Abstract
Protracted social isolation in laboratory animals causes stress, which induces a variety of behavioral abnormalities including increased aggressiveness, anxiety-related behaviors, cognitive deficits and hyper locomotion. Many of these disorders are similar to the symptoms found in psychiatric disorders, such as depression, anxiety, premenstrual dysphoria and posttraumatic stress disorders (PTSD). Recent studies have demonstrated that male mice that have been socially isolated for more than 4 weeks show: (a) reduced responsiveness of GABAA receptors (GABAA-R) to the administrations of GABA mimetic drugs at GABAA-R; (b) downregulated biosynthesis of 3α,5α-tetrahydroprogesterone (3α,5α-THP) (allopregnanolone: ALLO), a neurosteroid with a potent positive allosteric modulatory effect on the action of GABA on GABAA-R; and (c) alterations in the expression of GABAA-R subunits (i.e. a decrease of α1/α2 and γ2 subunits and an increase of α4 and α5 subunits).
The selective serotonin reuptake inhibitor (SSRI) fluoxetine (FLX) and its congener norfluoxetine (Nor-FLX), when administered systemically at nmol/kg doses, normalize the reduced content of brain ALLO and the reduced responsiveness of GABAA-R to GABA mimetic drugs (i.e. pentobarbital) and also attenuate aggressive behavior in socially isolated mice in a stereospecific manner. Although these compounds inhibit ex vivo serotonin reuptake into brain tissue, their SSRI activities require high μmol/kg dose ranges and are not stereospecific. These studies suggest that in socially isolated mice, abnormalities of GABAA-R signal transduction are attributable to the downregulation of ALLO production and to a switch in heteropentameric GABAA-R subunit assembly composition. Hence, the normalization of ALLO biosynthesis may be a new target for the development of drugs effective for psychiatric disorders related to neurosteroid biosynthesis downregulation.
Introduction
Long-term social isolation stress induces behavioral disturbances, including aggression, hyperlocomotion, cognitive deficits, and anxiety-related behavior in laboratory animals (Hatch et al. Citation1963; Valzelli Citation1973; Gentsch et al. Citation1988; Jones et al. Citation1991). These disturbances are also typical symptoms expressed in neuropsychiatric disorders, such as depression, panic, anxiety, premenstrual dysphoria and posttraumatic stress disorders (PTSD). Hence, socially isolated laboratory animals have been used as models to study neurochemical mechanisms underlying the phenotypic aspects of these psychiatric disorders (Pashko et al. Citation1980; Hilakivi et al. Citation1989; Kim and Kirkpatrick Citation1996; Matsumoto et al. Citation1996; Guidotti et al. Citation2001; Pinna et al. Citation2006b; Serra et al. Citation2007).
Changes in various neurotransmitter systems, including noradrenergic, dopaminergic and serotonergic systems in the brain, were first described after rodents were experimentally housed in social isolation experimentally (Hodge and Butcher Citation1975; Matsumoto et al. Citation1991; Ojima et al. Citation1995; Hall Citation1998; Heidbreder et al. Citation2000; Weiss et al. Citation2001). Recent reports from our and other groups (Matsumoto et al. Citation1996; Ojima et al. Citation1997; Serra et al. Citation2000; Dong et al. Citation2001; Pinna et al. Citation2003, Citation2004) reveal that protracted social isolation-induced stress in rodents produces a dysfunction of GABAA receptor (GABAA-R) signal transduction. Several lines of evidence suggest that this dysfunction is in part due to downregulation of the brain expression of 3α,5α-THP (allopregnanolone: ALLO), a neurosteroid synthesized in the brain from progesterone by the activity of two sequential enzymes, 5α-reductase type I and 3α-hydroxysteroid oxidoreductase (3α-HSD).
Recent studies indicate that 5α-reductase type I and 3α-HSD are primarily localized in pyramidal neurons (Agis Balboa et al. Citation2006) and that their distribution and the content of ALLO in the brain are not uniform (Cheney et al. Citation1995; Pinna et al. Citation2000; Dong et al. Citation2001).
In nmolar concentrations, ALLO acts as a positive allosteric modulator of the action of GABA at various GABAA-R subtypes (Puia et al. Citation1990, Citation1993, Citation2003; Belelli and Lambert Citation2005). Deficiency of ALLO in the brain results in a downregulation of GABAA-R function (Pinna et al. Citation2000; Puia et al. Citation2003), whereas administration of ALLO or administration of psychotropic drugs that reverse the decrease of ALLO brain content induced by social isolation reverses these behavioral abnormalities (Matsumoto et al. Citation1999; Pinna et al. Citation2003, Citation2004, Citation2006a).
During the last decade, clinical and preclinical studies revealed that changes in the brain content of neurosteroids, including ALLO, are important factors involved in the pathophysiology of some mental disorders, such as depression (Romeo et al. Citation1998; Uzunova et al. Citation1998, Citation2006; Frye et al. Citation2006; Pinna et al. Citation2006b), anxiety, premenstrual dysphoric disorders (Girdler et al. Citation2001; van Broekhoven and Verkes Citation2003; Pisu and Serra Citation2004; Dubrovsky Citation2005), aggression (Miczek et al. Citation2003; Pinna et al. Citation2003) and PTSD (Rasmusson et al. Citation2006), raising the possibility that ALLO brain content downregulation is a risk factor and may even be the cause of certain aspects of psychiatric morbidities (Guidotti and Costa Citation1998; Guidotti et al. Citation2001; Uzunova et al. Citation2004). Therefore, social isolation of rodents provides a useful model to study the roles of ALLO and GABAA-R function downregulation in the expression of symptoms characteristic of affective disorders. This review will focus on the dysfunction of GABAA-R elicited by protracted social isolation in connection with the physiological and pathophysiological relevance of altered endogenous brain ALLO content and changes in the heteropentameric GABAA-R subunit composition.
