Abstract
Stressor activation of the sympathetic nervous system and the hypothalamic–pituitary–adrenal axis can have profound effects on bone and also appetite and metabolism. We tested in rats the response of plasma osteocalcin (pOC, a bone biomarker that is acutely stress responsive), corticosterone, and leptin to (1) ethanol consumption (5% w/v) in a liquid diet (compared with ad libitum and pair-fed rats), (2) acute restraint, and (3) acute (once, 1 h) and (4) chronic (1 h/day for 7 weeks) social aggression. Basal pOC concentration did not differ with ethanol diet or social interaction, but was elevated by both foot restraint immobilization (Imo) and restraint in wire mesh cylinders (WMR). As previously reported for chronic Imo, ingestion of ethanol blunted the pOC response to Imo. Plasma corticosterone concentration was increased by acute WMR and acute social interaction but was unaltered by chronic social interaction. Plasma leptin concentration was markedly increased by Imo in ad libitum fed, but only slightly in ethanol or pair-fed rats. In contrast, the data reflect significant differences between acute and chronic stressor effects since chronic social stress had little effect on pOC or plasma corticosterone, but tended to decrease leptin level in relation to dominance. Lack of significant impact of prolonged ethanol intake or social aggression suggests physiological adaptation.
Introduction
In earlier experiments, we documented decrements in bone growth in rats repeatedly subjected to foot restraint immobilization (Imo). These changes were attributed primarily to repeated stimulation of the hypothalamic–pituitary–adrenal (HPA) axis and associated increase of endogenous glucocorticoid levels (Patterson-Buckendahl et al. Citation2001). It is now clear that Imo and numerous other psychological stressors (as distinguished from those of mechanical forces on the musculoskeletal system that are also referred to as stress) as well as the neural inputs associated with the former stimuli have important influences on bone. These inputs are receiving increasing attention from bone researchers.
Activation of hormones and neurotransmitters of the sympathetic nervous system (SNS) is likely to influence the skeleton via receptors on both bone-forming osteoblasts and bone-resorbing osteoclasts. Moore et al. (Citation1993) described beta-adrenergic receptors on osteoblasts and demonstrated that beta-receptor agonists could stimulate bone resorption in organ culture. Togari et al. (Citation1997) reported gene expression for neuropeptide Y, substance P, calcitonin gene-related protein, and beta-adrenergic receptors on human osteoblasts. They also demonstrated that stimulation of osteoblasts by beta receptor agonists induced secretion of a factor that stimulated osteoclastogenesis (Takeuchi et al. Citation2001). Consequently, direct influence on bone formation and indirect influence on bone resorption are both stimulated by activation of the SNS.
In addition to the SNS, the HPA axis also exerts multiple regulatory influences on bone metabolism (Canalis Citation2005). It is well established that pharmacological doses of glucocorticoids are associated with osteoporosis. More relevant to the present investigations is that stimulation of glucocorticoid secretion in response to stressful stimuli has many effects on bone metabolism and can cause bone loss. Humans diagnosed with major depression may have osteoporosis associated with high cortisol levels (Michelson et al. Citation1996). The extreme glucocorticoid production associated with Cushing's disease is associated with osteoporosis (Shaker and Lukert Citation2005). Anorexic patients also tend to have very high cortisol and leptin levels, and osteoporosis (Hebebrand et al. Citation2006). Leptin, a peptide produced by adipose cells, was initially presumed to exert primary regulation of adipose tissue mass and metabolism. However, Ducy et al. (Citation2000) found that both leptin deficient and leptin receptor deficient mice are not only obese but also have increased bone formation leading to high bone mass, helping to explain the correlation of human bone mass with body mass. Takeda et al. (Citation2002) showed that the leptin effect was mediated through a hypothalamic relay and could be reversed by intracerebroventricular infusion of leptin.
In response to some kinds of stressors, humans as well as animals used to model alcohol abuse often increase consumption of alcohol and other drugs (Pohorecky Citation1981, Citation1990, Citation1991). Alcohol consumption may then activate adrenomedullary catecholamine synthesis, as shown by increased gene expression for enzymes of the catecholamine biosynthetic pathway and increased plasma levels of catecholamines (Pohorecky et al. Citation1974; Eisenhofer et al. Citation1983; Patterson-Buckendahl et al. Citation2004, Citation2005). When taken to an extreme, chronic alcohol consumption and abuse may ultimately result in osteoporosis (Bikle Citation1993), decreased bone mass and increased bone fragility (Sampson Citation1998; Hogan et al. Citation1999; Sampson and Spears Citation1999).
One way to monitor bone turnover is to measure plasma levels of the bone protein osteocalcin (OC) (Gundberg Citation2000). This extracellular calcium-binding protein is synthesized and secreted almost exclusively by osteoblasts with most being bound onto the bone mineral as it is deposited (Hauschka et al. Citation1989). However, a relatively consistent amount of OC is released into the circulation (plasma OC, pOC). The usefulness of pOC concentration as a biomarker for bone turnover is complicated by the less recognized finding that psychological stimuli having little to do directly with bone cell activity may, both acutely and chronically, affect synthesis and secretion of OC. Napal et al. (Citation1993) reported that in highly anxious patients hospitalized for stabilization prior to surgery pOC levels were significantly lower than normal, and this was attributable to elevated cortisol. Nicholson et al. (Citation2002) studied effects of three different anesthetics on patients undergoing hip replacement. One, etomidate, known to inhibit the cortisol elevation induced by anesthetics, successfully inhibited the patients' cortisol response, but failed to prevent a decrease in pOC after surgery. The authors concluded that other factors, such as cytokines or catecholamines must be responsible for the post-operative decline in pOC.
