Abstract
Controversies exist regarding the impact of psychological stress on the functioning of the immune system in humans. The aim of the present study, therefore, was to evaluate whether the condition of a pre-exam stress may or not modify resting lymphocyte subsets, as well as blood pressure and heart rate. About 22 medical residents of both sexes not suffering from any medical or psychiatric disorder were included in the study. Anxiety levels were measured by means of the Hamilton rating scale for anxiety (HRSA) and anxiety traits by means of the panic-agoraphobic spectrum self-report (PAS-SR) version and the obsessive-compulsive spectrum self-report (OBS-SR) version. The results showed that systolic blood pressure and heart rate increased significantly just before sitting an examination (t1) in all subjects, as compared with a calm situation (t2), in parallel with the increase in the HRSA total score, while no significant difference was observed in lymphocyte subsets at the two assessment times. However, men had a higher number of CD4+ cells than women at t1 and t2, while women showed a higher heart rate at t1. In addition, significant correlations between CD4+ lymphocyte count and heart rate at t1 or HRSA at t2 were detected.
These findings indicate that the acute stress determined by sitting for examination provokes changes in autonomic nervous system parameters, such as blood pressure and heart rate, as well as in the subjective feeling of anxiety, as shown by the increased HRSA total scores, which were not paralleled by modifications of lymphocyte subsets. However, individual differences, related to both sex and personality traits yet to be identified, seem to have an impact in shaping the stress response.
Introduction
The existence of close relationships between stress and qualitative and quantitative changes in immunological parameters has been extensively described, although the findings are conflicting (Kronfol and Remick Citation2000; Gruzelier Citation2002). The different results obtained may have been due to the types of stress examined, such as hyperacute, acute or chronic, different design of the studies, or heterogeneity of subjects included. Generally, experimental studies where stressors are artificially administered employ hyperacute stimuli, while observational studies, evaluating stressors as they occur naturally, include acute and chronic conditions (Zorrilla et al. Citation2001). The most replicated findings regarding immune cells include an absolute lymphocytosis and an absolute increase in the cytotoxic lymphocyte subsets, such as CD8+ and natural killer (NK) cells, leading to a relative reduction of T lymphocytes and of the CD4+/CD8+ ratio; a lowered T-cell proliferative response to mitogenic stimulation and an increased leukocyte adhesion has also been widely reported (Fittschen et al. Citation1990; Arber et al. Citation1991; Bachen et al. Citation1992; Herbert and Cohen Citation1993; Cacioppo Citation1994; Herbert et al. Citation1994; Mills et al. Citation1995; Zorilla et al. Citation2001). However, several discrepancies in the results have been found for most of the immune measures. While experimental studies on hyperacute stressors showed increased blood levels of cytotoxic lymphocytes, most of the observational studies reported decreased levels of these cells and of NK cytotoxicity, with an increased CD4+/CD8+ ratio (Schlesinger and Yodfat Citation1988, Citation1991; Irwin et al. Citation1990; Brosschot et al. Citation1991; Schedlowski et al. Citation1993; Zorrilla et al. Citation2001). This pattern may represent a biphasic response to stress exposure, since the initial response would consist of a demargination of cytotoxic lymphocytes resulting in their increased blood levels and lytic activity, whereas, after a few hours, they would begin to enter the tissues with a consequent reduction in their blood levels (Brosschot et al. Citation1992; Schedlowski et al. Citation1993; Dhabhar et al. Citation1999; Dhabhar Citation2003).