Physiological relevance of brain ALLO
Neuropharmacological studies have elucidated the actions of ALLO and its congeners and the neuroactive steroid 3α,21-dihydroxy-5α-pregnan-20-one (tetrahydrodeoxycorticosterone, THDOC) on recombinant GABAA-R subtypes transfected into cell lines or on intrinsic GABAA-R expressed in neuronal cultures or in brain slices. It has been established that ALLO and its 3α-pregnane steroid congeners act as potent positive allosteric modulators of GABA action at the GABAA-R (Puia et al. Citation1990, Citation1991; Lambert et al. Citation2003) but these steroids have no modulatory effects on other neurotransmitter receptors, such as glycine, N-methyl-d-aspartate, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, or ion channels such as voltage-sensitive sodium or calcium channels (Lambert et al. Citation2003). Benzodiazepines are positive allosteric modulators of GABAA-R function and require the hetero-pentameric assembly of two α subunits (α1–3, or α5), two β subunits (β1, β2, or β3) and one (γ2) subunit and are ineffective on α4 or α6-containing GABAA-R (Costa and Guidotti Citation1996). In contrast, ALLO facilitates GABAA-R function independently of GABAA-R subunit composition (Puia et al. Citation1990) and it is even more active as a positive allosteric modulator of GABA action at GABAA-R expressing α4, α6, and δ subunits (Belelli and Lambert Citation2005). These findings allow us to infer that the spectrum of the pharmacological actions of ALLO on GABAA-R is broader than that of benzodiazepines (Costa and Guidotti Citation1996).
Importantly, physiologically relevant concentrations (nM) of ALLO and its congeners are detected in the brain (Cheney et al. Citation1995; Pinna et al. Citation2000; Dong et al. Citation2001) and at those concentrations, ALLO is capable of facilitating GABA actions at various GABAA-R subtypes (Puia et al. Citation1990, Citation1993, Citation2003; Lambert et al. Citation2003; Belelli and Lambert Citation2005). Indeed, pharmacological and electrophysiological studies using a potent inhibitor (SKF105,111) of 5α-reductase type I have provided substantial evidence that a decrease in brain ALLO levels reduces behavioral and synaptic responses to GABA mimetics, such as muscimol and pentobarbital (Matsumoto et al. Citation1999; Pinna et al. Citation2000; Guidotti et al. Citation2001; Puia et al. Citation2003) and potentiates behavioral responses to GABAA antagonists (Matsumoto et al. Citation2003). Furthermore, in brain slices from mice in which the ALLO content was decreased by SKF105,111 administration, GABAA-R-mediated postsynaptic currents evoked by spontaneously released GABA showed a faster decay and a lower extent of total charge transfer, resulting in diminished inhibitory strength. In addition, the effect of SKF105,111 treatment was reversed by applications of low concentrations of ALLO (Puia et al. Citation2003). These studies suggest that levels of endogenous ALLO exert a physiologically permissive role at the GABAA-R and regulate GABAergic inhibitory tone in the brain (Puia et al. Citation2003).
Relevance of brain ALLO in the pathogenesis of psychiatric disorders
The clinical relevance of the downregulation of neurosteroid expression, particularly ALLO, has been studied in patients with unipolar depression (Romeo et al. Citation1998; Uzunova et al. Citation1998; Stroehle et al. Citation1999, Citation2000), premenstrual dysphoria (Girdler et al. Citation2001), and generalized anxiety (Semeniuk et al. Citation2001). Reduced levels of ALLO in cerebrospinal fluid (CSF) were observed in depressed patients and the improvement of depression symptoms by treatment with fluoxetine (FLX) lasting 8–10 weeks was correlated with a normalization of ALLO content in the CSF (Uzunova et al. Citation1998). These studies suggest that a decrease in ALLO level in specific brain areas may be a relevant pathogenetic factor for clinical symptoms, such as anxiety and dysphoria in depressed patients (Guidotti and Costa Citation1998; Uzunova et al. Citation1998). Further, these findings are consistent with those reported by other groups that have determined a correlation between plasma neuroactive steroids and symptoms of depression following antidepressant treatment (Romeo et al. Citation1998; Stroehle et al. Citation1999). However, non-pharmacological antidepressant treatment, including partial sleep deprivation, failed to affect the plasma levels of ALLO (Schule et al. Citation2003; Eser et al. Citation2006). Indeed, one important issue in the above-mentioned studies that have determined plasma neuroactive steroids is the extent to which changes of blood levels of neuroactive steroids, or the lack thereof, may reflect similar changes in the brain and may be related to brain function. Although, in some experimental models, parallel changes have been observed in blood and brain, this was only partial or non-existent in other models. Most investigators measure neurosteroids and neurosteroidogenic enzyme expression/activity in specific regions of the rat/mouse brain and not just in blood because of these findings.
A recent study by Marx et al. (Citation2006) showed alterations in brain neurosteroid levels in the postmortem brain of subjects with schizophrenia and bipolar disorder. Considering the potent facilitatory role of endogenous ALLO in the regulation of GABAA-R function, these data allow the postulation that the beneficial actions of FLX and congeners on mood disorders may involve endogenous ALLO-mediated positive modulation of GABA action at GABAA-R (see ) (Guidotti and Costa Citation1998; Krystall et al. Citation2002; Dubrovsky Citation2005; Kendell et al. Citation2005; Pinna et al. Citation2006b).
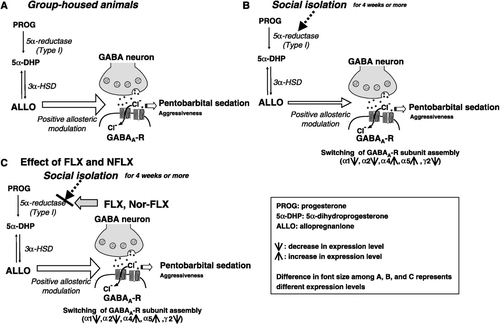
Figure 1 Schematic drawing of putative mechanism underlying social isolation-induced behavioral abnormalities and neurosteroid downregulation. (A) GABAergic neurotransmission in group-housed mice. ALLO synthesized by 5α-reductase type I and 3α-HSD at physiologically relevant concentrations positively modulates the action of GABA at GABAA-R, thereby exhibiting permissive action in the regulation of pentobarbital-induced sedation (bold, larger font) and suppressing aggression (smaller font), respectively. (B) GABAergic neurotransmission in socially isolated male mice: protracted social isolation (for 4 weeks or more) causes i) downregulation of 5α-reductase I expression and reduced brain ALLO content (indicated by a smaller font) and ii) alterations of GABAA-R subunit expression (i.e. decreases in α1-, α2-, and γ2-subunit and increases in α4- and α5-subunits), thereby reducing GABAA-R function. The decrease in GABAA-R-mediated signal transduction contributes to behavioral abnormalities; i.e. decreases in pentobarbital sedation (small font) and increases in aggressiveness (bold font). (C) FLX and Nor-FLX-induced amelioration of neurosteroid downregulation and behavioral abnormalities caused by social isolation. FLX and Nor-FLX ameliorate pentobarbital sedation and reduce aggressiveness, presumably by activating 5α-reductase type I and by correcting the reduced level of brain ALLO content, thereby normalizing GABAA-R-mediated signal transduction.
