Abstract
Glucocorticoids contribute fundamentally to the maintenance of basal and stress-related homeostasis in all higher organisms. These hormones influence a large percentage of the expressed human genome and their effects spare almost no organs or tissues. Glucocorticoids influence many functions of the central nervous system, such as arousal, cognition, mood and sleep, the activity and direction of intermediary metabolism, the maintenance of a normal cardiovascular tone, the activity and quality of the immune and inflammatory reaction, including the manifestations of the sickness syndrome, as well as growth and reproduction. The numerous actions of glucocorticoids are mediated by a set of at least 16 glucocorticoid receptor (GR) isoforms forming homo- or hetero-dimers. The GRs consist of multifunctional domain proteins operating as ligand-dependent transcription factors that interact with many other cell signaling systems. The presence of multiple GR monomers and dimers expressed in a cell-specific fashion at different quantities with quantitatively and qualitatively different transcriptional activities suggests that the glucocorticoid signaling system is highly stochastic. Based on ample evidence, we present our conception that glucocorticoids are heavily involved in human pathophysiology and influence life expectancy. Common psychiatric and/or somatic complex disorders, such as anxiety, depression, insomnia, chronic pain and fatigue syndromes, obesity, the metabolic syndrome, essential hypertension, diabetes type 2, atherosclerosis with its cardiovascular sequelae, and osteoporosis, as well as autoimmune inflammatory and allergic disorders, all appear to have a glucocorticoid component.
Introduction
Glucocorticoids are among the most pervasive hormones in mammalian organisms (Chrousos Citation2001; Franchimont et al. Citation2003). These steroid molecules reach all tissues, including the brain, readily penetrate the cell membrane, and interact with ubiquitous cytoplasmic/nuclear glucocorticoid receptors (GR), through which they exert markedly diverse actions. Using DNA microarray technology, we found that about 20 percent of the expressed human leukocyte genome was positively or negatively affected by glucocorticoids (Galon et al. Citation2002). This is many-fold higher than the proportion of genes that change in the transformation of a normal cell to a tumor cell and involves a broad array of functions, affecting every aspect of resting and stress-related homeostasis, as well as a large number of genes expressed by the immune system (Chrousos Citation1998; Galon et al. Citation2002). The pervasive nature of glucocorticoids, the rapid advances in our general knowledge of the human and other mammalian genomes, and the massive amount of information that increasingly accumulates, dictate a new model of thinking and testing of hypotheses regarding the actions of these hormones and their involvement in human physiology and pathophysiology.
Glucocorticoid receptor gene polymorphisms and complex human pathophysiology
Wust et al. (Citation2004) recently reported a convincing association between the hypothalamic–pituitary–adrenal (HPA) axis response to a standardized socio–emotional stimulus (Trier test) and polymorphisms of the GR gene. This study followed others that used a similar rationale and examined HPA axis indices and other end-points, such as arterial blood pressure, body mass index (BMI) and markers of the metabolic syndrome, and bone mineral density (Weaver et al. Citation1992; Buemann et al. Citation1997; Huizenga et al. Citation1998; Panarelli et al. Citation1998; Lin et al. Citation1999; Rosmond et al. Citation2000; Dobson et al. Citation2001; Ukkola et al. Citation2001). These studies have in part overlapped and have produced mostly concordant results, but have also shown inconsistencies. This should have been expected because they were performed on a limited number of subjects in different ethnic populations, and because the altered GR would have been expected to function differently in the context of different genetic backgrounds characterized by different panels of genes with differing epistatic effects upon the ability of the GR to exert its actions (Chrousos Citation2000b).