Environmental conditions considered anxiogenic, activating primarily the HPA axis, increase plasma corticosterone (the dominant glucocorticoid in rodents), and decrease pOC concentrations (Patterson-Buckendahl et al. Citation1988). However, rats subjected for 2 h to the stress of acute foot restraint Imo, which induces rapid activation of both the SNS and HPA axes, displayed elevations of levels of plasma catecholamines and glucocorticoid within a few minutes (Kvetnansky and Mikulaj Citation1970). Under these conditions, unlike with anxiogenic stressors, we have consistently seen a rapid and dramatic increase in pOC that peaks within 30–60 min and then decreases toward or below basal levels (Patterson-Buckendahl et al. Citation1995). When this stressor was applied repeatedly up to 42 times daily, the acute increase of pOC level was blunted but still present, and the animals had reduced bone growth (Patterson-Buckendahl et al. Citation2001).
Here we present data for pOC and leptin levels in rats stressed acutely by Imo and influenced by prior consumption of a liquid diet with or without alcohol. We also present data on effects of a milder restraint stimulus (10 min in wire mesh cylinders) on pOC and corticosterone levels as well as the effect on pOC, corticosterone, and leptin of acute and chronic social aggression in rats housed in groups of three or in isolation.
Methods
All protocols were reviewed and approved by Institutional Animal Care and Use Committee of Rutgers University. The Imo experiment was also reviewed and approved by the Animal Care and Use Committee of the Institute for Experimental Endocrinology (IEE), Bratislava.
Experiment 1-effect of alcohol diet on response to Imo
Experimental groups and diets
The protocol for this experiment has been published (Patterson-Buckendahl et al. Citation2005). In brief, male Sprague Dawley derived rats were obtained from Charles River Laboratories, Wiga, Germany. They were housed in the vivarium of the IEE, four per cage for 1 week prior to beginning the experiment. Temperature was maintained at 22 ± 2°C with a 12/12 h light/dark cycle, lights on at 6 AM. At the start of the diet study, the rats weighed 340 ± 2 g and were randomized by weight into three diet groups, housed two per cage on opposite sides of a Plexiglas™ divider that held the liquid diet feeding tubes. The present data are from subjects subjected to Imo as a part of an experiment previously reported (Patterson-Buckendahl et al. Citation2005). One group received ad libitum a liquid diet containing ethanol, 5% w/v, 35% of calories (ethanol, n = 8), one group received a similar isocaloric liquid diet ad libitum (Ad lib, n = 7), and the third group received the isocaloric diet restricted to the average volume of food consumed by the ethanol group on the previous day (pair-fed, n = 7). Rats were accustomed to the diet for 1 week, during which ethanol concentration was gradually increased in the ethanol group from 0 to 5%. For these rats, mean ( ± SEM) daily consumption during the 8 days prior to cannulation surgery (see below) was 8.56 ± 0.15 g ethanol/kg body weight. On the date of surgery, weight gained (mean ± SEM) by the Ad lib, ethanol, and pair-fed rats, respectively, was 65 ± 6, 13 ± 2, and 17 ± 6 g. Mean daily food consumption ( ± SEM) for the same rats was 130 ± 3 ml for Ad lib, 65 ± 1 ml for ethanol, and 77 ± 1 ml for pair-fed.
Surgery and blood sampling
On day 7 of full strength ethanol diet, each rat was anesthetized with a combination of ketamine (60–80 mg/kg BW) and xylazine (8–10 mg/kg BW) for placement of a permanent cannula in the tail artery (Chiueh and Kopin Citation1978). The cannula was passed subcutaneously along the dorsum, exteriorized at the base of the neck, and passed through a 30 cm long steel coil secured with bandage tape around the neck. From there, the cannula was passed through the cage top and closed with a 21-gauge needle and syringe. This procedure allows the rat free movement in the cage, shields the cannula from scratching or pulling, and after overnight recovery, allows blood samples to be obtained remotely through the cannula without handling the animal.
After overnight recovery, basal blood samples (0.5 ml each) were drawn without handling the rats. Following all blood sampling, fluid was replaced with an equal volume of isotonic saline containing 50 IU heparin per milli litre. After initial blood samples were obtained rats were acutely stressed by foot restraint according to the method of Kvetnansky and Mikulaj (Citation1970). Briefly, each rat was restrained in the prone position on the Imo board by securing its feet to raised supports with bandage tape. The rat's torso was unrestricted and capable of considerable movement and muscular contraction in attempts to free its feet. Additional blood samples were drawn remotely via the cannula during Imo after 5, 20, 60, and 120 min (2.5 ml in total). Plasma was separated from cells by centrifugation at 4°C and stored at − 70°C until analyzed. Stress hormone levels in these samples have been published (Patterson-Buckendahl et al. Citation2005).