Socially isolated mice as a model of GABAA receptor dysfunction
Impaired neurosteroidogenesis
On the basis of our recent studies, we have proposed that socially isolated mice provide a model to study: (a) molecular mechanisms that lead to the reduction of GABAA-R signal transduction; this reduction probably occurs as a consequence of impaired neurosteroidogenesis (Matsumoto et al. Citation1999; Pinna et al. Citation2006b); and (b) behavioral responses elicited by some antidepressant drugs that have a neurosteroidogenic action as their principal pharmacological property (Puia et al. Citation2003; Pinna et al. Citation2006b). Indeed, exposure of male mice to protracted social isolation stress for 4–6 weeks elicited an approximately 50% decrease of ALLO content in the frontal cortex and olfactory bulb ((B)) without decreasing the brain content of progesterone and pregnenolone, which are precursors of ALLO (Matsumoto et al. Citation1999; Dong et al. Citation2001; Pinna et al. Citation2003, Citation2004). A social isolation-elicited decrease in neurosteroid levels, including ALLO, has also been observed in socially isolated rats (Serra et al. Citation2000, Citation2007).
Compared to group-housed mice, mice with reduced brain ALLO content elicited either by protracted social isolation or by inhibition of 5α-reductase type I with SKF105,111 exhibit reduced responsiveness to the sedative actions of GABAA-R active drugs, such as pentobarbital, diazepam and ethanol (Matsumoto et al. Citation1997, Citation1999; Ojima et al. Citation1997; Serra et al. Citation2000; Pinna et al. Citation2004) (see also (B)). The effect of social isolation on pentobarbital-induced sedation is reversed by administering ALLO or THDOC, systemically or intracerebroventricularly (Matsumoto et al. Citation1996). In contrast, socially isolated mice are more susceptible than group-housed mice to seizures induced by the GABAA-R antagonist, picrotoxin (Matsumoto et al. Citation2003). The increased susceptibility to picrotoxin-induced seizures is reversed in socially isolated mice by systemic administration of ALLO at a dose that has no effect on picrotoxin-induced seizures in group-housed mice. Since social isolation fails to alter seizure susceptibility to other convulsant drugs such as kainic acid and strychnine (Matsumoto et al. Citation2003), these findings suggest that protracted social isolation induces a selective decrease in GABAA-R function that is attributable to the reduction of the positive modulatory influence of ALLO on GABA action at GABAA-R (see (A) and (B)) (for a review see Guidotti et al. Citation2001; Matsumoto et al. Citation2005; Pinna et al. Citation2006b).
Our investigation, which is aimed at clarifying the mechanism(s) by which social isolation downregulates the expression of brain neurosteroids, has demonstrated that male mice socially isolated for six weeks have a reduction of about 70% in the rate of ALLO biosynthesis compared to group-housed control animals () (Dong et al. Citation2001). Social isolation reduces the expression of 5α-reductase type I mRNA and its protein but it does not change: (a) 3α-HSD mRNA expression; (b) diazepam binding inhibitor (DBI) mRNA expression (Dong et al. Citation1999); (c) mitochondrial benzodiazepine receptor (MBR) mRNA expression (DBI and MBR are endogenous factors involved in cholesterol transport and pregnenolone formation in mitochondrial outer membranes); and (d) the brain levels of pregnenolone and progesterone. Thus, it is likely that the contribution of pregnenolone and progesterone to the social isolation-induced decrease in ALLO brain content is minor, if any. It is therefore very likely that this neurosteroid biosynthesis downregulation is primarily attributable to the reduced expression of 5α-reductase type I (Dong et al. Citation2001) ().
Table I. Social isolation-induced decrease in the turnover rate of 5α-DHP and 3α,5α-THP biosynthesis in the brain.
Table II. Social isolation reduces brain ALLO content by downregulating 5α-reductase type I expression in the brain.
Changes of GABAA receptor subunit expression
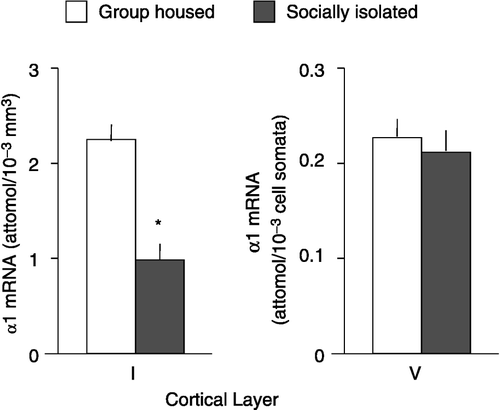
Numerous studies using postmortem subjects with psychiatric disorders have demonstrated the involvement of a GABAergic dysfunction in psychiatric disorders such as schizophrenia and depression (Akbarian et al. Citation1995; Dean et al. Citation1999; Lewis Citation2000; Ishikawa et al. Citation2004; Guidotti et al. Citation2005). Our recent study demonstrates that social isolation of male mice for more than four weeks causes alterations in the subunit expression of GABAA-R in the cortex and hippocampus; i.e., the expression level of the mRNAs encoding α1, α2, and γ2 GABAA-R subunit subtypes was reduced by 40–50% while that of the mRNAs encoding α4 and α5 subunit subtypes was increased by about 130%, compared to the values quantified in group-housed animals (). Western blot analysis of synaptic membrane preparations also revealed that social isolation causes a downregulation of α1 subunits ( − 40%) and an upregulation of α5 subunit expression (+100%) in the frontal cortex and hippocampus compared to expression levels measured in group-housed mice (Pinna et al. Citation2006a). To clarify whether the decline of GABAA-R α1 subunit expression in the frontal cortex during social isolation is layer- or cell-specific, we employed a laser microdissection technique coupled with nested RT-PCR amplification (Costa et al. Citation2002). In socially isolated mice, the expression levels of α1 subunit mRNA in frontal cortex layer I, which includes GABAergic interneurons and neuropil formed by the convergence of afferent thalamic fibers with apical dendrites of pyramidal neurons, showed about a 50% decrease, whereas the expression of α1 subunit mRNA in the pyramidal neurons of layer V was not influenced by social isolation (). These findings suggest that the changes in GABAA-R subunit mRNA expression caused by protracted social isolation are not uniformly distributed in the brain but rather are brain-region specific.
