti-rabbit PNMT antibody and anti-rabbit Alexa 488-conjugated secondary antibody; (C), same myocytes as in (B), but illuminated in polarized light; and (D), negative control (no primary antibody).
Discussion
6-OHDA is a potent neurotoxin that destroys catecholaminergic terminals (He et al. Citation2000). Administration of 6-OHDA to rats produces anatomical and functional noradrenergic denervation and induces compensatory hyperreactivity of the adrenal medullary chromaffin cells. These effects are manifested by an increase in the activity of catecholamine synthesizing enzymes (Kostrzewa and Jacobowitz Citation1974). In our experiments, we observed significantly lower (4.5-times) levels of NA in the LV of rats treated with 6-OHDA compared to control, untreated rats. However, Adr levels in control and 6-OHDA treated rats were only 2.3-times lower, which suggests that PNMT activity might be increased, or transcription and translation of the PNMT gene might be enhanced. Elayan et al. (Citation1990) have also found that 6-OHDA administration to bilaterally demedullated rats caused marked depletion of atrial and ventricular NA, but was less effective in decreasing cardiac Adr content. Differences in ratio between NA and Adr levels in control and 6-OHDA-treated rats correspond with those observed by Torda et al. (Citation1987), who found an increase in PNMT activity after chemical sympathectomy. Nevertheless, these results did not report data about PNMT gene expression and protein levels in cardiac atria and ventricles.
Here, we have clearly shown that PNMT is expressed also in cardiomyocytes. Firstly, we detected PNMT mRNA in cultured cardiomyocytes, which according to the culture protocol and media supplementation should be free of contamination by other cells. More importantly, we observed a clear and specific PNMT signal in cardiomyocytes by immunofluorescence, using independently two specific PNMT antibodies.
The first studies to describe cardiac PNMT mRNA levels were performed on fetal rat hearts (Ebert et al. Citation1996; Huang et al. Citation2005). The developing heart is likely to be a major source of catecholamines in the mammalian embryo (Pfeifer et al. Citation2004). In fetal hearts, PNMT mRNA was found in specialized cardiac cells, capable of adrenergic paracrine signaling in the mammalian heart and named ICA cells (Huang et al. Citation1996). These cells are non-neuronal cardiocytes that might provide an alternative adrenergic supply to maintain cardiac contractile and pacemaker function at rest and during stress in the absence of sympathetic innervation (Huang et al. Citation2005). We previously showed the presence of PNMT mRNA in the adult rat heart (Krizanova et al. Citation2001; Kvetnansky et al. Citation2004), with the predominant expression in the atria rather than in the ventricles. Similar results were observed on adult human heart (Goncalvesova et al. Citation2004). Because of the much greater abundance of the PNMT mRNA in atria, we proposed that PNMT is expressed predominantly in neuronal cardiac ganglia, which are localized in the atria (Krizanova et al. Citation2001). Since a relatively small amount of the PNMT mRNA was observed also in the ventricles, we anticipated that this small amount of PNMT mRNA might originate from cardiomyocytes. PNMT gene expression was observed also in transplanted hearts, with significantly higher amounts in the first three years after heart transplantation (Goncalvesova et al. Citation2004). Since transplanted hearts are completely lacking innervation, it can be proposed that PNMT mRNA originated from non-neuronal sources, e.g. cardiomyocytes. Also, we have recently published that modulation of PNMT mRNA expression by different stressors differs between the cardiac atria and ventricles (Tillinger et al. Citation2006). These observations would suggest that in the atria, PNMT mRNA expression is more sensitive to regulation by different stress stimuli and thus might contribute to coping with stress-induced changes in the atria, while PNMT in the ventricles might be involved in modulation of the basic metabolic demands of the heart. PNMT from cardiomyocytes might play a crucial role when availability of circulating Adr is restricted. Nevertheless, the functional importance of PNMT in cardiomyocytes remains to be elucidated. Also, multiple cellular origins of cardiac PNMT mRNA is supported by the finding that immobilization provokes different effects on the PNMT mRNA in the ganglionic and nonganglionic compartments of the atrium (Kvetnansky et al. Citation2004). Administration of 6-OHDA did not induce any changes in PNMT gene expression in normal, control conditions compared to non-treated rats, but prevented immobilization-induced increase of the PNMT mRNA. This observation would suggest that in the heart stress-induced increase in PNMT mRNA expression is rather by neuronal cells than by cardiomyocytes. This observation allows us to propose that although PNMT mRNA is expressed also in cardiomyocytes, inducible PNMT gene expression in neuronal cells is physiologically more important.
In summary, we have clearly shown that besides neuronal cells and ICA cells, PNMT is expressed also in cardiomyocytes. We observed clear and specific signal in cardiomyocytes with specific PNMT antibodies and immunofluorescent detection and we detected PNMT mRNA in purified cardiomyocytes. Chemical sympathectomy by 6-OHDA, which destroys noradrenergic terminals and might affect also ICA cells, did not alter PNMT gene expression in non-purified cardiomyocytes. We observed an effect of 6-OHDA only on the immobilization-induced increase of PNMT mRNA expression in the rat heart. Physiological relevance of the PNMT expression in cardiomyocytes is not clear. We hypothesize that this PNMT might play a role in a compensatory mechanism during development of pathological states in the heart. Nevertheless, further experiments are needed to test this hypothesis and to determine the physiological consequences of our findings.