Experiment 2-effect of social stress, triad and single housing
Subjects and environment
The protocol for this experiment has been described in detail elsewhere (Pohorecky et al. Citation2004). Briefly, 48 male Long Evans rats (Harlan Sprague Dawley, Indianapolis, Indiana) were individually housed in hanging wire-mesh, stainless steel cages for several weeks prior to the study, during which time they were extensively handled. Rats were transferred to the appropriate new housing cages on day 1 of the experiment and provided with Purina chow and water ad libitum throughout the study. The vivarium was kept at 21 ± 1° C, with controlled humidity and a reverse light/dark cycle (12 h each, lights off at 12:30 PM). The animal facilities were certified by AALAC.
Triad housing
Rats were housed three per experimental Plexiglas™ cage measuring 26 cm wide × 82 cm long × 19 cm high, with three individual compartments separated by two removable Plexiglas™ dividers. The bottom 6 cm of the dividers consisted of a wire mesh screen, allowing rats to maintain sensory contact even when separated. Dividers were removed daily for 1 h to allow the rats to interact and maintain rank status. Singly housed control rat cages were 25 × 25 × 30 cm, kept in a room separate from the triad caged animals. Although randomly assigned to triad or individual housing, triad subjects were matched based on body weights to avoid a possible effect of body size on dominance development. Initial body weights (mean ± SEM) were 554.8 ± 15.69, 542.3 ± 15.14, 546.4 ± 11.63 and 535.6 ± 13.02 g for dominant (n = 12), subdominant (n = 12), subordinate (n = 12) and singly housed (n = 10) rats, respectively, and were monitored daily.
Behavioral testing
To rate behaviors, assigned rats were placed into their novel cage and social interactions were recorded over the next 30 min. Using a modification of the method previously described (Pohorecky et al. Citation1999), the frequency of 24 behaviors was scored as previously reported (Pohorecky et al. Citation2004). These behaviors, classified into three major categories related to aggression, were re-scored multiple times throughout the study to monitor for potential changes in social hierarchy.
Restraint stress
Blood was collected from each rat by tail cut, following which the rat was restrained in a wire mesh cylinder for 10 min. Cylinders were 7.5 cm in diameter, 20 cm long, constructed from commercial wire mesh (0.5 in. mesh), closed at one end by an aluminum cup with a hole drilled in the center to assure breathing space. The rat entered the cylinder and was secured by crossed bamboo chopsticks to restrict its movement and prevent escape. A second tail blood sample was collected from the rat in the restrainer, prior to release to the home cage. This procedure was conducted 8 weeks prior to triad formation and again after 7 weeks of triad social housing.
Plasma analysis
Osteocalcin was quantified by the radioimmunoassay method of Patterson-Allen et al. (Citation1982). 125Iodine was obtained from Amersham for radiolabeling OC tracers. Intra-assay variation was less than 7%. Plasma corticosterone was quantified by 125I radioimmunoassay with commercial reagents (ICN Diagnostics, Orangeburg, NY #07-120103), for which intra-assay variation was less than 6%. Leptin was quantified by radioimmunoassay with commercial reagents (Rl-83K, Linco Research, St Charles Missouri), for which intra-assay variation was less than 5%. For all assays, samples from one experiment were assayed together.
Statistical analysis
Data were analyzed for statistical significance by ANOVA or ANOVA for repeated measures, with post-hoc analysis by Fishers LSD method when appropriate, with Statview software for Macintosh (SAS Institute). Data sets yielding values for p < 0.05 were considered significantly different.
Results
Experiment 1: Effect of foot restraint immobilization
As previously reported, the liquid diet had relatively little effect on plasma epinephrine and norepinephrine levels in pair-fed rats compared with Ad lib fed rats, and both groups responded with a typical elevation of approximately 20-fold above baseline after 5 min Imo. However, both catecholamines were approximately two-fold elevated in plasma obtained from the ethanol consuming rats before Imo. In plasma obtained during the subsequent Imo, both epinephrine and norepinephrine concentrations were also approximately twice as high as in Ad lib or pair-fed rats (Patterson-Buckendahl et al. Citation2005).
In the same rats, , the left panel shows that baseline pOC concentration (time 0) did not differ among the three diet groups (176 ± 12, 155 ± 11, and 184 ± 18 ng/ml for Ad lib, ethanol, and pair-fed respectively, p = 0.25). Consistent with previous experiments using this model, pOC was 43 ± 4% above baseline within 5 min of imposition of Imo (p < 0.0001) and returned toward baseline levels after 120 min. ANOVA with repeated measures indicated that the main effect of Imo was highly significant (F[4,76] = 49.5, p < 0.0001). There was a near-significant trend toward an effect of diet (F[2,76] = 3.053, p = 0.071), and a significant interaction of Imo with diet (F[8,76] = 2.127, p = 0.043). Although pair-fed tended to have higher pOC concentrations than Ad lib fed rats, they did not differ significantly (F[1,48] = 0.757, p = 0.40, in spite of greater food consumption by Ad lib rats (130 ± 3 vs. 77 ± 1 ml/day) (Patterson-Buckendahl et al. Citation2005). Because the pOC concentrations in Ad lib and pair-fed rats did not differ significantly, we evaluated the primary effects of ethanol consumption by comparing the ethanol group data with the combined data for rats not consuming ethanol, that is both Ad lib and pair-fed. This yielded a significant main effect of alcohol (F[1,80] = 5.41, p = 0.031) and a significant interaction of Imo and alcohol consumption (F[4,80] = 3.64, p < 0.01).