Table III. Expression levels of GABAA-R subunit mRNAs in the frontal cortex of group-housed and socially isolated mice.
Figure 2 Quantitative nested PCR analysis coupled with laser capture microdissection of α1 subunit mRNA in frontal cortical layers I and V of mice that were group-housed or socially isolated for 4 weeks. Cortical layer I and pyramidal cell somata located in layer V were microdissected using a laser capture method. GABAA-R α1 subunit mRNA in total RNA extracted from dissected tissue was determined by competitive RT-RTPCR associated with nested PCR. Each datum represents the mean ± SEM. of four mice. *P < 0.01 compared with group-housed mice.
Protracted treatment of rodents with positive allosteric modulators of GABAA-R function (Impagnatiello et al. Citation1996; Smith et al. Citation1998a; Kumar et al. Citation2004), or protracted exposure to environmental factors (Orchinik et al. Citation1995, Citation2001; Caldji et al. Citation2004) alters GABAA-R subunit expression in the brain. These alterations result in functional changes of GABAA-R responses to GABA mimetic drugs. Thus, we investigated whether changes in GABAA-R subunit expression in socially isolated mice could be translated into functional changes in GABAA-R responses to GABAA-R agonists, antagonists, or modulators. In behavioral studies, socially isolated mice were resistant to the sedative effects of diazepam and zolpidem, positive allosteric GABAA-R modulators that have high affinity for α1, α2, α3, or α5 subunit-containing GABAA-R and for α1 subunit-containing GABAA-R, respectively (Costa and Guidotti Citation1996; Guidotti et al. Citation2005). Mutational studies of GABAA-R subtypes have provided evidence that α1 subunit-containing GABAA-R mediate the sedative action of diazepam and zolpidem (Rudolph et al. Citation1999; Crestani et al. Citation2000; Rudolph and Mohler Citation2004). Thus, it is likely that the reduced responsiveness to the sedative action of diazepam in socially isolated mice is attributable to the decrease in α1-containing GABAA-R subtypes. Paradoxically, diazepam, which unlike zolpidem acts at α5-containing GABAA-R, increases the locomotor activity of socially isolated mice. Imidazenil, which fails to modulate α1-, α4-, and α6-containing GABAA-R but is a selective positive allosteric modulator of α5-containing GABAA-R, also increases locomotor activity in socially isolated mice (Pinna et al. Citation2006a). Importantly, socially isolated mice responded to muscimol, 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3(2H)-one (THIP), and ALLO in a manner similar to that of the group-housed mice. These data suggest that a switch (a decrease in α1/α2 and γ2 and an increase in α4 and α5 subunits) in the composition of the heteropentameric GABAA-R subunit assembly without a change in total GABAA-R number occurs during social isolation. Thus, the paradoxical actions of diazepam and imidazenil in socially isolated mice appear to be elicited by an allosteric modulation of GABAA-R that overexpress α subunits (Pinna et al. Citation2006a).
In contrast to GABAA-R, including α1, α2, α3, and α5 subunit subtypes, α4 subunit-containing GABAA-R exhibit unusual pharmacological profiles in vitro, including insensitivity to positive allosteric modulatory benzodiazepines, high sensitivity to flumazenil and bretazenil, and a high sensitivity to ethanol (Wafford et al. Citation1996). Although the expression level of GABAA-R containing α4 subunits is low in the brain, except in some regions such as the thalamus and dentate gyrus, enhanced expression of this GABAA-R subtype is observed in animal models of premenstrual syndrome (Smith et al. Citation1998a), epilepsy (Banerjee et al. Citation1998) and alcohol withdrawal (Cagetti et al. Citation2003). Similarly, social isolation induced an increase of α4 subunit-containing GABAA-R expression in the frontal cortex. However, this increase appeared to be irrelevant to the behavioral or pharmacological alterations observed in socially isolated animals, since GABA agonists such as THIP, which shows selectivity for the GABAA-R-containing α4/δ-subunit, failed to elicit stimulatory effects on locomotor activity (Pinna et al. Citation2006a). Thus, the switch of GABAA-R subunit assembly that may occur during protracted social isolation in mice suggests abnormalities in the molecular architecture of GABAA-R and is likely the cause of the dysfunction of the GABAA-R-mediated signal transduction observed in response to GABAmimetic drugs.
The observation in mice that social isolation induces a decrease in ALLO levels in several telencephalic structures and also changes the mRNA expression of GABAA-R subunits, poses the question of whether the changes in the GABAA-R subunit mRNA expression are mediated by social isolation-induced brain ALLO level downregulation. Several recent reports have described changes (e.g. increase of α4-containing GABAA-R subunit expression) in the expression of various GABAA-R subunits as a consequence of decreased brain levels of neurosteroids (e.g. progesterone withdrawal or blockade of 5α-reductase type I) (Smith et al. Citation1998a,Citationb). In our work, it remains to be clarified whether social isolation directly or indirectly affects the mRNA expression of GABAA-R subunits with a mechanism mediated by neurosteroids, for example by changing the brain content of 5α-dihydroprogesterone (5α-DHP), which by binding with high affinity to nuclear progesterone receptors affects the expression of target genes (e.g. GABAA-R subunit expression).
