Abstract
Lipopolysaccharide (LPS), an endotoxin released from the outer membranes of Gram-negative bacteria, triggers cells to synthesize and release inflammatory cytokines that may progress to septic shock in vivo. We found that LPS enhances tetrahydrobiopterin (BH4) biosynthesis by inducing the biosynthetic enzyme GTP cyclohydrolase I (GCH) in vitro in the mouse neuroblastoma cell line N1E-115. Furthermore, we observed that gene expression of GCH in the locus coeruleus (LC) in mice was enhanced by peripheral administration of LPS, resulting in increased concentrations of BH4, and norepinephrine, and its metabolite 4-hydroxy-3-methoxyphenylglycol (MHPG). These results suggest that tyrosine hydroxylase (TH) activity is increased by increased content of BH4 due to enhanced mRNA expression of GCH in the LC resulting in the increase in norepinephrine in the LC during endotoxemia. LPS in blood may act as a stressor to increase norepinephrine biosynthesis in the mouse LC.
| Abbreviations | ||
| AADC | = | aromatic l-amino acid decarboxylase |
| BH4 | = | tetrahydrobiopterin |
| DA | = | dopamine |
| DBH | = | dopamine β-hydroxylase |
| GCH | = | GTP cyclohydrolase I |
| GTP | = | guanosine triphosphate |
| IL-1β | = | interleukin-1β |
| L-DOPA | = | 3,4-dihydroxy-l-phenylalanine |
| l-Tyr | = | l-tyrosine |
| NE | = | norepinephrine |
| NH2P3 | = | dihydroneopterin triphosphate |
| PPH4 | = | 6-pyruvoyl-tetrahydropterin |
| PTPS | = | 6-pyruvoyltetrahydropterin synthase |
| SPR | = | sepiapterin reductase |
| TH | = | tyrosine hydroxylase |
| TNF-α | = | tumour necrosis factor-α |
Introduction
GTP cyclohydrolase I (GCH) catalyzes the first and rate-limiting step of biosynthesis of tetrahydrobiopterin (BH4, (6R)-l-erythro-1′, 2′-dihydroxypropyl-2-amino-4-hydroxy-5,6,7,8-tetrahydropteridine; Kaufman Citation1963; Matsuura et al. Citation1985). BH4 is the natural cofactor of pteridine-dependent monooxygenases, such as phenylalanine hydroxylase (PAH), tyrosine hydroxylase (TH), tryptophan hydroxylase (TPH). BH4 is also an essential cofactor of nitric oxide synthase (NOS) (Nagatsu and Ichinose Citation1999). These pteridine-dependent monooxygenases produce neurotransmitters and hormones in the brain and periphery: e.g. TH for catecholamines (dopamine, norepinephrine and epinephrine); TPH for serotonin and melatonin; and NOS for NO. BH4 is synthesized de novo from GTP by GCH, 6-pyruvoyltetrahydropterin synthase (PTPS) and sepiapterin reductase (SPR) ().
Figure 1 Schematic model of the activation of the norepinephrine synthesis pathway in the mouse locus coeruleus triggered by an i.p. administration of lipopolysaccharide (LPS; endotoxin).
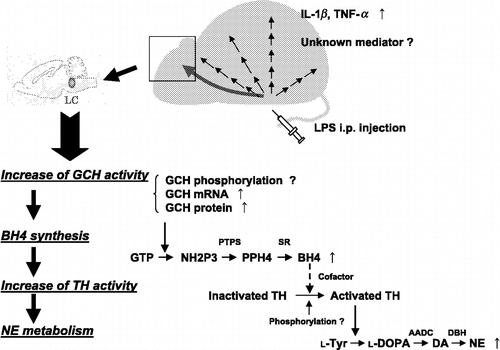
The expression of genes for catecholamine system enzymes is altered under stress. Catecholamine biosynthetic enzymes, especially TH, dopamine β-hydroxylase (DBH) and phenylethanolamine N-methyltransferase (PNMT), are induced under strong stressful stimulation such as immobilization stress (Kvetnansky et al. Citation1970a,Citationb). The transcription of GCH that supplies the BH4 cofactor for TH is also increased by the glucocorticoid stress hormone in PC12 cells in cultures and in rat adrenal medulla in vivo (Serova et al. Citation1997), and in the locus coeruleus (LC) and the nucleus tractus solitarii (NTS) in vivo by immobilization stress (Serova et al. Citation1999, Citation2005).