Figure 1 Plasma osteocalcin (left panel) and leptin (right panel) concentrations (mean ± SEM) in rats subjected to acute foot restraint Imo. Rats were provided unlimited access to a control liquid diet (Ad lib), a similar isocaloric diet containing 5% ethanol w/v (ethanol), or were restricted to the average amount of isocaloric liquid diet consumed by ethanol rats on the previous day (pair-fed). Each rat was implanted with a cannula in the tail artery to allow blood sampling without handling or otherwise disturbing the animal. Following collection of baseline samples, rats were subjected to Imo for 2 h, during which additional blood samples were collected. Numbers in parentheses indicate number of animals sampled for each measure. There were no differences in basal levels of either parameter. Within each treatment, (b) indicates significant difference from mean basal level for that group, p < 0.05. (e) and (p) indicate significant difference from ethanol or pair-fed at the time point measured, p < 0.05.
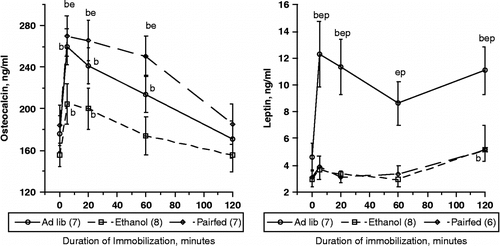
A more striking response was seen in plasma leptin concentration after Imo (, right panel). Here also, baseline leptin concentrations did not differ significantly among the three groups (4.59 ± 1.04, 2.96 ± 0.56, 3.05 ± 0.59 ng/ml for Ad lib, ethanol, and pair-fed respectively, p = 0.27). However, analysis of samples obtained during Imo, using repeated measures ANOVA, indicated a significant main effect of Imo (F[4,64] = 6.05, p < 0.001), and of diet (F[2,64] = 14.7, p < 0.001, and a marginal interaction of diet and Imo (F[8,64] = 1.94, p = 0.069). Ad lib rats differed significantly at all times during Imo from both ethanol fed and pair-fed (p < 0.001 for both). However, ethanol and pair-fed did not differ from each other at any time point. Analyzed separately, Ad lib rat leptin concentrations increased by 234% within 5 min Imo, remained significantly elevated after 20, dipped slightly after 60 min, and increased again by 120 min of Imo (p < 0.01). Leptin concentrations in the ethanol group were significantly 26% above basal levels after 5 and 20 min Imo (p < 0.05), had returned to baseline by 60 min, then were again elevated after 120 min (p < 0.05). Although pair-fed rat leptin levels were 27% above baseline levels after 5 min, the change from baseline did not differ significantly at that or any subsequent sampling time during Imo (p>0.1).
Experiment 2—effects of social interaction and housing
Response to acute restraint
Restraint for 10 min in wire mesh cylinders prior to initiation of social interactions between rats induced a typical increase in both plasma corticosterone and pOC concentrations ( and C). Values were analyzed with regard to eventual social rank to determine whether there was any predisposition to aggression-related differences. Repeated measures ANOVA indicated a significant main effect of restraint on plasma corticosterone concentration (F[1,40] = 39.3, p < 0.0001), but no effect of future housing condition (triad, by rank, or single, F[3,40] = 0.305, p = 0.82, and no interaction of rank and restraint (F[3,40] = 0.57, p = 0.64). Similarly, restraint induced a significant increase in pOC (F[1,40] = 28.5, p < 0.0001). There was no significant effect of future rank (F[3,40] = 2.16, p = 0.11) or interaction with restraint (F[3,40] = 0.831, p = 0.48).
Figure 2 Effect of 10 min restraint in wire mesh cylinders on plasma corticosterone and osteocalcin concentrations (mean ± SEM). Panels A and C show values from blood samples taken from tail cuts before and after 10 min restraint 8 weeks prior to formation of triad social housing. Following initial restraint testing, the dominance ranking was determined by observation of behaviors during 30 min interaction. After 8 weeks, singly housed control rats were transferred to single cages and placed in a separate room. Triad-housed rats were placed in cages as described and allowed to interact directly for 1 h per day, but were separated by dividers that allowed sensory but not physical contact for the remaining 23 h. Panels B and D are from blood samples taken 7 weeks after initiation of triad housing, 15 weeks after initial restraint test. Significant differences between pre- (dark shaded bars) and post-restraint (light shaded bars) samples within housing and social rank status are indicated by *p < 0.05; **p < 0.01; ***p < 0.001. Values are shown relative to established social rank in triads, indicated by “Dom”, socially dominant rats, “Subdom” subdominant rats, “Subord”, subordinant rats, or “Single”, singly housed rats. The percent increase in corticosterone induced by restraint of long-term triad housed rats, indicated by numbers in post-restraint bars, was significantly greater (p < 0.01) than in rats tested 8 weeks prior to triad formation. The percent increase in osteocalcin induced by restraint did not differ between tests and is not shown.