Acknowledgements
This work was supported by: SP 51/028 08 00/028 08 02, Slovak Grant Agency VEGA 2/5125 and 2/6078 and APVT 51-027-404.
References
- Axelrod J. Purification and properties of phenylethanolamine N-methyltransferase. J Biol Chem 1962; 237: 1657–1660
- Ebert SN, Thompson RP. Embryonic epinephrine synthesis in the rat heart before innervation: Association with pacemaking and conduction tissue development. Circ Res 2001; 88: 117–124
- Ebert SN, Baden JM, Mathers LH, Siddall BJ, Wong DL. Expression of phenylethanolamine N-methyltransferase in the embryonic rat heart. J Mol Cell Cardiol 1996; 28: 1653–1658
- Ebert SN, Rong Q, Boe S, Thompson RP, Grinberg A, Pfeifer K. Targeted insertion of the cre-recombinase gene at the phenylethanolamine n-methyltransferase locus: A new model for studying the developmental distribution of adrenergic cells. Dev Dyn 2004; 231: 849–858
- Elayan HH, Kennedy BP, Ziegler MG. Cardiac atria and ventricles contain different inducible adrenaline synthesizing enzymes. Cardiovasc Res 1990; 24: 53–56
- Goldstein DS. Stress, catecholamines and cardiovascular diseases. Oxford University Press, New York 1995
- Goncalvesova E, Micutkova L, Mravec B, Ksinantova L, Krizanova O, Fabian J, Kvetnansky R. Changes in gene expression of phenylethanolamine N-methyltransferase in the transplanted human heart. Ann NY Acad Sci 2004; 1018: 430–436
- He Y, Lee T, Leong SK. 6-Hydroxydopamine induced apoptosis of dopaminergic cells in the rat substantia nigra. Brain Res 2000; 858: 163–166
- Huang MH, Bahl JJ, Wu Y, Hu F, Larson DF, Roeske WR, Ewy GA. Neuroendocrine properties of intrinsic cardiac adrenergic cells in fetal rat heart. Am J Physiol Heart Circ Physiol 2005; 288: 497–503
- Huang MH, Friend DS, Sunday ME, Singh K, Haley K, Austen KF, Kelly RA, Smith TW. An intrinsic adrenergic system in mammalian heart. J Clin Invest 1996; 98: 1298–1303
- Johnson MD, Grignolo A, Kuhn CM, Schanberg SM. Hypertension and cardiovascular hypertrophy during chronic catecholamine infusion in rats. Life Sci 1983; 33: 169–180
- Kanemoto N, Imaoka C, Hiramatsu K, Goto Y. A case of normotensive pheochromocytoma masquerading as a dilated cardiomyopathy. Jpn Circ J 1988; 50: 1128–1132
- Kennedy B, Ziegler MG. Cardiac epinephrine synthesis. Regulation by a glucocorticoid. Circulation 1991; 84: 891–895
- Kostrzewa RM, Jacobowitz D. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev 1974; 26: 199–288
- Krizanova O, Micutkova L, Jelokova J, Filipenko M, Sabban EL, Kvetnansky R. Existence of cardiac PNMT mRNA in adult rats: Elevation by stress in a glucocorticoid-dependent manner. Am J Physiol Heart Circ Physiol 2001; 281: 1372–1379
- Kvetnansky R, Micutkova L, Kubovcakova L, Sabban EL, Palkovits M, Krizanova O. Localization and regulation of phenylethanolamine N-methyltransferase gene expression in the heart of rats and mice during stress. Ann NY Acad Sci 2004; 1018: 405–417
- Kvetnansky R, Mikulaj L. Adrenal and urinary catecholamines in rats during adaptation to repeated immobilization stress. Endocrinology 1970; 87: 738–743
- Peuler JD, Johnson GA. Simultaneous single isotope radioenzymatic assay of plasma noradrenaline, adrenaline and dopamine. Life Sci 1977; 21: 625–636
- Pfeifer K, Boe SP, Rong Q, Ebert SN. Generating mouse models for studying the function and fate of intrinsic cardiac adrenergic cells. Ann NY Acad Sci 2004; 1018: 418–423
- Sláviková J, Kuncová J, Reisching J, Dvořáková M. Catecholaminergic neurons in the rat intrinsic cardiac nervous system. Neurochem Res 2003; 28: 593–598
- Tillinger A, Bruderova V, Kubovcakova L, Zeman M, Kopacek J, Kvetnansky R, Krizanova O. Gene expression of the phenylethaolamine N-methyltransferase is differently modulated in cardiac atria and ventricles. Gen Physiol Biophys 2006; 25: 357–368
- Torda T, Culman J, Petrikova M. Distribution of phenylethanolamine N-methyltransferase in the rat heart: Effect of 6-hydroxydopamine. Eur J Pharmacol 1987; 141: 305–308