Glucocorticoid effects—physiology, pathophysiology
Empirically, experimentally and intuitively, physicians and scientists have made major advances in the general understanding of glucocorticoids and their involvement in human physiology and pathophysiology, and in using these hormones extensively and effectively in the treatment of a wide spectrum of human diseases (Chrousos Citation2001; Franchimont et al. Citation2003). As the end product of the HPA axis, glucocorticoids are clearly involved in every organ system of the human organism, in almost every physiologic, cellular and molecular network, and in many crucial modules of these networks (Chrousos Citation2000b; Galon et al. Citation2002; Franchimont et al. Citation2003). Glucocorticoids, furthermore, participate in a pivotal fashion in the unfolding of vital biological programs employing several networks synchronously or in tandem, including the behavioral and physical response to stress, the inflammatory reaction and the consequent “sickness syndrome”, i.e. the collections of “nonspecific symptoms” caused by excessive inflammatory cytokines during infectious or inflammatory illness, as well as the process of sleep, and long-term functions, such as growth and reproduction (Chrousos Citation1998, Citation2000b).
As happens with many other homeostatic systems, too much, as well as too little, of HPA axis and/or glucocorticoid activity signify pathology, for instance Cushing's syndrome vs. Addison's disease, respectively (Chrousos et al. Citation1993; McEwen Citation1998; Chrousos Citation2000a). Since the responsiveness of the target tissues to glucocorticoids is crucial for the end-effect of these hormones, similar pathology may result from hypersensitivity or resistance of these target tissues to these hormones, respectively (Chrousos et al. Citation1993; Kino et al. Citation2003b) (). However, because the brain and the pituitary are also targets for glucocorticoids, and because the organism strives for homeostasis during time-integrated free cortisol exposure, any generalized change in the glucocorticoid signaling system would be followed by corrective, “compensatory” changes in the activity of the HPA axis (). However, absence of complete compensation, be it slightly excessive or deficient, could result in allostasis leading to target tissue pathology, as occurs in chronically stressed or depressed individuals (Chrousos Citation2000a; Gold and Chrousos Citation2002). But this has been known for several decades. A key question is whether it is possible to have discordance between HPA axis feedback regulation by glucocorticoids and peripheral target tissue sensitivity to these hormones in totally normal individuals.
Table I. Expected clinical manifestations in target tissue hypersensitivity or resistance to glucocorticoids.
Figure 1 A: Feedback regulation of the HPA axis. ACTH, Adrenocorticotropic hormone; AVP, arginine vasopressin; CRH, corticotropin-releasing hormone; DOC, deoxycorticosterone; B, corticosterone. B: Feedback-regulated compensatory changes in the activity of the HPA axis and their effects in peripheral tissues, such as the liver, fat and blood vessels. Note that glucocorticoid sensitivity in the HPA axis and the peripheral tissues can be independently regulated and the former determines the serum free cortisol levels, thus combination of their directions of change from normal influence net peripheral action of this hormone. Modification from Kino et al. (Citation2001), Charmandari et al. (Citation2004).
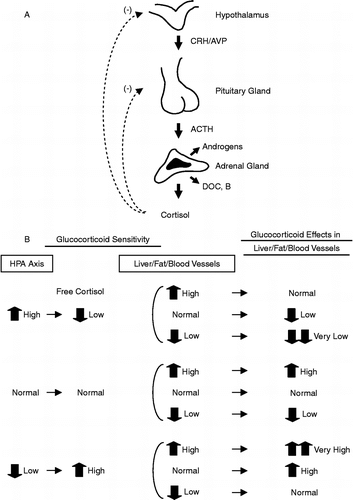
We propose that this is indeed so and elaborate below. The glucocorticoid signaling system of the suprahypothalamic, hypothalamic and pituitary glucocorticoid-sensing network is different from the signaling systems of the reward, arousal, associative, cardiovascular, metabolic and immune systems that are influenced by glucocorticoids. The hypothalamic-pituitary axis senses and thus determines the circulating glucocorticoid levels, while the rest of the tissues passively accept the actions of the secreted glucocorticoids. Indeed, any change in one or more molecules or processes that participate in the glucocorticoid signaling system could potentially have a different impact in the HPA feedback system and the other target tissues. Such discrepancy in the glucocorticoid sensing network between the HPA axis and peripheral tissues could, thus, produce peripheral tissue hypercortisolism or hypocorticosolism depending on their combinations ( and ) (Chrousos et al. Citation1993). In a recent study by Alevizaki et al. (Citation2007), both high HPA axis reactivity to stress and increased peripheral tissue sensitivity to glucocorticoids were associated with increased severity of coronary artery disease. However, naturally the GR is not alone in defining the sensitivity of the feedback system and other tissues to glucocorticoids. Numerous GR isoforms with different activities, other molecules or processes with important input into the activity of the cellular glucocorticoid signaling system have been described () (Kino et al. Citation2003a; Kino et al. Citation2003b; Chrousos and Kino Citation2005; Lu and Cidlowski Citation2005; Kino et al. Citation2006).