Social isolation-induced neurosteroid downregulation and behavioral abnormalities can be attenuated by fluoxetine and its congeners via a serotonin-independent mechanism
Relevance of neurosteroid downregulation to aggressiveness caused by social isolation
Aggression is a behavioral characteristic that can be induced in male mice by protracted social isolation (Valzelli Citation1981; Matsumoto et al. Citation1991, Citation1995, Citation2005; Pinna et al. Citation2003). Our previous studies demonstrate that aggressive behavior induced by social isolation was inversely related to both the decrease in responsiveness to the sedative action of pentobarbital (Ojima et al. Citation1995) and to the extent of the ALLO content downregulation in the olfactory bulb, frontal cortex and hippocampus but not the cerebellum (Pinna et al. Citation2003). Moreover, when administered systemically, ALLO attenuates the aggressive behaviors of socially isolated mice against an intruder mouse in a dose-dependent manner, leading to the suggestion that enhanced aggressiveness may be a behavioral consequence of the neurosteroid downregulation observed in socially isolated male mice (Guidotti et al. Citation2001; Pinna et al. Citation2003; Matsumoto et al. Citation2005). In contrast, female mice do not show aggressive behavior or neurosteroid expression downregulation even after protracted social isolation, but exhibit aggressiveness associated with decreases of brain ALLO content and 5α-reductase type I mRNA expression after daily treatment with testosterone propionate following a 3 week social isolation period (Pinna et al. Citation2005). Therefore, one may infer that impaired neurosteroidogenesis may be an important factor in the increase of aggressiveness caused by social isolation and that testosterone may play a role in the expression of aggressiveness and neurosteroid downregulation in socially isolated female mice.
Fluoxetine and its congeners reverse social isolation-induced behavioral abnormalities by a specific neurosteroidogenic action
FLX and paroxetine and other serotonin reuptake inhibitors (SSRIs) administered in nmol/kg doses to group-housed rats or mice increase brain ALLO without altering pregnenolone or progesterone content (Uzunov et al. Citation1996, Citation2006). Interestingly, when FLX was systemically administered as a racemic mixture of S- and R-enantiomers in nmol/kg doses in socially isolated mice, it normalized the reduced brain content of ALLO as well as the reduced responsiveness to the sedative action of pentobarbital ((C)). It must be noted that at these low doses, FLX did not alter either the ALLO content or the duration of pentobarbital-induced sedation in group-housed animals (Matsumoto et al. Citation1999). We investigated whether the racemic FLX-induced normalization of both brain ALLO content and pentobarbital-induced sedation was modified in socially isolated mice pretreated with p-chlorophenylalanine, which inhibits serotonin synthesis and reduces brain serotonin content by about 80%. This treatment failed to affect the actions of FLX, raising the possibility that the actions of racemic FLX in socially isolated mice are independent from the inhibition of serotonin reuptake (Matsumoto et al. Citation1999) and that FLX may have a characteristic pharmacological profile which contributes to neurostroidogenesis in the brain.
These findings led us to hypothesize that FLX may abolish behavioral abnormalities associated with protracted social isolation by normalizing the decreased brain levels of ALLO rather than by inhibiting serotonin reuptake. To further test this hypothesis, we took advantage of the stereoisomers (R- and S-forms) of FLX and norfluoxetine (Nor-FLX) to investigate whether FLX and Nor-FLX ameliorate social isolation-induced behavioral abnormalities, including pentobarbital sedation and aggressive behavior, in a manner related to neurosteroidogenesis but not to serotonin reuptake inhibition.
We reported that FLX and Nor-FLX at nmol/kg doses normalized the reduced duration of pentobarbital-induced loss of the righting reflex and inhibited the aggressive behaviors observed in socially isolated mice in a stereostructure-dependent manner. Indeed, with regard to aggressive behavior and pentobarbital-induced sedation in social isolated animals, the ED50 values calculated from dose–response curves revealed that S-FLX and S-Nor-FLX are two-to-seven times more potent than their R-enantiomers and that S-Nor-FLX is about three times more effective than S-FLX (Pinna et al. Citation2003, Citation2004, Citation2006b). We also found that at the same dose ranges as those in which these stereoisomers corrected social isolation-induced behavioral abnormalities, S-isoforms of FLX and Nor-FLX normalized the reduced levels of brain ALLO in socially isolated mice whereas R-enantiomers of FLX and Nor-FLX failed to change or slightly increased the ALLO content in the brain. Therefore, these findings provide pharmacological evidence that FLX and Nor-FLX stereospecifically ameliorate the behavioral abnormalities and neurosteroid downregulation caused by social isolation and that the S-enantiomers of FLX and Nor-FLX contribute to the action of the racemic forms of these compounds in socially isolated animals.
Importantly, ex vivo studies in which we measured [14C]-serotonin reuptake into cortical brain slices indicated that S-enantiomers of FLX and Nor-FLX dose-dependently inhibited serotonin transport with ED50 values of 8.3–13.7 μmol/kg (i.p.), which were about 10-(S-FLX) and 50-(S-Nor-FLX) fold higher than those required to ameliorate behavioral abnormalities and decrease neurosteroid content in socially isolated animals. In addition, no differences in the inhibitory activity of serotonin transporters were observed between the S- and R-enantiomers of FLX and Nor-FLX (Pinna et al. Citation2004), although other studies have reported conflicting results in terms of the stereospecificity of Nor-FLX enantiomers in their SSRI action (Wong et al. Citation1993). From these findings, one may infer that in doses that stimulate ALLO biosynthesis rather than block serotonin reuptake, FLX and its congeners may have an effect on psychiatric symptoms. Indeed, the tricyclic antidepressant drug imipramine failed to affect pentobarbital sedation, aggressive behavior, or the reduced levels of brain ALLO content at doses that almost completely inhibited ex vivo serotonin reuptake (Guidotti et al. Citation2001; Pinna et al. Citation2004). Failure of imipramine to affect brain ALLO content is consistent with previous reports (Griffin and Mellon Citation1999; Uzunov et al. Citation1996). A clinical report has found an increase of plasma neuroactive steroid levels in depressed patients following imipramine treatment. Thus, we must question whether peripheral fluctuation of neuroactive steroids following pharmacological treatments are representative of brain neurosteroid changes (Romeo et al. Citation1998).
