Abstract
Neuronal inputs from the forebrain and the brainstem to sympathetic preganglionic neurons in the spinal cord were investigated by the transneuronal retrograde tracing technique using pseudorabies virus in intact and brainstem-lesioned rats. After unilateral subcutaneous viral inoculations into the hind limb of intact rats, infected neurons were then visualized by immunostaining. At 3.5 days after inoculation, infected neurons appeared in the thoracic (T10) intermediolateral (IML) cell column. On the 4th day, infected neurons were present in the C1, A5, A6, A7 catecholamine cell groups and the rostral ventromedial medulla (RVMM). On the 5th day, viral labeling was seen in the hypothalamic paraventricular and arcuate nuclei and the lateral hypothalamic area. In all of these nuclei, the infected cells appeared bilaterally. However, the appearance of virus-labeled cells in these nuclei was unilateral following unilateral coronal sections between the medulla and the spinal cord (depending on the side of hemisection, but not on the site of virus inoculation). Midsagittal sections throughout the entire medulla oblongata did not alter the topographical pattern of virus-infected neurons in the forebrain or the brainstem. These findings indicate that descending fibers to the spinal neurons may not cross over in the lower brainstem but that they decussate within the spinal cord.
Introduction
Brainstem and spinal neurons that participate in neuronal organization of stress responses, including sympatho-neuronal and sympatho-adrenomedullary transmission, receive descending facilitatory and inhibitory modulatory input from supraspinal structures (Fields and Basbaum Citation1978; Fields et al. Citation1991; Dampney Citation1994; Sun Citation1995; Palkovits Citation1999; Saper Citation2002). Descending supraspinal nerve fibers terminate on sympathetic preganglionic neurons of the intermediolateral (IML) cell columns of the T10 spinal cord (Chiba and Masuko Citation1986; Milner et al. Citation1988; Zagon and Smith Citation1993; Pyner and Coote Citation1998; Tóth et al. Citation1999). By using conventional or transneuronal retrograde tracers, it has been demonstrated that neurons in the rostral ventromedial (RVMM) and ventrolateral medulla (RVLM), the pontine tegmentum, the midbrain central gray matter, and in the hypothalamus project to the spinal cord. The details of the termination of these descending axons have been extensively studied in the past two decades. However, our knowledge about the fine topography of these descending fibers from the rostral cells of their origin down to the spinal target neurons is still incomplete. Observations that one side viral inoculation in the hind limb infects forebrain and brainstem neurons retrogradely on either side, and the finding that a single injection of formalin into one hind limb elicits ipsilateral c-fos expression in the spinal cord but bilateral c-fos expression in supraspinal neurons (Palkovits et al. Citation1997, Citation1999) indicate that the descending supraspinal fibers may cross over somewhere on their way down to the spinal cord. The exact knowledge of the fine topography of cross over of fibers in the brain stem or within the spinal cord is of importance in the correct evaluation of anatomical and functional consequences of unilateral experimental manipulations (e.g. surgery, tract-tracing, recording of cell activities) or unilateral changes in certain regions of the brain stem or the spinal cord.
In the present study, to localize supraspinal neurons that innervate the spinal cord we injected pseudorabies virus (Bartha strain) subcutaneously in a hind limb of rats, exactly the same way (superficial subcutaneous injection) and into the same area where formalin was injected in previous studies (Palkovits et al. Citation1997, Citation1999). Since the major aims of this study were to localize supraspinal neurons that innervate hind limb-projecting spinal neurons, and to localize the levels where their fibers cross over on the way down to the spinal cord, mid-sagittal surgical knife cuts were performed in the medulla oblongata, and unilateral coronal cuts between the spinal cord and the medulla, two weeks before virus was injected into the hind limb.
Material and methods
Adult male Wistar rats, 250–350 g body weight were used (n = 55). The animals were kept under standard laboratory conditions (room temperature 22 ± 1°C, with a 12-h light-dark cycle, with lights on at 06:00 h). Regular rat chow and tap water were provided ad libitum. Experiments were carried out in accordance with the Guide for Care and Use of Laboratory Animals of the Ethics Committee of Semmelweis University, Budapest (based on the European Communities Council Directive of 24 November 1986 (86/609/EEC) regarding the care and use of animals for experimental procedures. The experimental protocol was approved by the Institutional Review Board of the Semmelweis University.