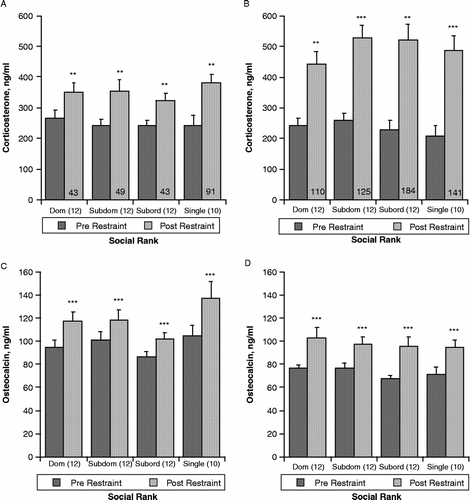
The restraint procedure was repeated 7 weeks after initiation of triad housing (15 weeks after initial restraint procedure), when social hierarchy was well established. As shown in and D, 10 min of restraint again induced significant increases in both plasma corticosterone (F[3,36] = 89.9, p < 0.0001) and pOC concentrations (F[1,33] = 29.8, p < 0.0001). Samples from some rats were not analyzed due to insufficient sample size. Again there were no significant rank-related differences or any interaction between rank and restraint.
There was, however, a significant effect of long-term triad housing on the magnitude of the response of plasma corticosterone to restraint imposed pre- and post-triad formation. As can be seen in and B, baseline corticosterone levels did not differ between restraint tests; however, as indicated by the percent increase shown on the post-restraint bars, the response of long-term triad housed and singly housed control rats to the second restraint was significantly greater than to the first (F[1,33] = 25.4, p < 0.001).
In contrast to corticosterone, although all post-restraint levels of pOC were greater than pre-restraint, there was no significant difference between the two restraint procedures in the magnitude of the pOC response. It should be noted that lower pre-restraint concentrations of osteocalcin for the second restraint and in final trunk blood levels reflect a well documented age-related decline in this parameter. This decrease does not, however, diminish the capability of pOC to respond to an acute stressor.
Effect of acute social interaction
We evaluated the effect of acute social interaction itself on corticosterone and pOC before and 24 h after formation of triad housing groups. As depicted in , left panel, ANOVA indicated that plasma corticosterone concentration was significantly elevated in triad-housed rats 24 h following initial social interaction (F[1,33] = 22.4, p < 0.0001). Student's t-test for within rank changes indicated that, although as a group, corticosterone was increased in triad-housed rats, only the subdominant rats differed significantly from their basal levels (p < 0.01).
Figure 3 Effect of social interaction at the initiation of triad housing on plasma corticosterone (A) and osteocalcin (B) concentrations (mean ± SEM). Social rank was determined as defined in , based on observations during this 1 h test. Blood was obtained from tail cuts one day before change of housing conditions and again 24 h after rats were placed in their respective triad or single caging. Values are shown relative to established social rank in triads, or single housing. **p < 0.01, from baseline within social rank.
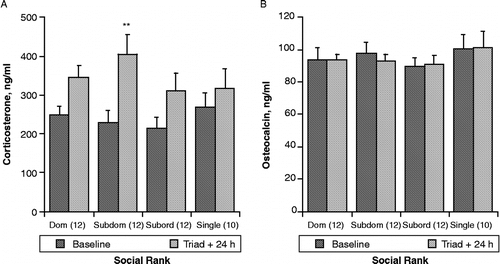
Concentrations of pOC in blood samples taken before and 24 h after initiation of triad housing ( right panel) did not differ due to change in housing status (F[1,33] = 0.077, p = 0.78).
Effect of long-term housing on final plasma corticosterone, leptin, and osteocalcin concentrations
shows corticosterone, leptin, and pOC levels in rats housed for 7 weeks in either triad cages and allowed social interaction for 1 h per day, or isolated one per cage in a separate room. Corticosterone in triad rats, killed immediately (by conscious decapitation) following their daily interaction, did not differ significantly relative to either social rank within triads (F[2,33] = 0.422, p = 0.66) or with triad housing vs. single housing (F[3,43] = 1.18, p = 0.33, ). As shown in , there was a near significant trend for significant differences in plasma leptin among the four groups (F[3,39] = 2.47, p = 0.076, with singly housed rats having the highest and triad dominant rats having the lowest leptin concentrations. shows plasma osteocalcin levels, which did not differ significantly with either rank or housing status (F[3,41] = 0.239, p = 0.87).
Figure 4 Plasma corticosterone (A), leptin (B), and osteocalcin (C) concentrations (mean ± SEM) in trunk blood collected after a final 1 h social interaction at the termination of the experiment. Social rank was defined as described in , and was re-evaluated several times during the experiment to monitor potential changes in social hierarchy. Values are shown relative to established social rank in triads, or single housing. Corticosterone and osteocalcin concentrations did not differ among groups; however, there was a trend toward a dominance related decrease in leptin concentrations, p = 0.072.
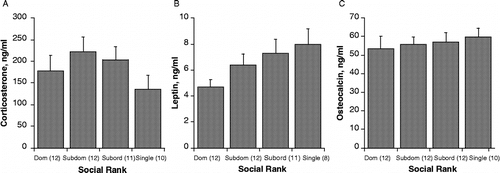
As noted above, pOC decreased significantly over the entire course of the experiment from the baseline level in samples taken prior to the first restraint session until those in trunk blood obtained when the experiment was terminated. This normal decline more likely reflects a slowing of bone formation rate as the animal ages rather than any effect of social or isolation housing.