There is more agreement regarding the effect of chronic stress that mainly reduces some immune parameters in humans: protracted stressful conditions, such as bereavement, work-related or marriage problems, heavy chronic exercise and depression, have been shown to provoke a decrease in B and T-cell counts, NK activity and reduced lymphocyte responses to mitogens (Schleifer et al. Citation1983; Dorian and Garfinkel Citation1987; Kiecolt-Glaser et al. Citation1987; Bennett and Cohen Citation1993; Maes et al. Citation1994; Zisook et al. Citation1994; Bauer et al. Citation1995; Perna et al. Citation1997; Irwin Citation1999; Chu et al. Citation2002). Furthermore, a relationship between severity of depression and immunosuppression has been described, that is with more severe depression there is a more marked immunosuppression (Schleifer et al. Citation1984, Citation1989; Ader et al. Citation1991). However, hyperacute stress has been extensively reported to provoke a potentiating effect on different immunological parameters (Bachen et al. Citation1992; Herbert et al. Citation1994; Zorrilla et al. Citation2001). One of the most investigated conditions is students undertaking examinations, which is the subject of the majority of the studies reporting activation of lymphocytes, increase in NK and CD8+ lymphocytes and decreased CD4+/CD8+ ratio (Brosschot et al. Citation1994; Benschop et al. Citation1995; Maes et al. Citation1998, Citation1999). It is interesting to note that such phenomena do not seem to be typical of all subjects, but only of those showing a strong reaction to events, as demonstrated by increased blood pressure and heart rate (Bachen et al. Citation1992), or of those with particular personality traits. Thus, those subjects with stable personality traits and low baseline anxiety show an increase, while others, characterized by anxious traits, would show a decrease in immune activity in response to stress (Borrella et al. Citation1999). In terms of immune, cardiovascular and endocrine responses, acute severe aerobic exercise stress seems to have the same effects as acute psychological stressors. Immune system responses include increases in NK cell number and cytotoxic lymphocytes and a decreased proliferative response to mitogens, while the recovery period for either acute psychological stress or exercise is characterized by biphasic changes, with impairment during recovery (Perna et al. Citation1997). In a sample of parachutists, increases within lymphocyte subsets and in functional NK capacity have been reported immediately after jumping, followed by a decrease in the same immunological parameters 1 h later (Schedlowski et al. Citation1993).
Moreover, stronger correlations between stressors and some immune parameters (absolute number of lymphocytes, number of CD3+ and CD4+ lymphocytes) have been found in studies with a prevalence of women and of older subjects (Zorrilla et al. Citation2001): these findings suggest that gender and age are confounding factors that should be taken into account when analyzing such data (Schlesinger et al. Citation1993; Souza et al. Citation2001).
In order to provide a further contribution to this area, we measured resting lymphocyte subsets, blood pressure and heart rate, before and after the acute stress encountered when sitting their yearly examination, in a group of medical residents of both sexes, characterized by similar habits and life-styles. Possible relationships between these different parameters were sought.
Materials and methods
Subjects
About 22 residents (11 women and 11 men, between 26 and 33 years of age, mean ± SD: 27.9 ± 2.3 years) who were sitting the yearly examinations at the Specialty School of Psychiatry at Pisa University, were studied. They were selected on the basis of the absence of any positive personal or family history for psychiatric disorders, as assessed by a detailed psychiatric interview, carried out by senior psychiatrists (DM, LD). In addition, the subjects had neither axis II disorders, as assessed by the structured clinical interview for axis II disorders (SCID-II), nor any concomitant or past severe medical disorder. They were drug-free and had never taken psychotropic drugs. They were not heavy smokers: only two subjects smoked at all (about five cigarettes/day), nor did they belong to any AIDS-risk group. No woman was going through her ovulatory phase or took contraceptive pills. Their anxiety levels were measured by means of the Hamilton rating scale for anxiety (HRSA, Hamilton Citation1959). Anxiety traits were evaluated by means of the panic-agoraphobic spectrum self-report version (PAS-SR, Shear et al. Citation2001) and the obsessive-compulsive spectrum self-report version (OBS-SR, Dell'Osso et al. Citation2002). All subjects gave their written informed consent for the study, which had been approved by the Ethics Committee at Pisa University.
Methods
Systolic and diastolic blood pressures were measured with a mercury sphygmomanometer.
For lymphocyte subset preparations, 10 ml of venous blood was drawn and collected into plastic tubes containing heparin and the analyses were performed within 1 h of collection, by immunologists who were blind to the sampling conditions. Cytofluorimetric analysis was carried out on a two-colour Facstar Flow Sorter apparatus (Becton-Dickinson, USA), equipped with Consort 30 software, using a dual-angle light scatter lymphocyte gate. The following fluorescin (FITC) or phycoerythrin (PE)-conjugated monoclonal antibodies (MoAbs), were used: CD4+ (Leu-3a; Coulter-Clone), CD8+ (Leu-2a; Coulter-Clone), CD3+ (Leu-4: Coulter-Clone), CD19+ (Leu-12; Coulter-Clone), CD56+ (Leu-19: Becton-Dickinson) and HLA-DR+. NK cells were identified by means of CD56+ and CD8/CD56+. The balance between T-helper and T-suppressor lymphocytes was evaluated by means of the ratio CD4+/CD8+ (all values are given in percentages). Quality control was performed according to the criteria prescribed by the first European Quality Control of Cellular Phenotyping by Flow Cytometry (1990).