GCH upregulation increases catecholamine levels indirectly as the result of increased BH4 concentration by increasing the activity of the rate-limiting enzyme TH (Nagatsu Citation1981) and also by affecting stability of the TH protein (Flatmark et al. Citation1999; Sumi-Ichinose et al. Citation2001). For basic studies on the regulation of catecholamine system enzymes under stress, we cloned full-length complementary DNAs (cDNAs) and genomic DNAs of the human and mouse catecholamine-synthesizing enzymes, i.e. TH, aromatic l-amino acid decarboxylase (AADC), DBH and PNMT (Nagatsu Citation1991; Nagatsu et al. Citation1995). We also cloned cDNAs (Togari et al. Citation1992) and genes for human and mouse GCH, the cofactor that regulates TH (Ichinose et al. Citation1995).
We found that lipopolysaccharide (LPS), which induces inflammation, increased BH4 levels and the gene expression of GCH, PTPS and SPR in mouse neuroblastoma N1E-115 cells in cultures (Mori et al. Citation1997) and that LPS given by intraperitoneal (i.p.) administration in mice increased the contents of BH4, norepinephrine and its metabolites 4-hydroxy-3-methoxyphenylglycol (MHPG) and 4-hydroxy-3-methoxymandelic acid (VMA) in the LC, as well as increased mRNA expression of GCH (Kaneko et al. Citation2001). The inflammation that is induced by LPS in the circulation blood well understood to be a stressor, and increased transcription of GCH in the LC may be one of the earliest events in the response to inflammation stress. Here we review the effects of LPS in cell culture and in vivo on the mouse LC.
Lipopolysaccharide (LPS) enhances tetrahydrobiopterin (BH4) biosynthesis in a murine neuroblastoma cell line
LPS released from the outer membrane of Gram-negative bacteria triggers cells to synthesize and release the inflammatory mediators that may progress to septic shock in vivo (Glauser et al. Citation1991). Werner-Felmayer et al. (Citation1993) showed widespread induction of GCH, which finally led to increased total biopterin levels in tissues including the brain and adrenal gland, in rats after peripheral administration of LPS. Moreover, Sakai et al. (Citation1995) reported that the synthesis of both BH4 and NO in microglia was induced by LPS and/or cytokines closely in parallel.
As reviewed by Werner-Felmayer et al. (Citation2002), the pharmacological effects of manipulating intracellular BH4 levels have been examined in non-neural cells such as rat cardiac myocytes and the murine proerythroblastoid Friend erythroleukemia cell line. As the first step toward the full understanding of the mechanisms controlling the LC under an inflammatory cascade of events triggered by an LPS challenge, we estimated the modulation of the BH4-synthesizing enzyme system in cultured neuronal cells incubated with LPS (Ota et al. Citation1996). Murine neuroblastoma N1E-115 cells were used for the purpose. The cell line was cloned as being predominantly adrenergic in nature (Amano et al. Citation1972) from C-1300 neuroblastoma cells which had spontaneously arisen in the A/J mouse and had been sustained in situ or in tissue culture. N1E-115 cells possess abundant amounts of BH4 (Bräutigam et al. Citation1984) and TH protein revealing almost identical properties of the catalytic reaction to those of purified TH from normal tissue (Richelson Citation1976). The cell line can synthesize norepinephrine in the absence of an inhibitor of aromatic l-amino acid decarboxylase (Amano et al. Citation1972). The effects of dopamine, norepinephrine and serotonin on N1E-115 cells have been intensively studied (Kato and Narahashi Citation1982; Neijt et al. Citation1986; Horn and Mirkin Citation1990). Activation of neuronal N1E-115 cells with 1 μg/ml LPS resulted in significant increases in intracellular BH4 content and the activity (Vmax) of GCH. We then analyzed the signal transduction pathway from LPS to the BH4 biosynthetic system in N1E-115 cells with specific inhibitors. We first used oxanosine, an IMP dehydrogenase inhibitor, which results in the exhaustion of intracellular GTP and in specific inhibition of intracellular events mediated by G-proteins, including Ras (Itoh et al. Citation1989; Watanabe et al. Citation1994). Furtheremore, in order to elucidate whether or not protein tyrosine kinases are involved in the intracellular signaling pathway that underlies the process, we used two inhibitors of protein tyrosine kinases: erbstatin (an inhibitor of receptor-type tyrosine kinase; Umezawa et al. Citation1986); and herbimycin-A (an inhibitor of non-receptor-type tyrosine kinase; Uehara et al. Citation1985). Our data indicate the following conclusions: (a) protein tyrosine kinase systems are involved in mediating LPS signal to BH4 production and (b) there might be a cross-talk between GTP-binding protein and the protein tyrosine kinase system in mediating the LPS signal. These observations suggested that a neuronal cell such as N1E-115, which barely expresses LPS receptor CD14 on its cell surface, responded to LPS like macrophages, monocytes and microglia in the absence of soluble CD14.