Table II. Factors influencing GR functions.
Glucocorticoids and the metabolic syndrome
Endogenous or exogenous Cushing's syndrome is associated with the full metabolic profile of the metabolic syndrome and with substantially increased cardiovascular morbidity and mortality (Friedman et al. Citation1996; Miller and Chrousos Citation2001). Glucocorticoids directly cause insulin resistance of peripheral target tissues in proportion to their levels and to the particular target tissue's sensitivity to these hormones. Over time, glucocorticoids also cause progressive accumulation of visceral fat, leading to worsening manifestations of the metabolic syndrome. Thus, when polymorphisms of the glucocorticoid receptor gene lead to an unfavorable discordance between the activity of the HPA axis and the sensitivity of muscle, fat and liver to glucocorticoids, the increased glucocorticoid effect in these tissues could influence the metabolic profile and the longevity of humans in a negative fashion, similar to what occurs in Cushing's syndrome (Chrousos Citation2000a; Dobson et al. Citation2001; Stevens et al. Citation2004; Buemann et al. Citation2005; DeRijk and de Kloet Citation2005; Marti et al. Citation2006).
Genetic and developmental factors, nutrition, lifestyle and cumulative chronic or intermittent stress may lead to development of obesity, primarily of the visceral type, and hence the metabolic syndrome with its components of insulin resistance, dyslipidemia, chronic smoldering inflammation, blood hypercoagulation and hypertension (). These changes lead to endothelial inflammation, atherosclerosis and cardiovascular disease, ultimately resulting in premature cardiovascular morbidity and death. Glucocorticoids contribute to the pathogenesis of obesity and the metabolic syndrome not only through unfavorable genetic variations that increase both the activity of the HPA axis and the sensitivity of tissues to glucocorticoids, but also because of fetal programming of the HPA axis by an adverse intrauterine environment, which may lead to a postnatally hyperactive axis, and because of chronic cortisol hypersecretion owing to real or perceived stress (Chrousos Citation1998, Citation2000a; Phillips et al. Citation1998).
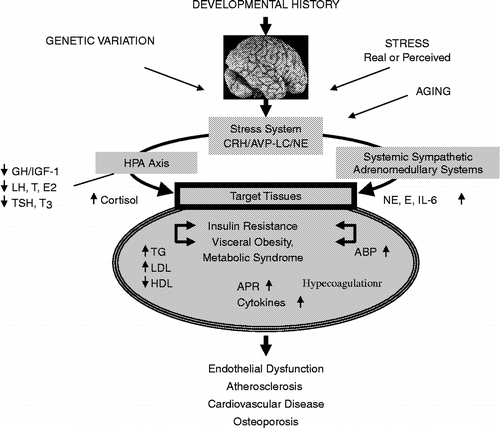
Figure 2 Endogenous/exogenous inputs to the stress system and their effects on the metabolic and cardiovascular systems and bone. ABP, arterial blood pressure; APR, acute phase reactants; AVP, arginine vasopressin; CRH, corticotropin-releasing hormone; E, epinephrine; E2, estradiol; GH, growth hormone; HDL, high-density lipoprotein; HPA axis, hypothalamic–pituitary–adrenal axis; IGF-1, insulin-like growth factor-1; IL-6, interleukin-6; LC, locus caeruleus; LDL, low-density lipoprotein; LH, luteinizing hormone; NE, norepinephrine; T, testosterone; T3, triiodothyronine; TG, triglyceride; TSH, thyroid-stimulating hormone.