Discussion
The data presented here show that responses of the parameters we studied differed significantly with respect to the type of stressor (alcohol, restraint, social aggression), and to the duration of stress (acute vs. chronic stimuli). In rodents, restraint by either Imo or confinement in wire mesh or plastic containers reliably induces increased secretion of corticosterone, catecholamines, and other stress-associated hormones. Furthermore, there was a difference in the magnitude of the response to an acute stressor following the chronic exposure to a heterotypic stressor.
Stressor effects on plasma osteocalcin
We previously reported that ethanol-consuming rats from experiment 1 had significantly elevated basal levels of plasma norepinephrine and epinephrine and marginally increased basal corticosterone levels. There were greater increases in these hormones in ethanol rats due to Imo than in either Ad lib fed or pair-fed rats. In addition, adrenomedullary gene expression for enzymes of the catecholamine synthetic pathway was also significantly increased by ethanol in the diet, and potentiated by Imo. Those data suggested that ethanol consumption itself constituted a stressor that markedly activates SNS output, and that the subsequent novel stress of Imo further increased that effect (Patterson-Buckendahl et al. Citation2005).
The effect of the liquid diets on pOC concentration was less pronounced than on the stress hormones. Basal pOC did not differ among the three treatments, although there was a significant interaction of diet with the subsequent response to Imo. The blunted response of pOC to Imo of rats that consumed high amounts of ethanol for over 1 week prior to Imo, compared with controls consuming isocaloric diet suggests a possible down-regulation of osteoblastic activity in the days prior to Imo. This could reflect a decrease in bone formation rate, which would be expected to lead to a decreased amount of bone after even longer exposure to alcohol, as has been documented with long term alcohol consumption in animal models as well as in humans (Bikle Citation1993; Dyer et al. Citation1998; Sampson Citation1998; Hogan et al. Citation1999; Sampson and Spears Citation1999). The exact origin of the stress-induced pOC increase is unknown. These increases occur too rapidly to be a result of new synthesis. It has been suggested that they might arise directly from the bone surface as a result of non-cellular release related to or regulated by plasma ionized calcium levels (Gundberg et al. Citation1991). If that were the case, then decreased synthesis and bone accumulation during the previous week could decrease availability of exposed bone surface protein during stress.
That chronic stress could affect bone formation is less appreciated. A similar blunting of the pOC response to Imo was seen in rats subjected to repeated Imo from 7 to more than 40 times (Patterson-Buckendahl et al. Citation1995, Citation2001). Because glucocorticoids are known to decrease OC synthesis (Beresford et al. Citation1984), the reduced levels of pOC in rats exposed to repeated Imo had been attributed to their repeatedly increased corticosterone levels. However, subsequent data showing increased adrenomedullary expression of genes for catecholamine synthetic enzymes (Kvetnansky et al. Citation2003; Kvetnansky Citation2004; Patterson-Buckendahl et al. Citation2005) suggested that increased catecholamine production might also have played an important role in reducing the pOC response to both repeated Imo and to Imo following chronic alcohol consumption. Importantly, Yirmiya et al. (Citation2006) documented decreased bone formation, increased plasma corticosterone, increased norepinephrine in the bone, and bone loss in mice due to chronic mild stress (a model of depression). However, if the mice were given propranolol, a beta-adrenergic receptor antagonist, thus blocking effects of elevated norepinephrine, the bone loss was reversed. Although neither pOC nor bone OC was measured in those mice, OC synthesis and therefore pOC level, would likely have been decreased in relation to the elevated hormones induced by chronic stress.
Social interaction represents a completely different type of stressor. Predictably, both plasma corticosterone and pOC were significantly elevated by 10 min of restraint in wire mesh cylinders prior to triad formation. When the same stressor was applied after 7 weeks of triad housing, both corticosterone and pOC responses were accentuated regardless of social rank, suggesting that in rats chronically experiencing aggressive social interactions, the response to the novel stressor is enhanced. Such sensitization to a novel stressor in chronically stressed animals has been reported by Kvetnansky (Citation2004) and Kvetnansky et al. (Citation2003) among many others.
The lack of difference in pOC concentration in rats 24 h after initiation of social aggression at the time of triad formation probably indicates a return to normal levels during the 23 h following the initial interaction, similar to that seen in samples taken 24 h following repeated Imo (Patterson-Buckendahl et al. Citation2001). Because pOC peaks within the first hour of Imo and then returns toward normal, it is possible that samples taken within 10–30 min of initiation of social interactions would show increases, especially in the most aggressive groups.
The levels of corticosterone and pOC in blood obtained at the termination of the experiment showed no significant differences relative to either housing or social interactions. This is particularly interesting, given that rats were removed from the social activity and killed immediately, with no opportunity for recovery from social interactions. Alternatively, because aggressive behavior in long-term colonies is sporadic, they may have already returned to basal levels before being killed. Dominance hierarchy was maintained and adrenomedullary gene expression for enzymes for catecholamine synthesis was increased in a rank-related manner (Pohorecky et al. Citation2004). In spite of that activation, the glucocorticoid and osteocalcin responses apparently had adapted to the established social order.