Experimental protocols
Experimental group #1: Injections of pseudorabies virus into the hind limb (n=17)
Bartha strain of pseudorabies virus (Ba-Prv, 109 plaque-forming units [PFU]/ml in 0.5 ml/rat) was inoculated subcutaneously into the hind limb of intact rats. The rats were killed 3 (n = 5), 3.5 (n = 5), 4 (n = 4) and 5 (n = 3) days after viral inoculations. In previous studies, these time intervals were found to be optimal for the detection of the trans-synaptic routes of transfer of the virus through the first, second and tertiary order of infected neurons (Boldogköi et al. Citation2004). Rats were anesthetized with i.p. ketamine and xylazine-hydrochloride 2%, and perfused intracardially with a Bouin fixative solution (4% paraformaldehyde, 0.19% [saturated] picric acid in 0.1 M phosphate buffer [PB], pH 7.35) after a brief saline rinse. The brains were removed and post-fixed in the same solution at 4°C overnight, then cryoprotected in 20% sucrose for 24 h. Serial coronal sections of 50 μm thickness were cut through the forebrain, brainstem and spinal cord with a freezing microtome (Frigomobil, Reichert–Jung, Heidelberg, Germany) at − 20°C and further processed for the detection of the viral antigen by immunohistochemistry.
Experimental group #2: Neurosurgical interventions
Rats were anaesthetized with a combination mixture of i.p. ketamine (80 mg/kg) and xylazine-hydrochloride (15 mg/kg), placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). Two types of transections were performed:
Coronal hemisections at the spino-medullary border
Right and left side hemisections were performed (n = 12 in each group). The heads of the rats were fixed in a maximal (about 45°) nose-down position. At the level of the medulla/spinal cord border, the medulla was unilaterally transected by a perpendicular penetration of a 3.5-mm wide “glass knife” cut out from a histological coverslip (Palkovits et al. Citation1982). After separating the neck muscles, the atlanto-occipital membrane was exposed, cleaned and then transected. The posterior arch of the atlas was used as a landmark for the penetration, and the knife was lowered along the proximal edge of the bone vertically down to the internal surface of the skull. Thus, one half of the medulla was completely separated from the spinal cord. After surgery, rats were allowed to recover for 14 days (by that time, 19 of 24 rats survived the surgery). Then, all of the rats were anaesthetized with ketamine and xylazine (as above) and Ba-Prv was inoculated subcutaneously into the right hind limb, as for the rats in experimental group #2 (Ba-Prv, 109 PFU/ml in 0.5 ml). Both groups (10 left and 9 right hemisected) were further divided into 3 subgroups and perfuse-fixed under anesthesia as in experiment #2, 3.5, 4, 5 days after inoculation. The brains were removed and processed for the detection of the viral antigen by immunohistochemistry.
Midsagittal medullary transections
The medulla was exposed under surgical anesthesia as in experimental #2a, but the cut was performed with a 4.0 mm wide “glass knife” in the midline from the caudal tip of the fourth ventricle to as caudal as the spino-medullary border (n = 14). The vertical penetration reached the base of the brainstem, by which cut the two sides of the caudal half of the medulla were separated. After surgery, rats were allowed to recover for 14 days before viral inoculations. Rats that survived the surgery (n = 10) were virus-injected, treated and killed like those in experiment # 2a.
The exact topographical locations of the transections were determined in histological sections. Following perfusion-fixation, 50 μm coronal (perpendicular to the brainstem axis) sections were cut from the transected areas of the medulla and spinal cord and stained with cresyl violet. Rats with incomplete transactions or large hemorrhages (2 rats in experimental group #2a, and 3 rats in group #2b) were excluded from further study. In general, as an advantage of the use of “glass knives”, the surgical damage was minimal and the recovery from the procedure was rapid.
Immunohistochemistry
The free-floating sections were washed in 0.1 M PB, incubated in 3% H2O2 for 10 min, rinsed again in PB, and incubated in 0.3% Triton X-100 at 4°C overnight. Then the sections were rinsed three times in PB and placed in 10% normal goat serum (Dakopatts, Glostrup, Denmark) for 1 h at room temperature. Virus-infected cells were identified using Ba-Prv polyclonal antiviral antibody directed against rabies virus nucleocapsids, raised in rabbit (courtesy of Miselis, Philadelphia, code number Rb134), as the primary antibody. The antibody was used at a dilution of 1:5000 in 0.1 M PB containing 0.1% bovine serum albumin and 0.05% sodium azide for an incubation period of 48 h at 4°C. The immunoperoxidase detection of biotin-labeled secondary antibodies (anti-rabbit IgG 1:500 for 1 h at room temperature) was performed with an Elite Vectastain ABC Kit (Vector Labs., Burlingame, CA, USA). Immunoreactivity was revealed with the nickel-DAB method (50 mmol/l Tris–HCl, pH 7.6, containing 0.02% 3,3'-diaminobenzidine, 0.0045% H2O2, and 0.6% nickel ammonium sulfate for 3 min). Sections were mounted onto gelatin-coated slides and counterstained with Nuclear Fast Red (Fluka).