The opposite result is possible as well. Patients may be protected from obesity, the metabolic syndrome, and premature death because of favorable genetic variations causing a decreased activity of their HPA axis and their tissue sensitivity to glucocorticoids, as well as by advantageous fetal programming and, or, decreased exposure to real or perceived stress (Chrousos Citation1998, Citation2000a; Phillips et al. Citation1998). In several studies favorable genetic variations in the glucocorticoid receptor gene, in which carriers of a particular polymorphism had peripheral target tissues with decreased sensitivity to glucocorticoids, were found to result in increased sensitivity of the same tissues to insulin and hence a healthier metabolic profile (van Rossum et al. Citation2002, Citation2004; van Rossum and Lamberts Citation2004; DeRijk and de Kloet Citation2005; Syed et al. Citation2006).
Beyond glucocorticoids and the metabolic syndrome
Despite their obvious importance, glucocorticoids and their signaling system are only one of several physiologic and molecular networks that participate in the development of obesity and the metabolic syndrome, with a resultant adverse effect on longevity. Other major hormones of the stress system and their receptors also participate in these phenomena () (Chrousos Citation1998; McEwen Citation1998).
The stress system includes brain nuclei, such as the paraventricular nucleus of the hypothalamus, and the brainstem locus caeruleus–norepinephrine/autonomic nervous system nuclei, and two powerful peripheral neuroendocrine limbs, the HPA axis and the systemic sympathetic and adrenomedullary systems. The main central molecular mediators of the stress system are corticotropin-releasing hormone, arginine vasopressin, and norepinephrine. The key peripheral molecular mediators are corticotropin, cortisol, arginine vasopressin, norepinephrine, epinephrine and interestingly, interleukin-6 (IL-6) (Chrousos Citation1995, Citation1998, Citation2000a). The genes that code for the synthesis, regulation, actions and metabolism of these mediators and their receptors are major participants in the adaptation to stress. The stress system is activated in a coordinated fashion during stress, influencing central and peripheral functions that are important for adaptation and survival (Chrousos Citation1998). Chronic activation of the stress system, however, is associated with many negative sequelae, including obesity/metabolic syndrome and loss of bone mineral density, i.e. osteopenia or osteoporosis (Chrousos Citation1998).
In addition to noninflammatory stress, even very mild, asymptomatic inflammation stimulates secretion of IL-6 and other inflammatory cytokines, while adipose tissue is a major source of circulating tumor necrosis factor-α and IL-6 (Chrousos Citation1995, Citation2000a; Papanicolaou et al. Citation1998). Both glucocorticoids and IL-6 synergistically stimulate the acute phase response, including C-reactive protein, fibrinogen and plasminogen activator inhibitor 1, all of which increase the ability of blood to coagulate and through their pro-atherosclerosis action have a negative effect on longevity. Thus, chronic stress, an indolent infection, an active autoimmune process and visceral obesity are all associated with mild hypercytokinemia and low-grade inflammation, which ultimately results in blood hypercoagulability, endothelial dysfunction, atherosclerosis and cardiovascular disease. Finally, it is evident that the metabolic syndrome, regardless of its cause, is a major risk factor for the development of diabetes type 2 and the polycystic ovary syndrome in patients with a genetic propensity to develop these very common disorders.
We have survived and been “selected” as a species because we were able to adapt to potentially lethal evolutionary stressors during our life on earth. Thus, selective pressures on our genome have produced adaptive changes that, at this time in our evolutionary history, have become somewhat maladaptive in a large proportion of the population (Chrousos Citation1995, Citation1998; Papanicolaou et al. Citation1998; Gold and Chrousos Citation2002) (). Thus, gene networks dedicated to adaptation and survival, with a finite number of members, are probably responsible for much of the contemporary nosology of Western societies presented in . Even though cancer is not included in this Table, it is evident that modification of the immune system and the inflammatory reaction by stress could increase the susceptibility of the organism to certain neoplasias.