Stressor effects on leptin
Leptin, initially presumed to act primarily on regulation of adipose tissue, is now appreciated for many more actions, both in the central nervous system and in the periphery. Because food consumption by the rats given liquid diet in experiment 1 differed greatly, basal plasma leptin levels might have been expected to differ among diet groups. This was not the case. The subsequent marked elevation of leptin by Imo of Ad lib fed rats may be related to regulation of leptin release by catecholamines (Kosaki et al. Citation1996; Nye et al. Citation2000; Tsigos and Chrousos Citation2002), and would tend to decrease appetite, a well known effect of severe stress such as Imo (Sapolsky et al. Citation2000). On the other hand, the ethanol and pair-fed rats exhibited a blunted leptin response to Imo. Possibly, increased catecholamine synthesis, as reflected by increased gene expression for the synthetic enzymes (Patterson-Buckendahl et al. Citation2005), is responsible for this decreased response. The Ad lib fed rats had ample food and a greater weight gain during the experiment, as well as a lesser change in gene expression for tyrosine hydroxylase, dopamine beta-hydroxylase, and phenylethanolamine N-methyl transferase than that of either ethanol or pair-fed rats, and would likely better tolerate loss of appetite.
The effects of social aggression tended toward a significant rank relationship, with singly housed rats having the highest leptin levels and the dominant rats having the lowest levels. These trends were similar to those of adrenal gene expression for enzymes of the catecholamine synthetic pathway (Pohorecky et al. Citation2004), though not directly correlated. The triad groups were not equally aggressive, and leptin levels might show greater differences if all triads were highly aggressive.
The unremarkable differences in pOC, corticosterone, and leptin in long-term triad and singly housed rats suggest that there would be negligible effects on bone metabolism after this length of time. In fact, no differences in weight or OC content of the third lumbar vertebrae were noted in these rats (Patterson-Buckendahl, unpublished observations). The acute response of pOC, leptin, and corticosterone to either mode of restraint used in these experiments is less likely to represent a major effect on bone and more likely that of an extraskeletal influences. An extraskeletal role for pOC remains to be elucidated.
Acknowledgements
The authors would like to thank Dale Buckendahl for fabricating and adapting the cage dividers to hold feeding tubes for the liquid diet study. Research was conducted with support from: National Institutes of Health, National Institute for Alcoholism and Alcohol Abuse: R21 AA 12705-01 and R21 AA 14399-01A2 (PP-B), Slovak Grant Agency for Science #5125, SP Grant 51/0280800-2004, and APVV grant No. 0148-06 (RK), and funds from the Center of Alcohol Studies, Rutgers, The State University of New Jersey (LAP).
References
- Beresford JN, Gallagher JA, Poser JW, Russell RG. Production of osteocalcin by human bone cells in vitro. Effects of 1,25(OH)2D3, 24,25(OH)2D3, parathyroid hormone, and glucocorticoids. Metab Bone Dis Relat Res 1984; 5(5)229–234
- Bikle DD. Alcohol-induced bone disease. World Rev Nutr Diet 1993; 73: 53–79
- Canalis E. Mechanisms of glucocorticoid action in bone. Curr Osteoporos Rep 2005; 3(3)98–102
- Chiueh CC, Kopin IJ. Hyperresponsivitiy of spontaneously hypertensive rat to indirect measurement of blood pressure. Am J Physiol 1978; 234(6)H690–H695
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell 2000; 100(2)197–207
- Dyer SA, Buckendahl P, Sampson HW. Alcohol consumption inhibits osteoblastic cell proliferation and activity in vivo. Alcohol 1998; 16(4)337–341
- Eisenhofer G, Lambie DG, Johnson RH. Effects of ethanol on plasma catecholamines and norepinephrine clearance. Clin Pharmacol Ther 1983; 34(2)143–147
- Gundberg CM. Biochemical markers of bone formation. Clin Lab Med 2000; 20(3)489–501
- Gundberg CM, Grant FD, Conlin PR, Chen CJ, Brown EM, Johnson PJ, LeBoff MS. Acute changes in serum osteocalcin during induced hypocalcemia in humans. J Clin Endocrinol Metab 1991; 72(2)438–443
- Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: Vitamin K-dependent proteins in bone. Physiol Rev 1989; 69(3)990–1047
- Hebebrand J, Muller TD, Holtkamp K, Herpertz-Dahlmann B. The role of leptin in anorexia nervosa: Clinical implications. Mol Psychiatry 2006; 12(1)23–25
- Hogan HA, Groves JA, Sampson HW. Long-term alcohol consumption in the rat affects femur cross-sectional geometry and bone tissue material properties. Alcohol Clin Exp Res 1999; 23(11)1825–1833
- Kosaki A, Yamada K, Kuzuya H. Reduced expression of the leptin gene (ob) by catecholamine through a G(S) protein-coupled pathway in 3T3-L1 adipocytes. Diabetes 1996; 45(12)1744–1749