All subjects, recruited in two different years, underwent two blood tests, one immediately (from 15 to 5 min) before the examination in mid-June (t1) while sitting in the same room at a constant temperature (23°C), and the second between 2 and 4 weeks after the examination, when they were calm, having sat in resting conditions for at least 30 min at the same room temperature (t2), between 9 and 10 a.m.
Statistical analyses
The differences in the immunological parameters and HRSA total scores before and after the stressful situation were assessed by non-parametric Wilcoxon signed ranks test, while the difference in blood pressure, heart rate and HRSA scores were analysed by paired two-tailed Student t-tests. The difference between the same parameters in men and women was evaluated by non-parametric Mann–Whitney analysis. Values are presented as means ± SD.
The correlation between immunological cell counts and characteristics of the subjects was explored through Pearson's method. All analyses were carried out using the SSPS, version 4.0 (Nie et al. Citation1998) by means of personal computer programmes.
Results
The HRSA total score was significantly greater at t1 than at t2 (; Z = − 4.1, p = 0.001), as were systolic blood pressure (t = 9.4, df = 21, p = 0.0001) and heart rate (t = 8.2, df = 21, p = 0.0001).
Table I. Immunological parameters, cardiovascular measures and anxiety scores in medical residents before (t1) and after (t2) a professional examination statistical analyses. Panel a: All subjects; Panel b: Men and Women.
No significant difference was detected for lymphocyte subsets and in the ratio CD4+/CD8+, measured as both percentages and absolute numbers, between the measurements performed at t1 and t2 (). Similarly, no modification of diastolic blood pressure at t1 and t2 was found (data not shown).
Men showed a significantly higher number of CD4+ lymphocytes than women at t1 (; mean ± SD: 46.4 ± 3.3 vs. 43.2 ± 3.8, Mann–Whitney test: U = 24.0, Z = − 2.4, p = 0.016) and t2 (mean ± SD: 47.2 ± 2.6 vs. 43.7 ± 2.8, Mann–Whitney test: U = 18.0, Z = − 2.8, p = 0.004). Women had a significantly higher heart rate than men at t1 (t = 2.2, df = 20, p = 0.039).
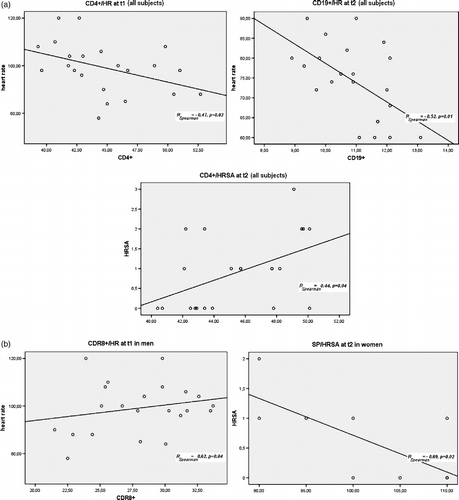
At t1, the number of CD4+ lymphocytes was significantly and negatively correlated with the heart rate of the whole sample (; r = − 0.47, p = 0.03), while at t2, a similar correlation was observed for the CD19+ subset (r = − 0.52, p = 0.01). Again at t2, the HRSA total score showed a significant and positive correlation with the CD4+ count (r = 0.44, p = 0.04). When the same analysis was carried out according to sex, a significant and positive relationship was observed between the CD8+ count and heart rate at t1 in men (; r = 0.62, p = 0.04) and a negative correlation between systolic blood pressure and HRSA total score at t2 in women (; r = − 0.69, p = 0.02). No other significant relationship was detected.
Figure 1 Correlations of different lymphocyte counts with heart rate or anxiety score. (a) Whole group; (b) by sex. Abbreviations: HR: heart rate; HRSA: Hamilton rating scale for anxiety; SP: systolic blood pressure; t1: time of examination; t2: 2–4 weeks after examination.