Semi-quantitative analysis of virus-labeled cells was carried out in 3–6 coronal sections from T10 to L1 segments of the spinal cord, 3–6 sections from the medulla oblongata 1.0–1.2 and 3.2–3.5 mm rostral to the obex (for the A1–A2/C2 and C1 cell groups, respectively), 3–5 sections at the medulla/pons junction (A5 cell group), 3 sections through the larger section profile of the locus coeruleus, and 3–6 sections from the hypothalamus at the levels of the paraventricular and the arcuate nuclei, 1.7–2.0 and 2.6–2.9 mm caudal to the level of the bregma, respectively. The number of virus-infected cells was counted on the two sides of the sections separately. In each experimental group, the smallest and the highest cell counts/section of the investigated areas are given.
Results
Topography of virus-infected cells in the brain and the spinal cord of the surgically intact rats
Three and a half days after viral injection, labeled cells were found in the T10 and lumbar (L1) segments of the spinal cord on the side of the inoculation. Infected cells were concentrated in the IML cell column, but infected cells were scattered in the intermediomedial nucleus and in the lateral funiculus, close to the “neck” of the dorsal horn (). The average number of virus-labeled cells on the side of the injection varied between 11 and 37 per section (8–22 cells/section in the IML) taken from segments T10–L1 (n = 5). Occasionally, a single labeled cell appeared on the other side.
Figure 1 T10 spinal cord. Coronal section. Ba-Prv virus-labeled neurons in the IML cell column 3.5 day after inoculation. Abbreviations: C, central canal; df, dorsal funiculus; dh, dorsal horn; lf, lateral funiculus; vh, ventral horn. Scale bar = 200 μm.
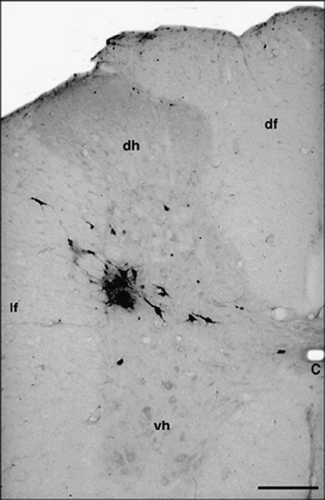
The viral infection progressed further by the 4th day after inoculation. Several neurons (19–45/section, n = 4) were infected in the ipsilateral IML cell column, and infected cells first appeared in the lower brainstem. Neurons in the RVLM, at the location of the rostral part of the C1 adrenergic cell group, showed signs of viral infection (). In all of these nuclei, infected cells were evident bilaterally (). No infected cells were seen in the caudal ventrolateral medulla, in the A1 or the caudal portion of the C1 catecholamine cell groups. Virus-infected cells were also seen in the RVMM, the raphe magnus, the giganto- and paragiganto-cellular reticular nuclei ().
Figure 2 Virus-infected cells in the hypothalamus (A,B) 5 days, and in the pons (C,D) and the medulla oblongata (E,F) 4 days after inoculation. (A) Paraventricular nucleus, dorsolateral parvicellular part. (B) Scattered cells in the lateral hypothalamic area and the arcuate nucleus. (C) Caudal part of the locus coeruleus, subcoeruleus area and the rostral part of the A5 cell group. (D) Caudal part of the pons: A5 cell group. (E) Labeled cells in the RVMM and scattered labeled cells in the C3 adrenaline cell group. (F) RVLM. Labeled cells are concentrated in the rostral part of the C1 adrenaline cell group. Abbreviations: Ar, arcuate nucleus; A5, A5 noradrenaline cell group; Ce, central amygdaloid nucleus; IO, inferior olive; LH, lateral hypothalamic area; p, pyramidal tract; PGi, paragigantocellular reticular nucleus; PVN, paraventricular nucleus; SC, subcoeruleus area; tp, trapezoid body; 3V, third ventricle; 4V, 4th ventricle; 7th, cranial portion of the facial nerve. Scale bars = 500 μm (A) μm and 1 mm (B–F).