Downregulation of the expression of 5α-reductase type I (mRNA and protein) seems the most plausible explanation for the decreased brain ALLO content in male mice socially isolated for >4 weeks since neither 3α-HSD mRNA expression nor progesterone levels changed in the brains of socially isolated mice. However, the exact mechanisms by which protracted social isolation downregulates 5α-reductase type I expression remain to be clarified. Moreover, the mechanisms by which the S-enantiomers of FLX and Nor-FLX elicit an increase of ALLO are also unclear. In vitro studies reported by Griffin and Mellon (Citation1999) showed that FLX and various other SSRIs fail to affect recombinant 5α-reductase type I activity in concentrations as high as 50 μM, whereas these drugs in a μM concentration range enhance the ability of 3α-HSD to convert 5α-DHP to ALLO by decreasing the Km value of this enzyme for 5α-DHP by 10- to 30-fold. However, in another study by Trauger et al. (Citation2002), these agents failed to activate the 3α-HSD enzyme with 50 μM concentrations of FLX and our data from behavioral and neurochemical studies showed no differences in the expression level of 3α-HSD mRNA in group-housed or socially isolated animals (). Moreover, we reported that both racemic and S-enantiomers of FLX, in doses that increased responsiveness of GABAA-R to GABA mimetics and corrected ALLO downregulation in socially isolated animals, failed to act in group-housed animals (Matsumoto et al. Citation1999; Pinna et al. Citation2004). Collectively, these findings thus suggest that the high potency and stereospecificity of FLX and Nor-FLX in ameliorating behavioral abnormalities and normalizing ALLO content in socially isolated animals may not be mediated by a mechanism that is exclusively dependent on 3α-HSD activation. Since socially isolated mice in which 5α-reductase type I expression is downregulated are substantially more sensitive to the neurosteroidogenic actions of FLX and Nor-FLX, it is possible that these compounds could indirectly induce the activity of 5α-reductase type I and thereby stimulate the production of brain ALLO in a socially isolated animal group ((C)). The molecular mechanisms underlying the FLX enhancement of neurosteroidogenesis remain to be investigated. Such investigations may be able to provide a new target for the development of drugs effective for psychiatric symptoms that are affected by ALLO expression downregulation.
Conclusion
Behavioral, neuropharmacological and neurochemical evidence has demonstrated that at physiologically relevant concentrations, ALLO plays a positive allosteric modulatory role in the regulation of GABAA-R-mediated signal transduction. The decrease in brain ALLO levels observed in socially isolated mice is associated with changes of GABAA-R subunit expression and causes impairments of GABAergic transmission, as shown by a reduced responsiveness of mice to GABAA mimetic drugs. Consistent with clinical reports that FLX exerts its beneficial effects on affective and anxiety disorders by increasing the levels of GABAergic neuroactive steroids (Romeo et al. Citation1998; Uzunova et al. Citation1998; Stroehle et al. Citation1999, Citation2000), preclinical studies using socially isolated mice have provided substantial evidence that FLX and Nor-FLX mitigate behavioral abnormalities with a mechanism dependent on neurosteroid biosynthesis. These effects are clearly dependent on the stereostructure of FLX and Nor-FLX and are elicited by doses that are devoid of significant inhibitory action on brain serotonin reuptake mechanisms. Thus, it is likely that the regulation of ALLO biosynthesis may be a new target for the development of drugs that would be effective for treatment of psychiatric disorders related to GABAergic dysfunctions caused by ALLO downregulation and changes in GABAA-R subunit assembly.
Acknowledgements
We thank Drs Erminio Costa and Alessandro Guidotti (Psychiatric Institute, Department of Psychiatry, University of Illinois at Chicago) for insightful comments on the manuscript. This work was supported by a Grant-in-Aid (Houga)(# 17659350) for Scientific Research from the Ministry of Education, Science, Culture and Sports, Japan (to K. M.) and by a Campus Research Board Award (to G. P.).
References
- Agis Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci USA 2006; 103: 14602–14607
- Akbarian S, Huntsman MM, Kim JJ, Tafazzoli A, Potkin SG, Bunney WEJ, Jones EG. GABAA receptor subunit gene expression in human prefrontal cortex: Comparison of schizophrenics and controls. Cereb Cortex 1995; 5: 550–560
- Banerjee PK, Tillakaratne NJ, Brailowsky S, Olsen RW, Tobin AJ, Snead OC, 3rd. Alterations in GABAA receptor alpha 1 and alpha 4 subunit mRNA levels in thalamic relay nuclei following absence-like seizures in rats. Exp Neurol 1998; 154: 213–223
- Belelli D, Lambert JJ. Neurosteroids: Endogenous regulators of the GABAA receptor. Nat Rev Neurosci 2005; 6: 565–575
- Cagetti E, Liang J, Spigelman I, Olsen R. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol 2003; 63: 53–64
- Caldji C, Diorio J, Anisman H, Meaney M. Maternal behavior regulates benzodiazepine/GABAA receptor subunit expression in brain regions associated with fear in BALB/c and C57BL/6 mice. Neuropsychopharmacology 2004; 29: 1344–1352
- Cheney DL, Uzunov DP, Guidotti A, Costa E. Gas chromatographic-mass fragmentographic quantitation of 3 alpha-hydroxy-5 alpha-pregnan-20-one (allopregnanolone) and its precursors in blood and brain of adrenalectomized and castrated rats. J Neurosci 1995; 15: 4641–4650
- Costa E, Guidotti A. Benzodiazepines on trial: A research strategy for their rehabilitation. Trends Pharmacol Sci 1996; 17: 192–200
- Costa E, Auta J, Grayson DR, Matsumoto K, Pappas GD, Zhang X, Guidotti A. GABAA receptors and benzodiazepines: A role for dendritic resident subunit mRNAs. Neuropharmacology 2002; 43: 925–937
- Crestani F, Martin JR, Mohler H, Rudolph U. Mechanism of acton of the hypnotic zolpidem in vivo. Br J Pharmacol 2000; 131: 1251–1254