- Kvetnansky R. Stressor specificity and effect of prior experience on catecholamine biosynthetic enzyme phenylethanolamine N-methyltransferase. Ann N Y Acad Sci 2004; 1032: 117–129
- Kvetnansky R, Mikulaj L. Adrenal and urinary catecholamines in rats during adaptation to repeated immobilization stress. Endocrinology 1970; 87(4)738–743
- Kvetnansky R, Rusnak M, Dronjak S, Krizanova O, Sabban EL. Effect of novel stressors on tyrosine hydroxylase gene expression in the adrenal medulla of repeatedly immobilized rats. Neurochem Res 2003; 28(3–4)625–630
- Michelson D, Stratakis C, Hill L, Reynolds J, Galliven E, Chrousos G, Gold P. Bone mineral density in women with depression. N Engl J Med 1996; 335(16)1176–1181
- Moore RE, Smith CK, Bailey CS, Voelkel EF, Tashjian AH, Jr. Characterization of beta-adrenergic receptors on rat and human osteoblast-like cells and demonstration that beta-receptor agonists can stimulate bone resorption in organ culture. Bone Miner 1993; 23(3)301–315
- Napal J, Amado JA, Riancho JA, Olmos JM, Gonzalez-Macias J. Stress decreases the serum level of osteocalcin. Bone Miner 1993; 21(2)113–118
- Nicholson G, Bryant AE, Macdonald IA, Hall GM. Osteocalcin and the hormonal, inflammatory and metabolic response to major orthopaedic surgery. Anaesthesia 2002; 57(4)319–325
- Nye EJ, Bornstein SR, Grice JE, Tauchnitz R, Hockings GI, Strakosch CR, Jackson RV, Torpy DJ. Interactions between the stimulated hypothalamic–pituitary–adrenal axis and leptin in humans. J Neuroendocrinol 2000; 12(2)141–145
- Patterson-Allen P, Brautigam CE, Grindeland RE, Asling CW, Callahan PX. A specific radioimmunoassay for osteocalcin with advantageous species crossreactivity. Anal Biochem 1982; 120(1)1–7
- Patterson-Buckendahl PE, Grindeland RE, Shakes DC, Morey-Holton ER, Cann CE. Circulating osteocalcin in rats is inversely responsive to changes in corticosterone. Am J Physiol 1988; 254(5 Pt 2)R828–R833
- Patterson-Buckendahl P, Blakley G, Kubovcakova L, Krizanova O, Pohorecky LA, Kvetnansky R. Alcohol alters rat adrenomedullary function and stress response. Ann N Y Acad Sci 2004; 1018: 173–182
- Patterson-Buckendahl P, Kubovcakova L, Krizanova O, Pohorecky LA, Kvetnansky R. Ethanol consumption increases rat stress hormones and adrenomedullary gene expression. Alcohol 2005; 37(3)157–166
- Patterson-Buckendahl P, Kvetnansky R, Fukuhara K, Cizza G, Cann C. Regulation of plasma osteocalcin by corticosterone and norepinephrine during restraint stress. Bone 1995; 17(5)467–472
- Patterson-Buckendahl P, Rusnak M, Fukuhara K, Kvetnansky R. Repeated immobilization stress reduces rat vertebral bone growth and osteocalcin. Am J Physiol Regul Integr Comp Physiol 2001; 280(1)R79–R86
- Pohorecky LA. The interaction of alcohol and stress. A review. Neurosci Biobehav Rev 1981; 5(2)209–229
- Pohorecky LA. Interaction of ethanol and stress: Research with experimental animals—an update. Alcohol Alcohol 1990; 25(2–3)263–276
- Pohorecky LA. Stress and alcohol interaction: An update of human research. Alcohol Clin Exp Res 1991; 15(3)438–459
- Pohorecky LA, Blakley GG, Kubovcakova L, Krizanova O, Patterson-Buckendahl P, Kvetnansky R. Social hierarchy affects gene expression for catecholamine biosynthetic enzymes in rat adrenal glands. Neuroendocrinology 2004; 80(1)42–51
- Pohorecky LA, Jaffe LS, Berkeley HA. Effects of ethanol on the adrenal medulla of the rat. Pharmacology 1974; 12(6)340–346
- Pohorecky LA, Skiandos A, Zhang X, Rice KC, Benjamin D. Effect of chronic social stress on delta-opioid receptor function in the rat. J Pharmacol Exp Ther 1999; 290(1)196–206
- Sampson HW. Alcohol's harmful effects on bone. Alcohol Health Res World 1998; 22(3)190–194
- Sampson HW, Spears H. Osteopenia due to chronic alcohol consumption by young actively growing rats is not completely reversible. Alcohol Clin Exp Res 1999; 23(2)324–327
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 2000; 21(1)55–89
- Shaker JL, Lukert BP. Osteoporosis associated with excess glucocorticoids. Endocrinol Metab Clin North Am 2005; 34(2)341–356, viii–ix
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002; 111(3)305–317
- Takeuchi T, Tsuboi T, Arai M, Togari A. Adrenergic stimulation of osteoclastogenesis mediated by expression of osteoclast differentiation factor in MC3T3-E1 osteoblast-like cells. Biochem Pharmacol 2001; 61(5)579–586
- Togari A, Arai M, Mizutani S, Mizutani S, Koshihara Y, Nagatsu T. Expression of mRNAs for neuropeptide receptors and beta-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci Lett 1997; 233(2–3)125–128
- Tsigos C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res 2002; 53(4)865–871
- Yirmiya R, Goshen I, Bajayo A, Kreisel T, Feldman S, Tam J, Trembovler V, Csernus V, Shohami E, Bab I. From the cover: Depression induces bone loss through stimulation of the sympathetic nervous system. Proc Natl Acad Sci USA 2006; 103(45)16876–16881