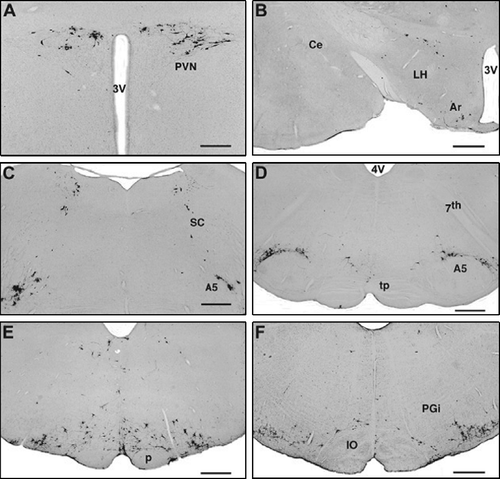
Table I. Number of Ba-Prv virus-infected cells (4 and 5 days after inoculation) in brain regions in intact (right and left side) and neurosurgically-operated rats (ipsi- and contra-lateral sides to the unilateral subcutaneous injection).
Infected cells were seen in the pons 4 days after inoculation. Labeled neurons occupied the territory of the A5 catecholamine cell group ( and D, ) along the root of the facial nerve. Here, virus-labeled small and medium-sized neurons formed an arch along the dorsolateral edge of the superior olivary complex. In the subcoeruleus area, a group of labeled neurons formed an arch between the most caudal part of the locus coeruleus and the A5 cell group. The number of virus-labeled cells in the subcoeruleus area varied between 14 and 29 (ipsilateral) and between 11 and 20 (contralateral) per section, while only 4–9 (ipsilateraal) and 5–12 labeled cells/section were seen in the locus coeruleus (). More rostral and lateral to these cells, another group of infected cells was seen in a location that represents the A7 catecholamine cell group. In the midbrain, virus-infected cells appeared in the ventral and lateral parts of the caudal half of the periaqueductal gray matter (not shown).
By the 5th post-inoculation day, infected cells were present in the hypothalamic paraventricular nucleus, mainly in its dorsal and dorsolateral subdivisions (). Infected cells were also seen in the arcuate nucleus and in the lateral hypothalamic area close to the fornix (). All of this labeling in the hypothalamus was bilateral with a slight ipsilateral dominance (). Occasionally, labeled cells were seen in the bed nucleus of the stria terminalis, but none were seen in the amygdala ().
Virus- infected cells in rats with spino-medullary hemisections
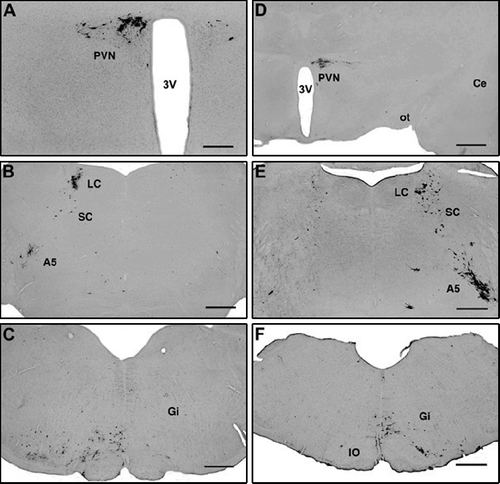
Rats with right (n = 3) and left (n = 4) hemisections at the spino-medullary border (at the caudal edge of the pyramidal decussation) all received virus injections into the right hind limb, 14 days after surgery. Four days after injections, the topography of the virus-infected cells in the brainstem and the forebrain was identical with that in intact rats, but only on one side. The unilaterally injected virus appeared in the rostral ventrolateral and ventromedial medulla ( and F), in the locus coeruleus, the subcoeruleus area, the A5 cell group ( and E), and in the paraventricular nucleus ( and D). Depending on the side of the transections, but quite apart from the side of the injections, labeled cells appeared almost exclusively only on one side: contralateral to the side of the transection (). Thus, in rats with transections on the right side, labeled cells were seen on the left side (–C), and transections on the left side resulted in virus labeling on the right side (–F). Since the virus is transported retrogradely from the nerve terminals to the perikaryon, the presence of the infected cells in the brain stem and the hypothalamus clearly indicates that the descending axons from neurons of these brain areas cross over caudal to the level of the transections (medulla/spinal junction), i.e. inside the spinal cord.