- Dean B, Hussain T, Hayes W, Scarr E, Kitsoulis S, Hill C, Opeskin K, Copolov DL. Changes in serotonin2A and GABAA receptors in schizophrenia: Studies on the human dorsolateral prefrontal cortex. J Neurochem 1999; 72: 1593–1599
- Dong E, Matsumoto K, Tohda M, Kaneko Y, Watanabe H. Diazepam binding inhibitor (DBI) gene expression in the brains of socially isolated and group-housed mice. Neurosci Res 1999; 33: 171–177
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, Guidotti A. Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci USA 2001; 98: 2849–2854
- Dubrovsky BO. Steroids, neuroactive steroids and neurosteroids in psychopathology. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29: 169–192
- Eser D, Romeo E, Baghai TC, di Michele F, Schule C, Pasini A, Zwanzger P, Padberg F, Rupprecht R. Neuroactive steroids as modulators of depression and anxiety. Neuroscience 2006; 138: 1041–1048
- Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3α-Hydroxy-5α-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience 2006; 138: 1007–1014
- Gentsch JM, Lichtsteiner M, Frischknecht HR, Feer H, Siegfried B. Isolation-induced locomotor hyperactivity and hypoalgesia in rats are prevented by handling and recovered by resocialization. Physiol Behav 1988; 43: 13–16
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry 2001; 49: 788–797
- Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci USA 1999; 96: 13512–13517
- Guidotti A, Costa E. Can the antidysphoric and anxiolytic profiles of selective serotonin reuptake inhibitors be related to their ability to increase brain 3α 5α-tetrahydroprogesterone (allopregnanolone) availability?. Biol Psychiatry 1998; 44: 865–873
- Guidotti A, Dong E, Matsumoto K, Pinna G, Rasmusson AM, Costa E. The socially-isolated mouse: A model to study the putative role of allopregnanolone and 5α-dihydroprogesterone in psychiatric disorders. Brain Res Brain Res Rev 2001; 37: 110–115
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: New treatment strategies on the horizon. Psychopharmacology (Berl) 2005; 180: 191–205
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol 1998; 12: 129–162
- Hatch AM, Balazs T, Wiberg GS, Grice HC. Long-term isolation in rats. Science 1963; 142: 507–508
- Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience 2000; 100: 749–768
- Hilakivi LA, Ota M, Lister RG. Effect of isolation on brain monoamines and the behavior of mice in tests of exploration, locomotion, anxiety and behavioral ’despair’. Pharmacol Biochem Behav 1989; 33: 371–374
- Hodge GK, Butcher LL. Catecholamine correlates of isolation-induced aggression in mice. Eur J Pharmacol 1975; 31: 81–93
- Impagnatiello F, Pesold C, Longone P, Caruncho H, Fritschy JM, Costa E, Guidotti A. Modifications of γ-aminobutyric acidA receptor subunit expression in rat neocortex during tolerance to diazepam. Mol Pharmacol 1996; 49: 822–831
- Ishikawa M, Mizukami K, Iwakiri M, Hidaka S, Asada T. GABAA receptor γ subunits in the prefrontal cortex of patients with schizophrenia and bipolar disorder. Neuroreport 2004; 15: 1809–1812
- Jones GH, Marsden CA, Robbins TW. Behavioral rigidity and rule-learning deficits following isolation-rearing in the rat: Neurochemical correlates. Behav Brain Res 1991; 43: 35–50
- Kendell SF, Krystal JH, Sanacora G. GABA and glutamate systems as therapeutic targest in depression and mood disorders. Expert Opin Ther Targets 2005; 9: 153–168
- Kim JW, Kirkpatrick B. Social isolation in animal models of relevance to neuropsychiatric disorders. Biol Psychiatry 1996; 40: 918–922
- Krystall JH, Sanacora G, Bumberg H, Anand A, Charney DS, Marek G, Epperson CN, Goddard A, Mason GF. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry 2002; 7: S71–S80
- Kumar S, Fleming RL, Morrow AL. Ethanol regulation of γ-aminobutyric acidA receptors: Genomic and nongenomic mechanisms. Pharmacol Ther 2004; 101: 211–226
- Lambert JJ, Belelli D, Peden DR, V AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol 2003; 71: 67–80
- Lewis DA. GABAergic local circuit neurons and prefrontal cortical dysfunction in schizophrenia. Brain Res Brain Res Rev 2000; 31: 270–276
- Marx CE, Stevens RD, Shampine LJ, Uzunova V, Trost WT, Butterfield MI, Massing MW, Hamer RM, Morrow AL, Lieberman JA. Neuroactive steroids are altered in schizophrenia and bipolar disorder: Relevance to pathophysiology and therapeutics. Neuropsychopharmacology 2006; 31: 1249–1263
- Matsumoto K, Cai B, Satoh T, Ohta H, Watanabe H. Desipramine enhances isolation-induced aggressive behavior in mice. Pharmacol Biochem Behav 1991; 39: 167–170
- Matsumoto K, Ojima K, Watanabe H. Noradrenergic denervation attenuates desipramine enhancement of aggressive behavior in isolated mice. Pharmacol Biochem Behav 1995; 50: 481–484
- Matsumoto K, Ojima K, Watanabe H. Neurosteroidal modulation of social-isolation stress-induced decrease in pentobarbital sleep in mice. Brain Res 1996; 708: 1–6
- Matsumoto K, Ojima K, Watanabe H. Central corticotropin-releasing factor and benzodiazepine receptor systems are involved in the social isolation stress-induced decrease in ethanol sleep in mice. Brain Res 1997; 753: 318–321
- Matsumoto K, Uzunova V, Pinna G, Taki K, Uzunov DP, Watanabe H, Mienville JM, Guidotti A, Costa E. Permissive role of brain allopregnanolone content in the regulation of pentobarbital-induced righting reflex loss. Neuropharmacology 1999; 38: 955–963
- Matsumoto K, Nomura H, Murakami Y, Taki K, Takahata H, Watanabe H. Long-term social isolation enhances picrotoxin seizure susceptibility in mice: Upregulatory role of endogenous brain allopregnanolone in GABAergic systems. Pharmacol Biochem Behav 2003; 75: 831–835
- Matsumoto K, Pinna G, Puia G, Guidotti A, Costa E. Social isolation stress-induced aggression in mice: A model to study the pharmacology of neurosteroidogenesis. Stress 2005; 8: 85–93
- Miczek KA, Fish EW, De Bold JF. Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm Behav 2003; 44: 242–257