Table III. Gene networks subserving functions important for human survival and species preservation, which may produce pathology in contemporary western societies.
Conclusions
To understand the roles of polymorphisms of multiple genes related to the HPA axis and the glucocorticoid signaling system in human physiology and pathophysiology, one will have to study large populations of normal subjects, including adequate numbers of representative racial and ethnic subpopulations, as well as populations of patients afflicted by states and diseases that may result from dysfunction of this system, which are summarized in Tables and . Once we have defined the crucial genes and their polymorphisms, we could employ existing new and constantly improving methods to screen for changes in the entire gene networks of choice, which, in the appropriate context, could predict the relative risk for developing common disorders. Also, granted that a large subgroup of this gene network plays a major role in regulating immune function, this information could be useful in predicting vulnerability to certain infections and tumors. Finally, this knowledge might help individualize medications and doses for subjects with the above conditions depending on their genetics in a rational way, an effort that is developing into the field of pharmacogenomics.
Acknowledgements
The work that has led to this article was funded by the Intramural Research Program of the National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD.
References
- Alevizaki M, Cimponeriu A, Lekakis J, Papamichael C, Chrousos GP. High anticipatory stress plasma cortisol levels and sensitivity to glucocorticoids predict severity of coronary artery disease in subjects undergoing coronary angiography. Metabolism 2007; 56(2)222–226
- Buemann B, Black E, Holst C, Toubro S, Echwald S, Pedersen O, Astrup A, Sorensen T. The N363S polymorphism of the glucocorticoid receptor and metabolic syndrome factors in men. Obes Res 2005; 13(5)862–867
- Buemann B, Vohl MC, Chagnon M, Chagnon YC, Gagnon J, Perusse L, Dionne F, Despres JP, Tremblay A, Nadeau A, Bouchard C. Abdominal visceral fat is associated with a BclI restriction fragment length polymorphism at the glucocorticoid receptor gene locus. Obes Res 1997; 5(3)186–192
- Charmandari E, Kino T, Chrousos GP. Glucocorticoids and their actions: An introduction. Ann NY Acad Sci 2004; 1024: 1–8
- Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 1995; 332(20)1351–1362
- Chrousos GP. Stressors, stress, and neuroendocrine integration of the adaptive response. Ann NY Acad Sci 1998; 851: 311–335, The 1997 Hans Selye Memorial Lecture
- Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: Neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord 2000a; 24(Suppl 2)S50–S55
- Chrousos GP. The stress response and immune function: Clinical implications. Ann NY Acad Sci 2000b; 917: 38–67, The 1999 Novera H. Spector lecture
- Chrousos GP. Glucocorticoid therapy. Endocrinology and metabolism4th ed., P Flig, L Frohman. McGraw-Hill, New York 2001; 609–632
- Chrousos GP. The glucocorticoid receptor gene, longevity, and the complex disorders of Western societies. Am J Med 2004; 117(3)204–207
- Chrousos GP, Kino T. Intracellular glucocorticoid signaling: A formerly simple system turns stochastic. Sci STKE 2005; 2005(304)pe48
- Chrousos GP, Charmandari E, Kino T. Glucocorticoid action networks—an introduction to systems biology. J Clin Endocrinol Metab 2004; 89(2)563–564
- Chrousos GP, Detera-Wadleigh SD, Karl M. Syndromes of glucocorticoid resistance. Ann Intern Med 1993; 119(11)1113–1124