The scores (mean ± SD) for the PAS-SR and of the OBS-SR were, respectively, mean ± SD: 15.4 ± 11.2 and 25.6 ± 15.8, at t1 and 14.7 ± 12.1 and 26.7 ± 14.9 at t2. A positive trend between a high score at the PAS-SR and systolic blood pressure and heart rate of all subjects was observed at t1, although it did not reach statistical significance (r = 0.31, p = 0.15).
Discussion
The results of the present study, carried out in a sample of healthy medical residents undergoing a stressful situation, namely that of sitting for their yearly examination, failed to detect any difference in cell number in lymphocyte subsets, as compared with measurements in the same subjects in a calm situation.
It might have been that the residents were not particularly stressed, since they were undergoing an examination with teachers whom they knew well: however, this was not the case, since all subjects experienced a marked and often disturbing subjective feeling of anxiety, as revealed and assessed by the “pathological” total score on the specific scale use to measure it. The presence of high levels of anxiety was supported by evidence of somatic symptoms, namely a significant increase in systolic blood pressure and heart rate, in all subjects. Furthermore, the residents expressed the view that it was more anxiogenic to sit for an examination with teachers and colleagues with whom they worked all the time and whose opinion about them could change, depending upon the results of the examination, than to sit the examination with examiners who were unfamiliar to them.
Our findings may be considered in line with those of other experimental studies reporting that acute, psychological stressful conditions do not seem to induce any alterations in the functioning of the immune system (Kiecolt-Glaser et al. Citation1986; Halvorsen and Vassend Citation1987; Landmann et al. Citation1991; Herbert and Cohen Citation1993; Zorrilla et al. Citation2001). Previously, the situation of academic examinations in university students has been reported to increase the number of cells in some T and B lymphocyte subsets, such as CD8+, CD19+, CD26+, and HLA-DR+; in addition these changes were parallelled by a significant decrease in the CD4+/CD8+T cell ratio (Maes et al. Citation1999).
According to other observations, experimental hyperacute stress would be characterized by a biphasic response with an initial demargination of cytotoxic lymphocytes which, in turn, results in increased blood levels and lytic activity (Brosschot et al. Citation1994; Benschop et al. Citation1995; Dhabhar et al. Citation1999; Perna et al. Citation1997; Marshall et al. Citation1998; Dhabhar Citation2003). More agreement exists with the notion that chronic stress would produce an immunodepression (Schleifer et al. Citation1983; Dorian and Garfinkel Citation1987; Kiecolt-Glaser et al. 1987; Zisook et al. Citation1994; Maes et al. Citation1994; Miller et al. Citation1999).
Stress-related modifications in the immune system have been linked even to individual differences (O'Leary Citation1990; Christensen et al. Citation1996; Borrella et al. Citation1999), or to cognitive activation consistent with left hemispheric preferential influences over the immune system which can be influenced by relaxation techniques (Whitehouse et al. Citation1996; Gruzelier et al. Citation2001, Citation2002). In our sample, we detected that heart rate at t1 was negatively correlated with CD4+ lymphocyte count just before the examination, and with the CD19+ count after it. Evidently, the response of the heart is linked to changes in some lymphocyte subsets, although it is not clear whether one provokes the other, or both are due to other changes, such as stress hormones (e.g. catecholamines or glucocorticoids) (Dhabhar and McEwen Citation1995; Dhabhar Citation2003). In the calm situation, the anxiety scale total scores were significantly correlated with some lymphocyte subset counts, negatively with CD3+ and positively with CD4+ counts: this indicates that low basal anxiety levels might be related to increased CD3+ and decreased CD4+ lymphocyte numbers and vice versa, while suggesting that the degree of the anxiety state of the subject is linked to distinct lymphocyte subsets. It is worth noting that changes in CD4+ counts have been observed in anxiety disorders where they have been proposed as a specific marker of these disorders, in contrast with major depression (Marazziti et al. 1999), or in psychologically frustating situations that offer no possibility of control and result in hopelessness and helplessness (Knapp et al. Citation1992; Hoffman-Goetz and Pedersen Citation1994).
When our data were analyzed according to the sex of the subjects, no difference between men and women was detected except for a significantly higher number of CD4+ lymphocytes in men at the two assessment times, while women presented a higher heart rate just before the examination. Specific sex-related correlations were also observed: stressed men showed a positive correlation between CD8+ cell number and heart rate just before the examination, while in resting women there was a negative correlation between systolic blood pressure and HRSA total scores. Previously, women have been reported to have higher CD4+ percentages than men of different ages and cultures (Burton et al. Citation1983; Afoke et al. Citation1993; Lee et al. Citation1996), but negative findings are also available in the literature (Roman et al. Citation1993; Schlesinger et al. Citation1993; Souza et al. Citation2001; Yovel et al. Citation2001). In any case, women in our sample tended to present a more marked autonomic response than men, as already described (Schlesinger et al. Citation1993).