Figure 3 Unilateral (right side) spino-medullary hemisection, ipsilateral (right side) viral injections into the hindlimb (A–C). Four/five days after inoculation, infected cells appear only on the contralateral (left) side in the hypothalamic paraventricular nucleus (A), the pons (B) and the rostral medulla oblongata (C). Unilateral (left side) spino-medullary hemisections, contralateral to viral injection into the right hind limb (D–F). Four/five days after inoculation, infected cells appear only on the side of the injection (right) in the hypothalamic paraventricular nucleus (D), the pons (E) and the rostral medulla oblongata (F). Abbreviations: A5, A5 noradrenaline cell group; Ce, central amygdaloid nucleus; Gi, gigantocellular reticular nucleus; IO, inferior olive; LC, locus coeruleus; ot, optic tract; PVN, paraventricular nucleus; SC, subcoeruleus area; 3V, third ventricle. Scale bars = 500 μm (A) and 1 mm (B–F).
Virus-infected cells in rats with midsagittal medullary sections
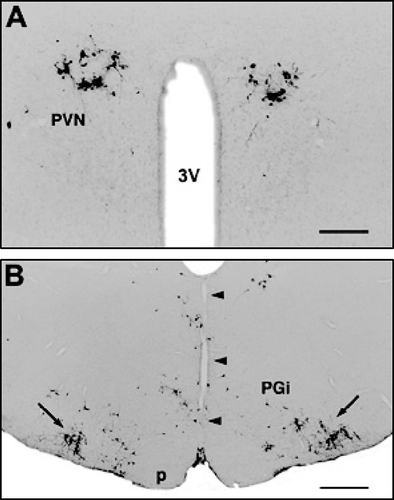
The two sides of the medulla oblongata were completely separated by a midsagittal cut which extended from the spino-medullary border up to the rostral edge of the inferior olive (). The transection did not influence the virus infection of cells in the brainstem and the hypothalamus, in any time period after virus injection into the hind limb. Their locations and the distribution pattern of infected cells were comparable to those in un-operated virus-infected rats (), in both sides of the medulla () and in the hypothalamic paraventricular () and arcuate nuclei, as well as in the lateral hypothalamic area ().
Figure 4 Midsagittal medullary transections. Bilateral distribution of viral infected cells (similar to unoperated rats) in the hypothalamic paraventricular nucleus (A) 5 days and (B) in the rostral medulla oblongata (arrows point to the dense populations of infected cells in the C1 cell group) 4 days after inoculation of the virus into the right hind limb. Abbreviations: p, pyramidal tract; PGi, paragigantocellular reticular nucleus; PVN, paraventricular nucleus; 3V, third ventricle. The arrowheads point to the mid-sagittal medullary transection (narrow vertical cut in the midline). Scale bars = 500 μm (A) and 1 mm (B).
Discussion
One of the major portions of the bulbospinal axons, especially to the preganglionic sympathetic neurons in the IML cell column arises in the RVLM (Loewy et al. Citation1979; Ross et al. Citation1981; Clark and Proudfit Citation1993; Sun Citation1995; Pyner and Coote Citation1998). This area in the medulla oblongata, from the level of the obex up to the caudal pons incorporates adrenergic (C1 cell group), noradrenergic (A5 cell group) and non-catecholamine neurons. By using different approaches, projections from these cells to the spinal cord have been investigated in detail. The majority of RVLM cells innervate IML preganglionic neurons in a strict topographical arrangement (Zagon and Smith Citation1993).
Neurons in the ventro-caudal part of the locus coeruleus and in the subcoeruleus area project to the spinal cord (Nygren and Olson Citation1977; Loughlin et al. Citation1986; Clark and Proudfit Citation1991a) and terminate in the ipsilateral IML cell column. Other pontine neurons, in the territory of the A7 noradrenergic cell group also became virus-infected 4 days after inoculation indicating their projections to the spinal cord, as previously reported by Clark and Proudfit (Citation1991b).
The majority of the descending hypothalamic fibers to the spinal cord arise in the paraventricular nucleus (Sawchenko and Swanson Citation1982; Luiten et al. Citation1985; Tucker and Saper Citation1985; Cechetto and Saper Citation1988; Strack et al. Citation1989a,Citationb; Hosoya et al. Citation1991; Shafton et al. Citation1998), but a substantial number of descending axons arise in the arcuate nucleus, the medial retrochiasmatic (Cechetto and Saper Citation1988; Elias et al. Citation1998) and lateral hypothalamic areas (Tucker and Saper Citation1985; Cechetto and Saper Citation1988; Strack et al. Citation1989a,Citationb).