We further found that the amounts of mRNA of three BH4-synthesizing enzymes; i.e. GCH, PTPS and SPR were increased in neuronal cell line N1E-115 in cultures with 1 μg/ml LPS (Mori et al. Citation1997).
These data suggested that LPS could activate the intrinsic pathway of BH4 biosynthesis resulting in enhanced expression of the genes for these enzymes to produce increased levels of BH4.
Peripheral lipopolysaccharide (LPS) administration in mice enhances expression of GTP cyclohydrolase I (GCH) and increases the contents of tetrahydrobiopteirn (BH4), norepinephrine, and its metabolites in the locus coeruleus (LC)
We further studied in vivo effects of peripheral administration of LPS on GCH expression and BH4 contents in the brain in mice (Kaneko et al. Citation2001). We investigated whether the endotoxemia caused by an i.p. injection of LPS can modulate BH4 production in the norepinephrine nuclei in the brain, i.e. the LC (A6 neurons) and the central caudal pons (A5 neurons) in C3H/HeN mice, and whether such a change in BH4, if any, can result in the modification of norepinephrine production in these nuclei. After a 5-μg i.p. administration of LPS, the protein expression of GCH and TH in both nuclei was examined by immunohistochemistry. The staining intensity of GCH-positive cells increased in the LC at 6 h, whereas no significant change in the staining intensity of TH-positive cells was detected. Similar immunohistochemical results on GCH and TH were also observed in A5 neurons. Next, we measured the contents of BH4, norepinephrine and its metabolites MHPG and VMA in the LC after i.p. LPS administration. The BH4 content increased to a statistically significant level at 2 and 4 h after the injection. The contents of norepinephrine, MHPG and VMA also increased with a similar time-course to that of BH4. These data suggested that an increased supply of BH4 in the LC increased TH activity and resulted in an increase in norepinephrine production rate at the site. This agreed with the report by Lacosta et al. (Citation1999) in which LPS induced an increase in the turnover of norepinephrine within the mouse LC.
We further examined changes in mRNA expression of GCH as the rate-limiting biosynthetic enzyme of BH4 in the LC after i.p. administration of LPS in mice (Kaneko et al. Citation2003). The expression level of GCH mRNA increased within 2 h, and reached a maximum level at 4 h after the LPS administration. However, the mRNA expression levels of PTPS and SPR, both of which are involved in BH4 biosynthesis, were not affected by the LPS administration. These results suggested that for the first 6 h after LPS i.p. administration GCH upregulation alone occurred first and was sufficient to modulate BH4 production in the LC. In addition, for the first 6 h after LPS i.p. administration the mRNA level of TH was not affected. All these data indicated that GCH plays a crucial role in regulating norepinephrine biosynthesis for the first 6 h after LPS i.p. administration by a pathway the activity of which is triggered by i.p. LPS.
Possible mechanism of activation of norepinephrine biosynthesis in the locus coeruleus (LC) in mice triggered by an intraperitoneal (i. p.) administration of lipopolysaccharide (LPS)
The above data indicated that norepinephrine biosynthesis rate (turnover rate) in the LC is increased by an i.p. administration of LPS in mice. Our speculation on the probable mechanism of increased turnover in norepinephrine by peripheral administration of LPS in mice is shown schematically in .
Our in vitro data on cultures of neuronal cells suggested that BH4 biosynthesis in neuronal cells could be directly enhanced by LPS like that in macrophages. However, it is unlikely that LPS penetrated the blood-brain barrier and readily reached the norepinephrine neurons in the LC within such a short time as 2 h. On the other hand, it might be possible for LPS to transmit its message via circumventricular organs where the blood-brain barrier is open to the LC through connections from the hypothalamus or brainstem. Alternatively, inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), or an unknown mediator which might be first produced in the periphery in the LPS-treated mice, could transmit the signal through circumventricular organs or via receptors on cerebral blood vessels, and thus reach the norepinephrine neurons in the LC. The transduced signal would stimulate by phosphorylation the activity of GCH, or increase the expression levels of GCH mRNA and protein. This activated or increased content of GCH would generate more BH4, which would activate TH and finally lead to the increase in norepinephrine turnover in the LC.