- DeRijk R, de Kloet ER. Corticosteroid receptor genetic polymorphisms and stress responsivity. Endocrine 2005; 28(3)263–270
- Dobson MG, Redfern CP, Unwin N, Weaver JU. The N363S polymorphism of the glucocorticoid receptor: Potential contribution to central obesity in men and lack of association with other risk factors for coronary heart disease and diabetes mellitus. J Clin Endocrinol Metab 2001; 86(5)2270–2274
- Franchimont D, Kino T, Galon J, Meduri GU, Chrousos G. Glucocorticoids and inflammation revisited: The state of the art. Neuroimmunomodulation 2003; 10(5)247–260, NIH clinical staff conference
- Friedman TC, Mastorakos G, Newman TD, Mullen NM, Horton EG, Costello R, Papadopoulos NM, Chrousos GP. Carbohydrate and lipid metabolism in endogenous hypercortisolism: Shared features with metabolic syndrome X and NIDDM. Endocr J 1996; 43(6)645–655
- Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, O'Shea JJ, Chrousos GP, Bornstein SR. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J 2002; 16(1)61–71
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs. low CRH/NE states. Mol Psychiatry 2002; 7(3)254–275
- Huizenga NA, Koper JW, De Lange P, Pols HA, Stolk RP, Burger H, Grobbee DE, Brinkmann AO, De Jong FH, Lamberts SW. A polymorphism in the glucocorticoid receptor gene may be associated with and increased sensitivity to glucocorticoids in vivo. J Clin Endocrinol Metab 1998; 83(1)144–151
- Ichijo T, Voutetakis A, Cotrim AP, Bhattachryya N, Fujii M, Chrousos GP, Kino T. The Smad6-histone deacetylase 3 complex silences the transcriptional activity of the glucocorticoid receptor: Potential clinical implications. J Biol Chem 2005; 280(51)42067–42077
- Kino T, Chrousos GP. Tumor necrosis factor alpha receptor- and Fas-associated FLASH inhibit transcriptional activity of the glucocorticoid receptor by binding to and interfering with its interaction with p160 type nuclear receptor coactivators. J Biol Chem 2003; 278(5)3023–3029
- Kino T, Chrousos GP. Glucocorticoid and mineralocorticoid receptors and associated diseases. Essays Biochem 2004; 40: 137–155
- Kino T, Charmandari E, Chrousos GP. Basic and clinical implications of glucocorticoid action -focus on development. Horm Metab Res 2003a; 35(10)628–648, National Institutes of Health, Bethesda, Maryland, USA. june 17–18, 2003. Introduction and abstracts
- Kino T, De Martino MU, Charmandari E, Mirani M, Chrousos GP. Tissue glucocorticoid resistance/hypersensitivity syndromes. J Steroid Biochem Mol Biol 2003b; 85(2–5)457–467
- Kino T, Ichijo T, Chrousos GP. FLASH interacts with p160 coactivator subtypes and differentially suppresses transcriptional activity of steroid hormone receptors. J Steroid Biochem Mol Biol 2004; 92(5)357–363
- Kino T, Souvatzoglou E, De Martino MU, Tsopanomihalu M, Wan Y, Chrousos GP. Protein 14-3-3sigma interacts with and favors cytoplasmic subcellular localization of the glucocorticoid receptor, acting as a negative regulator of the glucocorticoid signaling pathway. J Biol Chem 2003; 278(28)25651–25656
- Kino T, Souvatzoglou E, Charmandari E, Ichijo T, Driggers P, Mayers C, Alatsatianos A, Manoli I, Westphal H, Chrousos GP, Segars JH. Rho family guanine nucleotide exchange factor Brx couples extracellular signals to the glucocorticoid signaling system. J Biol Chem 2006; 281(14)9118–9126
- Kino T, Vottero A, Chrousos GP. Mineralocorticoid and glucocorticoid receptors. Nuclear receptor and genetic disease, TP Burris, ERB McCabe. Academic Press, London 2001; 297–307