- Ojima K, Matsumoto K, Tohda M, Watanabe H. Hyperactivity of central noradrenergic and CRF systems is involved in social isolation-induced decrease in pentobarbital sleep. Brain Res 1995; 684: 87–94
- Ojima K, Matsumoto K, Watanabe H. Flumazenil reverses the decrease in the hypnotic activity of pentobarbital by social isolation stress: Are endogenous benzodiazepine receptor ligands involved?. Brain Res 1997; 745: 127–133
- Orchinik M, Weiland NG, McEwen B. Chronic exposure to stress levels of corticosterone alters GABAA receptor subunit mRNA levels in rat hippocampus. Brain Res Mol Brain Res 1995; 34: 29–37
- Orchinik M, Carroll SS, Li YH, McEwen BS, Weiland NG. Heterogeneity of hippocampal GABAA receptors: Regulation by corticosterone. J Neurosci 2001; 21: 330–339
- Pashko S, DeTurck KH, Vogel WH. Use of the catheterized rat in studies on social interactions and plasma catecholamines. Pharmacol Biochem Behav 1980; 13: 471–473
- Pinna G, Uzunova V, Matsumoto K, Puia G, Mienville J-M, Costa E, Guidotti A. Brain allopregnanolone regulates the potency of the GABAA receptor agonist muscimol. Neuropharmacology 2000; 39: 440–448
- Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci USA 2003; 100: 2035–2040
- Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically facilitate pentobarbital sedation by increasing neurosteroids. Proc Natl Acad Sci USA 2004; 101: 6222–6225
- Pinna G, Costa E, Guidotti A. Changes in brain testosterone and allopregnanolone biosynthesis elicit aggressive behavior. Proc Natl Acad Sci USA 2005; 102: 2135–2140
- Pinna G, Agis-Balboa RC, Zhubi A, Matsumoto K, Grayson DR, Costa E, Guidotti A. Imidazenil and diazepam increase locomotor activity in mice exposed to protracted social isolation. Proc Natl Acad Sci USA 2006a; 103: 4275–4280
- Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology 2006b; 186: 362–372
- Pisu MG, Serra M. Neurosteroids and neuroactive drugs in mental disorders. Life Sci 2004; 74: 3181–3197
- Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron 1990; 4: 759–765
- Puia G, Vicini S, Seeburg PH, Costa E. Influence of recombinant gamma-aminobutyric acid—a receptor subunit composition on the action of allosteric modulators of gamma-aminobutyric acid-gated Cl− current. Mol Pharmacol 1991; 39: 691–696
- Puia G, Ducic I, Vicini S, Costa E. Does neurosteroid modulatory efficacy depend on GABAA receptor subunit composition?. Receptors Channels 1993; 1: 135–142
- Puia G, Mienville J-M, Matsumoto K, Takahata H, Watanabe H, Costa E, Guidotti A. On the putative physiological role of allopregnanolone on GABAA receptor function. Neuropharmacology 2003; 44: 49–55
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, Guidotti A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry 2006; 60: 704–713
- Romeo E, Strohle A, Spalletta G, diMichele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry 1998; 155: 910–913
- Rudolph R, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol 2004; 44: 475–498
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature 1999; 401: 796–800
- Schule C, di Michele F, Baghai T, Romeo E, Bernardi G, Zwanzger P, Padberg F, Pasini A, Rupprecht R. Influence of sleep deprivation on neuroactive steroids in major depression. Neuropsychopharmacology 2003; 28: 577–581
- Semeniuk T, Jhangri GS, Le Melledo JM. Neuroactive steroid levels in patients with generalized anxiety disorder. J Neuropsychiatry Clin Neurosci 2001; 13: 396–398
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, Usala L, Purdy RH, Biggio G. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABAA receptor function in rat brain. J Neurochem 2000; 75: 732–740
- Serra M, Sanna E, Mostallino MC, Biggio G. Social isolation stress and neuroactive steroids. Eur Neuropsychopharmacol 2007; 17: 1–11
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 1998a; 392: 926–930
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3α-OH-5α-pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci 1998b; 18: 5275–5284
- Stroehle A, Romeo E, Hermann B, Pasini A, Spalletta G, di Michele F, Holsboer F, Rupprecht R. Concentrations of 3α-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol Psychiatry 1999; 45: 274–277
- Stroehle A, Pasini A, Romeo E, Hermann B, Spalletta G, di Michele F, Holsboer F, Rupprecht R. Fluoxetine decreases concentrations of 3α, 5α-tetrahydrodeoxycorticosterone (THDOC) in major depression. J Psychiatr Res 2000; 34: 183–186
- Trauger JW, Jiang A, Stearns BA, LoGrasso PV. Kinetics of allopregnanolone formation catalyzed by human 3α-hydroxysteriod dehydrogenase type III (AKR1C2). Biochemistry 2002; 41: 13451–13459
- Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci USA 1996; 93: 12599–12604
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA 1998; 95: 3239–3244
- Uzunova V, Wrynn AS, Kinnunen A, Ceci M, Kohler C, Uzunov DP. Chronic antidepressants reverse cerebrocortical allopregnanolone decline in the olfactory-bulbectomized rat. Eur J Pharmacol 2004; 486: 31–34
- Uzunova V, Sampson L, Uzunov DP. Relevance of endogenous 3alpha-reduced neurosteroids to depression and antidepressant action. Psychopharmacology (Berl) 2006; 186: 351–361
- Valzelli L. The “Isolation Syndrome” in mice. Psychopharmacologia (Berl) 1973; 31: 305–320
- Valzelli L. Psychopharmacology of aggression: An overview. Int Pharmacopsychiatry 1981; 16: 39–48
- van Broekhoven F, Verkes RJ. Neurosteroids in depression: A review. Psychopharmacology (Berl) 2003; 165: 97–110
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human γ-aminobutyric acidA receptors containing the α4 subunit. Mol Pharmacol 1996; 50: 670–678
- Weiss IC, Domeney AM, Heidbreder CA, Moreau JL, Feldon J. Early social isolation, but not maternal separation, affects behavioral sensitization to amphetamine in male and female adult rats. Pharmacol Biochem Behav 2001; 70: 397–409
- Wong DT, Bymaster FP, Reid LR, Mayle DA, Krushinski JH, Robertson DW. Norfluoxetine enantiomers as inhibitors of serotonin uptake in rat brain. Neuropsychopharmacology 1993; 8: 337–344