A possible neuronal pathway from the hypothalamus to the LC may be from corticotropin-releasing factor (CRF) neurons. CRF neurons originating in the hypothalamus project their fibers to the LC, and CRF is known to be involved in the regulation of responses of norepinephrine neurons to stress. There are two types of CRF receptor, type 1 (CRFR1) and type 2 (CRFR2) (Chen et al. Citation1993; Lovenberg et al. Citation1995; Perrin et al. Citation1995). CRFR1 is more widely distributed in the central nervous system than CRFR2. We found that pre-treatment with CP-154526, a CRFR1 antagonist, attenuated the increase in expression level of GCH mRNA in the LC at 4 h after i.p. administration of LPS (Kaneko et al. Citation2005). However, no effects on the expression level of TH mRNA at the site were observed. These results indicated that CRFR1 attenuated the increase of norepinephrine turnover in the LC caused by peripheral LPS administration, suggesting the involvement of CRF neurons in the increased turnover by peripheral administration of LPS. However, we note that several catecholamine cell groups in the lower brainstem, one of which is the NTS, comprise the brain circuitry mediating stress reactions, as do the CRF-containing hypothalamic neurons (Ceccatelli et al. Citation1989). LC and NTS neurons have been shown to contain the glucocorticoid receptor (Härfstrand et al. Citation1986). I.P. LPS administration induces hypotensioin due to systemic vasodilation as one of the symptoms of endotoxemia, which led to the modulation of the baroreflex in NTS of rats (Lin et al. Citation1999). Peripheral LPS enhanced the contents of norepinephrine and serotonin as well as dopamine turnover rate in the rat NTS (Molina-Holgado and Guaza Citation1996). Serova et al. (Citation1999, Citation2004, Citation2005) have shown that the expression levels of TH mRNA and GCH mRNA in the catecholaminergic LC and NTS are upregulated in parallel in rats exposed to immobilization or to changes in the hormonal milieu. These results indicate the possibility that the NTS plays important role(s) in mediating the effects of peripheral derangements caused by i.p. LPS administration on the modulation of LC activity apart from the proposed route using a CRF neuronal relay from the hypothalamus to the LC.
Our studies indicated that peripheral administration of LPS to mice produces activation and increased expression of GCH and increases in BH4 content and norepinephrine turnover, without increases in the expression of the TH gene in the LC for the first 6 h after i.p. LPS administration. These results are different from those obtained from rats exposed to immobilization stress. Serova et al. (Citation1999) found increased TH, DBH and GCH mRNA levels as well as elevated DBH immunoreactive protein in the LC of rats exposed to single and repeated immobilization stress. We speculate that these differences in the results may reflect the different neuronal signaling mechanisms activated by immobilization stress and peripheral LPS administration, which may have stimulated the LC neurons with different intensities, leading to stimulation of transcription of genese for GCH, TH and DBH with the former stimulus, and increased transcription of only GCH mRNA with the latter. Our results suggest that in the early stage of endotoxemia enhanced expression in the LC of GCH causing increased levels of BH4 is the first event and is sufficient to induce increased biosynthesis of norepinephrine.