In addition, it is notable that we detected a trend towards more marked autonomic responses, i.e. systolic blood pressure and heart rate, in those subjects with a higher score on the PAS-SR, a questionnaire that examines anxiety traits. In any event, further studies seem necessary to clarify the impact of different stressful conditions on the immune system and the possible clinical (short- and long-term) consequences of such disturbances, as well as the impact of individual characteristics in the modulation of stress responses.
References
- Ader R, Felten DL, Cohern N. Psychoneuroimmunology2nd ed. Academic Press, London 1991
- Afoke AO, Eeg-Olofsson O, Hed J, Kjellman NI, Lindblom B, Ludvigsson J. Seasonal variation and sex differences of circulating macrophages, immunoglobulins and lymphocytes in healthy school children. Scand J Immunol 1993; 37: 209–215
- Arber N, Berliner S, Tamir A, Liberman E, Segal G, Pinkhas J, Aronson M. The state of leukocyte adhesiveness/aggregation in the peripheral blood: A new and independent marker of mental stress. Stress Med 1991; 7: 75–78
- Bachen EA, Manuck SB, Marsland AL, Cohen S, Malkoff SB, Muldoon MF, Rabin BS. Lymphocyte subset and cellular immune responses to a brief experimental stressor. Psychosom Med 1992; 54: 673–679
- Bauer ME, Gauer GJ, Luz C, Silveira RO, Nardi NB, Von Muhlen CA. Evaluation of immune parameters in depressed patients. Life Sci 1995; 57: 665–674
- Bennett HT, Cohen S. Depression and immunity: A meta-analytic review. Psych Bull 1993; 113: 472–483
- Benschop RJ, Godaert GLR, Genen R, Brosschot JF, De Smet M, Olff M, Heijnen CJ, Ballieux RE. Relationships between cardiovascular ad immunological changes in an experimental stress model. Psychol Med 1995; 25: 323–327
- Borella P, Bargellini A, Rovesti S, Pinelli M, Vivoli R, Solfrini V, Vivoli G. Emotional stability, anxiety, and natural killer activity under examination stress. Psychoneuroendocrinology 1999; 24: 613–627
- Brosschot JF, Smelt D, De Smet M, Heijnen CJ, Olff M, Ballieux RE, Godaert GLR. Effects of experimental psychological stress on T-lymphocytes and NK cells in man: An exploratory study. Psychophysiology 1991; 5: 59–67
- Brosschot JF, Benschop RJ, Godaert GLR, De Smet M, Olff M, Heijnen CJ, Ballieux RE. Effects of experimental psychological stress on distribution and function of peripheral blood cells. Psychosom Med 1992; 54: 394–406
- Brosschot JF, Benschop RJ, Godaert GLR, Olff M, Genen R, De Smet M, Heijnen CJ, Ballieux RE. Influence of life stress on immunological reactivity to mild psychological stress. Psychosom Med 1994; 56: 216–224
- Burton RC, Ferguson P, Gray M, Hall J, Hayes M, Smart YC. Effects of age, gender, and cigarette smoking on human immunoregulatory T-cell subsets: Establishment of normal ranges and comparison with patients with colorectal cancer and multiple sclerosis. Diagn Immunol 1983; 1(3)216–223
- Cacioppo JT. Social neuroscience: Anatomic, neuroendocrine, and immune responses to stress. J Psychophysiol 1994; 31: 113–128
- Christensen AJ, Edwards DL, Wiebe JS, Benotsch EG, McKelvey L, Andrews M, Lubaroff DM. Effect of verbel self-disclosure on natural killer cell activity: Moderating influence of cynical hostility. Psychosom Med 1996; 58: 150–155
- Chu L, Tian S, Chen H, Zhou Z. Study on natural killer cell subset and activity in patients with depression. Zhonghua Yi Xue Za Zhi 2002; 82: 830–831
- Dell'Osso L, Rucci P, Cassano GB, Maser JD, Endicott J, Shear MK, Sarno N, Saettoni M, Grochocinski VJ, Frank E. Measuring social anxiety and obsessive-compulsive spectra: Comparison of interviews and self-report instruments. Compr Psychiatry 2002; 43: 81–87