- Lanz RB, McKenna NJ, Onate SA, Albrecht U, Wong J, Tsai SY, Tsai MJ, O'Malley BW. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell 1999; 97(1)17–27
- Lin RC, Wang WY, Morris BJ. High penetrance, overweight, and glucocorticoid receptor variant: Case-control study. BMJ 1999; 319(7221)1337–1338
- Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell 2005; 18(3)331–342
- Marti A, Ochoa MC, Sanchez-Villegas A, Martinez JA, Martinez-Gonzalez MA, Hebebrand J, Hinney A, Vedder H. Meta-analysis on the effect of the N363S polymorphism of the glucocorticoid receptor gene (GRL) on human obesity. BMC Med Genet 2006; 7: 50–60
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med 1998; 338(3)171–179
- Miller WL, Chrousos GP. The adrenal cortex. Endocrinology and metabolism4th ed., P Felig, LA Frohman. McGraw-Hill, New York 2001; 387–524
- Panarelli M, Holloway CD, Fraser R, Connell JM, Ingram MC, Anderson NH, Kenyon CJ. Glucocorticoid receptor polymorphism, skin vasoconstriction, and other metabolic intermediate phenotypes in normal human subjects. J Clin Endocrinol Metab 1998; 83(6)1846–1852
- Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med 1998; 128(2)127–137
- Phillips DI, Barker DJ, Fall CH, Seckl JR, Whorwood CB, Wood PJ, Walker BR. Elevated plasma cortisol concentrations: A link between low birth weight and the insulin resistance syndrome?. J Clin Endocrinol Metab 1998; 83(3)757–760
- Rosmond R, Chagnon YC, Holm G, Chagnon M, Perusse L, Lindell K, Carlsson B, Bouchard C, Bjorntorp P. A glucocorticoid receptor gene marker is associated with abdominal obesity, leptin, and dysregulation of the hypothalamic-pituitary-adrenal axis. Obes Res 2000; 8(3)211–218
- Stevens A, Ray DW, Zeggini E, John S, Richards HL, Griffiths CE, Donn R. Glucocorticoid sensitivity is determined by a specific glucocorticoid receptor haplotype. J Clin Endocrinol Metab 2004; 89(2)892–897
- Syed AA, Irving JA, Redfern CP, Hall AG, Unwin NC, White M, Bhopal RS, Weaver JU. Association of glucocorticoid receptor polymorphism A3669G in exon 9beta with reduced central adiposity in women. Obesity (Silver Spring) 2006; 14(5)759–764
- Ukkola O, Rosmond R, Tremblay A, Bouchard C. Glucocorticoid receptor Bcl I variant is associated with an increased atherogenic profile in response to long-term overfeeding. Atherosclerosis 2001; 157(1)221–224
- van Rossum EF, Lamberts SW. Polymorphisms in the glucocorticoid receptor gene and their associations with metabolic parameters and body composition. Recent Prog Horm Res 2004; 59: 333–357
- van Rossum EF, Koper JW, Huizenga NA, Uitterlinden AG, Janssen JA, Brinkmann AO, Grobbee DE, de Jong FH, van Duyn CM, Pols HA, Lamberts SW. A polymorphism in the glucocorticoid receptor gene, which decreases sensitivity to glucocorticoids in vivo, is associated with low insulin and cholesterol levels. Diabetes 2002; 51(10)3128–3134
- van Rossum EF, Voorhoeve PG, te Velde SJ, Koper JW, Delemarre-van de Waal HA, Kemper HC, Lamberts SW. The ER22/23EK polymorphism in the glucocorticoid receptor gene is associated with a beneficial body composition and muscle strength in young adults. J Clin Endocrinol Metab 2004; 89(8)4004–4009
- Weaver JU, Hitman GA, Kopelman PG. An association between a Bc1I restriction fragment length polymorphism of the glucocorticoid receptor locus and hyperinsulinaemia in obese women. J Mol Endocrinol 1992; 9(3)295–300
- Wust S, Van Rossum EF, Federenko IS, Koper JW, Kumsta R, Hellhammer DH. Common polymorphisms in the glucocorticoid receptor gene are associated with adrenocortical responses to psychosocial stress. J Clin Endocrinol Metab 2004; 89(2)565–573