References
- Amano T, Richelson E, Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones. Proc Natl Acad Sci USA 1972; 69: 258–263
- Bräutigam M, Dreesen R, Herken H. Tetrahydrobioptein and total biopterin content of neuroblastoma (N1E-115, N2A) and pheochromocytoma (PC-12) clones and the dependence of catecholamine synthesis on tetrahydrobiopterin concentration in PC-12 cells. J Nreurochem 1984; 42: 390–396
- Ceccatelli S, Villar MJ, Goldstein M, Hökfelt T. Expression of c-Fos immunoreactivity in transmitter-characterized neurons after stress. Proc Natl Acad Sci USA 1989; 86: 9569–9573
- Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing factor receptor. Proc Natl Acad Sci USA 1993; 90: 8967–8971
- Flatmark T, Almås B, Knappskog PM, Berge SV, Svebak RM, Chehin R, Muga A, Martínez A. Tyrosine hydroxylase binds tetrahydrobiopterin cofactor with negative cooperativity, as shown by kinetic analyses and surface plasmon resonance detection. Eur J Biochem 1999; 262: 840–849
- Glauser MP, Zanetti G, Baumgartner J-D, Cohen J. Septic shock: Pathogenesis. Lancet 1991; 338: 732–739
- Horn PT, Mirkin BL. Modulation of in situ murine neuroblastoma tumor growth by the adrenergic nervous system: Differential response of clonal cell lines to chemical sympathectomy. Life Sci 1990; 47: 2251–2259
- Härfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wirkström A-C, Okret S, Yu Z-Y, Goldstein M, Steinbusch H, Verhofstad A, Gustafsson J-Å. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad sci USA 1986; 83: 9779–9783
- Ichinose H, Ohye T, Matsuda Y, Hori T, Blau N, Burlina A, Rouse B, Matalon R, Fujita K, Nagatsu T. Characterization of mouse and human GTP cyclohydrolase genes: Mutations in patients with GTP cyclohydrolase I deficiency. J Biol Chem 1995; 270: 10062–10071
- Itoh O, Kuroiwa S, Atsumi S, Umezawa K, Takeuchi T, Hori M. Induction by the guanosine analogue oxanosine of reversion toward the normal phenotype of K-ras-transformed rat kidney cells. Cancer Res 1989; 49: 996–1000
- Kaneko YS, Ikemoto K, Mori K, Nakashima A, Nagatsu I, Ota A. Expression of GTP cyclohydrolase I in murine locus ceruleus is enhanced by peripheral administration of lipopolysaccharide. Brain Res 2001; 890: 203–210
- Kaneko YS, Mori K, Nakashima A, Nagatsu I, Ota A. Peripheral administration of lipopolysaccharide enhances the expression of guanosine triphosphate cyclohydrolase I mRNA in murine locus coeruleus. Neurocsience 2003; 116: 7–12
- Kaneko YS, Mori K, Nakashima A, Nagatsu I, Nagatsu T, Ota A. Corticotropin-releasing factor receptor antagonist attenuates LPS-induced increase of GTP cyclohydrolase I expression at murine locus coeruleus. Biogenic Amines 2005; 19: 289–297
- Kato E, Narahashi T. Characteristics of the electrical response to dopamine in neuroblastoma cells. J Physiol 1982; 333: 213–226
- Kaufman S. The structure of phenylalanine hydroxylase cofactor. Proc Natl Acad Sci USA 1963; 50: 1085–1093
- Kvetnansky R, Weise VK, Kopin IJ. Elevation of adrenal tyrosine hydroxylase and phenylethanolamine N-Methyltransferase by repeated immobilization of rats. Endocrinology 1970a; 87: 744–749
- Kvetnansky R, Gerwitz GP, Weise VK, Kopin IJ. Enhanced synthesis of adrenal dopamine β-Hydroxylase induced by repeated immobilization in rats. Mol Pharmacol 1970b; 7: 81–86
- Lacosta S, Merali Z, Anisman H. Behavioral and neurochemical consequences of lipopolysaccharide in mice: Anxiogenic-like effects. Brain Res 1999; 818: 291–303
- Lin HC, Wan FJ, Kang BH, Wu CC. Systemic administration of lipopolysaccharide induces release of nitric oxide and glutamate and c-Fos expression in the nucleus tractus solitarii of rats. Hypertension 1999; 33: 1218–1224
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Charmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA 1995; 92: 836–840, Erratum in: Proc Natl Acad Sci USA 92: 5759
- Matsuura S, Sugimoto T, Murata S, Sugawara Y, Iwasaki H. Stereochemistry of biopterin cofactor and facile methods for the determination of the stereochemistry of a biologically active 5,6,7,8-tetrahydropterin. J Biochem 1985; 98: 1341–1348