- Dhabhar FS. Stress, leukocyte trafficking, and the augmenttaion of the skin immine function. Ann NY Acad Sci 2003; 992: 205–217
- Dhabhar FS, McEwen. Effects of stress on immune cell distribution: Dynamics and hormonal mechanisms. J Immunol 1995; 154: 5511–5527
- Dhabhar FS, Miller AH, McEwen BS, Spencer ML. Changes in blood leukocye distribution: Interactions between catecholamines and glucocorticoid hormones. Neuroimmunomodulation 1999; 6: 213–220
- Dorian B, Garfinkel PE. Stress, immunity and illness—a review. Psychol Med 1987; 17: 393–407
- Fittschen B, Schulz KH, Schulz H, Raedler A, Kerekjarto M. Changes of immunological parameters in healthy subjects under examination stress. Int J Neurosci 1990; 51: 241–242
- Gruzelier JH. A review of the impact of hypnosis, relaxation, guided imagery and individual differences on aspects of immunity and health. Stress 2002; 5: 147–173
- Gruzelier J, Smith F, Nagy A, Henderson D. Cellular and humoral immunity, mood and exam stress: The influence of self-hypnosis and personality predictors. J Psychophysiol 2001; 42: 55–71
- Halvorsen R, Vassend O. Effects of examination stress on some cellular immunity functions. J Psychosom Res 1987; 31: 693–701
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol 1959; 32: 50–55
- Herbert TB, Cohen S. Stress and immunity in humans: A meta-analytic review. Psychosom Med 1993; 55: 364–379
- Herbert TB, Cohen S, Marsland AL, Bachen EA, Rabin BS, Muldoon MF, Manuk SB. Cardiovascular reactivity and the course of immune response to an acute psychological stressor. Psychosom Med 1994; 56: 337–344
- Hoffman-Goetz L, Pedersen BK. Exercise and the immune system: A model of the stress response. Immunol Today 1994; 15: 382–387
- Irwin M. Immune correlates of depression. Adv Exp Med Biol 1999; 461: 1–24
- Irwin M, Patterson T, Smith TL, Caldwell C, Brown SA, Gillin JC, Grant I. Reduction of immune function in life stress and depression. Biol Psychiatry 1990; 27: 22–30
- Kiecolt-Glaser JK, Glaser R, Strain EC. Modulation of cellular immunity in medical students. J Behav Med 1986; 9: 5–21
- Kiecolt-Glaser JK, Fisher LD, Ogrocki P, Stout JC, Speicher CE, Glaser R. Marital quality, marital disruption, and immune function. Psychosom Med 1987; 49: 13–34
- Knapp PH, Levy EM, Giorni RG, Black PH, Fox BH, Heeren TC. Short-term immunological effects of induced emotions. Psychosom Med 1992; 54: 133–148
- Kronfol Z, Remick DG. Cytokines and the brain: Implications for clinical psychiatry. Am J Psychiatry 2000; 157: 683–694
- Landmann RMA, Muller FB, Perini CH, Buhler RF. Catecholamine and leukocyte phenotype responses to mental stress in subjects with anxiety or suppressed aggression. Stress and immunity, N Plotnikoff, A Murgo, R Faith, J Wybran. CRC Press, Boca Raton 1991; 235–246
- Lee BW, Yap HK, Chew FT, Quah TC, Prabhakaran K, Chan GS, Wong SC, Seah CC. Age- and sex-related changes in lymphocyte subpopulations of healthy Asian subjects: From birth to adulthood. Cytometry 1996; 26: 8–15
- Maes M, Meltzer HY, Stevens W, Calabrese J, Cosyns P. Natural killer cell activity in major depression: Relation to circulating natural killer cells, cellular indices of the immune response, and depressive phenomenology. Prog Neuropsychopharmacol Biol Psychiatry 1994; 18: 717–730
- Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, Smith RS. The effects of psychological stress on humans: Increased production of pro-inflammatory cytokines and a Th-1-like response in stress-induced anxiety. Cytokine 1998; 10: 313–318