- Molina-Holgado F, Guaza C. Endotoxin administration induced differential neurochemical activation of the rat brain stem nuclei. Brain Res Bull 1996; 40: 151–156
- Mori K, Nakashima A, Nagatsu T, Ota A. Effect of lipopolisaccharide on the gene expression of the enzymes involved in tetrahydrobiopterin de nove biosynthesis in murine neuroblastoma cell line N1E-115. Neurosci Lett 1997; 238: 21–24
- Nagatsu T. Biopterin cofactor and regulation of monoamine-synthesizing mono-oxygenases. Trends Pharmacol Sci 1981; 2: 276–279
- Nagatsu T. Genes for human catecholamine-synthesizing enzymes. Neurosci Res 1991; 12: 315–345
- Nagatsu I, Ichinose H. Regulation of pteridine-requiring enzymes by the cofactor tetrahydrobiopterin. Mol Neurobiol 1999; 19: 79–96
- Nagatsu T, Kobayashi K, Ichinose H. Alterations in catecholamine system enzymes. Ann N Y Acad Sci 1995; 771: 159–165
- Neijt HC, Vijverberg HP, van den Bercken J. The dopamine response in mouse neuroblastoma cells is mediated by serotonin 5HT3 receptors. Eur J Pharmacol 1986; 127: 271–274
- Ota A, Yoshida S, Nomura T, Matsui S, Hagino Y, Umezawa K, Katoh S, Nagatsu T. Tetrahydrobiopterin biosynthesis enhanced by lipopolisaccharide stimulation in murine neuroblastoma cell line N1E-115. J Neurochem 1996; 67: 2540–2548
- Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjan L, Sawchenko P, Vale W. Identification of a second neurotrophin receptor gene and characterizaton of a cDNA expressed in heart. Proc Natl Acad Sci USA 1995; 92: 2969–2973
- Richelson E. Properties of tyrosine hydroxylation in living mouse neuroblastoma clone N1E-115. J Neurochem 1976; 27: 1113–1118
- Sakai N, Kaufman S, Milstien S. Parallel induction of nitric oxide and tetrahydrobiopterin synthesis by cytokines in rat glial cells. J Neurochem 1995; 65: 895–902
- Serova L, Nankova B, Rivkin M, Kvetnansky R, Sabban EL. Glucocorticoids elevate GTP cyclohydrolase I mRNA levels in vivo and in PC12 cells. Mol Brain Res 1997; 48: 251–258
- Serova LI, Nankova BB, Feng Z, Hong J-S, Hutt M, Sabban EL. Heightened transcription for enzymes involved in norepinephrine biosynthesis in the rat locus coeruleus by immobilization stress. Biol Psychiatry 1999; 45: 853–862
- Serova LI, Maharjan S, Huang A, Sun D, Kaley G, Sabban EL. Response of tyrosine hydroxylase and GTP cyclohydrolase I gene expression to estrogen in brain catecholaminergic regions varies with mode of administration. Brain Res 2004; 1015: 1–8
- Serova LI, Maharjan S, Sabban EL. Estrogen modifies stress response of catecholamine biosynthetic enzyme genes and cardiovascular system in ovariectomized female rats. Neuroscience 2005; 132: 249–259
- Sumi-Ichinose C, Urano F, Kuroda R, Ohye T, Kojima M, Tazawa M, Shiraishi H, Hagino Y, Nagatsu T, Nomura T, Ichinose H. Catecholamines and serotonin are differently regulated by tetrahydrobiopterin. J Biol Chem 2001; 276: 41150–41160
- Togari A, Ichinose H, Matsumoto S, Fujita K, Nagatsu T. Multiple mRNA forms of human GTP cyclohydrolase I. Biochem Biophys Res Commun 1992; 187: 359–365
- Uehara Y, Hori M, Takeuchi T, Umezawa H. Screening of agents which convert “transformed morphology” of Rous sarcoma virus-infected rat kidney cells to “normal morphology”: Identification of an active agent as herbimycin and its inhibition of intracellular srk kinase. Jpn J Cancer Res 1985; 76: 672–675
- Umezawa H, Imoto M, Sawa T, Isshiki K, Matsuda N, Uchida T, Iinuma H, Hamada M, Takeuchi T. Studies on a new epidermal growth factor-receptor kinase inhibitor, erbstatin, produced by MH435-hF3. J Antibiot (Tokyo) 1986; 39: 170–173
- Watanabe Y, Shimada N, Nagatsu T, Umezawa K. Inhibition of G-Protein-Mediated differentiation of PC12 cells by an IMP dehydrogenase inhibitor, oxanosine. Biogenic Amines 1994; 10: 509–517
- Werner-Felmayer G, Prast H, Werner ER, Phillippu A, Wachter H. Induction of GTP cyclohydrolase I by bacterial lipopolisaccharide in the rat. FEBS Lett 1993; 322: 223–226
- Werner-Felmayer G, Golderer G, Werner ER. Tetrahydrobiopterin biosynthesis, utilization and pharmacological effects. Curr Drug Metab 2002; 3: 159–173