- Maes M, Van Bockstaele DR, Van Gastel A, Song C, Schotte C, Neels H, De Meester I, Scharpe S, Janca A. The effects of psychological stress on leukocyte subset distribution in humans: Evidence of immune activation in university students by academic examination. Neuropsychobiology 1999; 39: 1–9
- Marazziti D, Presta S, Pfanner C, Gemignani A, Rossi A, Sbrana S, Rocchi V, Ambrogi F, Cassano GB. Immunological alterations in adult obsessive-compulsive disorder. Biol Pschiatry 1999; 46: 810–815
- Marshall GD, Agarwal SK, Lloyd C, Cohen L, Henninger EM, Morris GJ. Cytokine dysregulation associated with exam stress in healty medical students. Brain Behav Immun 1998; 12: 297–307
- Miller GE, Cohen S, Herbert TB. Pathways linking major depression and immunity in ambulatory female patients. Psychosom Med 1999; 61: 850–860
- Mills PJ, Berry CC, Dimsdale JE, Ziegler MG, Nelesen RA, Kennedy BP. Lymphocyte subset redistribution in response to acute experimental stress: Effects of gender, ethnicity, hypertension, and the sympathetic nervous system. Brain Behav Immun 1995; 9: 61–69
- Nie NH, Hull CH, Steinbrenner K, Bent DH. Statistical package for the social science4th ed. Mc Graw-Hill, New York 1998
- O'Leary A. Stress, emotion, and human immune function. Psychol Bull 1990; 108: 363–382
- Perna FM, Schneiderman N, LaPerriere A. Psychological stress, exercise and immunity. Int J Sports Med 1997; 18: S78–S83
- Roman S, Moldovan I, Calugaru A, Regalia T, Sulica A. Lymphocyte subset reference ranges in Romanian adult Caucasians. Rom J Intern Med 1993; 33: 27–36
- Schedlowski M, Jacobs R, Stratmann G, Richter S, Hadicke A, Tewes U, Wagner TOF, Schmidt RE. Changes of natural killer cells during acute psychological stress. J Clin Immunol 1993; 13: 119–126
- Schleifer SJ, Keller SE, Camarino E, Thornton JC, Stein M. Suppression of lymphocyte stimulation following bereavement. J Am Med Assoc 1983; 250: 374–377
- Schleifer SJ, Keller SE, Meyerson AT, Raskin MJ, Davis KL, Stein M. Lymphocyte function in major depressive disorder. Arch Gen Psychiatry 1984; 41: 484–486
- Schleifer SJ, Keller SE, Bond RN, Cohen J, Stein M. Major depressive disorder and immunity. Arch Gen Psychiatry 1989; 46: 81–87
- Schlesinger M, Yodfat Y. Effect of psychosocial stress on natural killer cell activity. Cancer Detect Prev 1988; 12: 9–14
- Schlesinger M, Yodfat Y. The impact of stressfull life events on natural killer cells. Stress Med 1991; 7: 53–60
- Schlesinger M, Yodfat Y, Rabinowitz R, Bronner S, Kark JD. Psychosocial stress and NK cells among members of a communal settlement. Adv Exp Med Biol 1993; 335: 247–254
- Shear MK, Frank E, Rucci P, Fagiolini A, Grochocinski VJ, Houck P, Cassano GB, Kupfer DJ, Endicott J, Maser JD, Mauri M, Banti S. Panic-agoraphobic spectrum: Reliability and validity of assessment instruments. J Psychiatr Res 2001; 35: 59–66
- Souza SS, Castro FA, Mendonca HC, Palma PV, Morais FR, Ferriani RA, Voltarelli JC. Influence of menstrual cycle on NK activity. J Reprod Immunol 2001; 50: 151–159
- Whitehouse WG, Dinges DF, Orne EC, Keller SE, Bates BL, Bauer NK, Morahan P, Haupt BA, Carlin MM, Bloom PB, Zaugg L, Orne MT. Psychosocial and immune effect of self-hypnosis training for stress management throughout the first semester of medical school. Psychosom Med 1996; 58: 249–263
- Yovel G, Shakhar K, Ben-Eliyahu S. The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol Oncol 2001; 81: 254–262
- Zisook S, Shucter SR, Irwin M, Darko CF, Sledge P, Resovsky K. Bereavement, depression and immune function. Psychiatr Res 1994; 52: 1–10
- Zorilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assais: A meta-analytic review. Brain Behav Imm 2001; 15: 199–226