In the present study, the subcutaneous nociceptive and the sympathetic nerve endings were infected in the virus-injected area of the hind limb. Accordingly, 3.5 days later, infected neurons appeared at first in spinal cord segments T10–L1, in the IML cell column, on the side of the inoculation. A short time later, the virus from these cells spread over the supraspinal regions by retrograde axonal transport, and the bulbospinal projection neurons in the above listed brain areas became infected.
Descending bulbospinal axons pass through the medulla oblongata and they terminate bilaterally in the spinal cord. Most of the descending bulbospinal fibers enter the spinal cord in the lateral funiculus near to the dorsolateral surface of the spinal cord. In general, the descending bulbospinal fibers terminate predominantly in the ipsilateral side of the spinal cord (Shafton et al. Citation1998; Tóth et al. Citation1999; Moon et al. Citation2002). Some of the descending axons or their collaterals cross over in the central gray either dorsal or ventral to the central canal and terminate in the IML (Nygren and Olson Citation1977; Loewy et al. Citation1979; Clark and Proudfit Citation1993; Zagon and Smith Citation1993; Pyner and Coote Citation1998; Tóth et al. Citation1999).
The present study indicates that crossover occurs caudal to the spinal cord-medulla junction. Coronal hemisections of the medulla at the level of the spino-medullary border cut the fibers on one side and blocked the retrograde transport of the virus to the bulbospinal neurons on the side of the transection, apart from the side of the injection of the virus into the hind paw (). Thus, the descending fibers cross over, or may provide cross over collaterals at a level more caudal to the transection, i.e. between the spino-medullary border and the thoracolumbar spinal cord. Furthermore, cross over of the descending fibers in the caudal part of the medulla can be excluded since the mid-sagittal medullary cut, which separated the two sides of the medulla completely did not influence the viral labeling at higher levels, either in the rostral medulla, the pons, or in the hypothalamus.
Figure 5 Descending supraspinal pathways to the sympathetic preganglionic neurons in the spinal cord and their possible cross over in the spinal cord. Fibers arise in the hypothalamus (neurons in the paraventricular [PVN] and arcuate nuclei, and the lateral hypothalamic area [LH]), the pons (neurons in the A5, A7 cell groups, caudal portion of the locus coeruleus [LC] and the subcoeruleus area) and in the medulla oblongata (neurons in the C1 adrenaline cell group, the RVMM and the nucleus of the solitary tract). After cross over in the spinal cord, fibers terminate on the sympathetic preganglionic neurons in the IML cell column of the T10 spinal cord. 1, site of the Ba-Prv viral injection (primary nociceptive neurons); 2, unilateral transection between the spinal cord and the medulla oblongata; 3, midsagittal transection through the medulla oblongata.
![Figure 5 Descending supraspinal pathways to the sympathetic preganglionic neurons in the spinal cord and their possible cross over in the spinal cord. Fibers arise in the hypothalamus (neurons in the paraventricular [PVN] and arcuate nuclei, and the lateral hypothalamic area [LH]), the pons (neurons in the A5, A7 cell groups, caudal portion of the locus coeruleus [LC] and the subcoeruleus area) and in the medulla oblongata (neurons in the C1 adrenaline cell group, the RVMM and the nucleus of the solitary tract). After cross over in the spinal cord, fibers terminate on the sympathetic preganglionic neurons in the IML cell column of the T10 spinal cord. 1, site of the Ba-Prv viral injection (primary nociceptive neurons); 2, unilateral transection between the spinal cord and the medulla oblongata; 3, midsagittal transection through the medulla oblongata.](/cms/asset/d7f592a0-cd47-4148-a2cf-c11a4f4eb2d8/ists_a_242355_f0005_b.gif)
Conclusions
The present observations may give more accurate knowledge about the place of the decussation of descending, most probably sympathetic premotor fibers from the hypothalamus and the brain stem to the spinal cord. Such knowledge of the location of the decussating fibers is important for experimental surgical interventions (e.g. unilateral lesioning of the descending pathways), neuronal tract-tracing, or single cell recording to study spinal-supraspinal relations. Evaluation of changes in neurochemical character, functional activity or in gene expression of spinal and supraspinal neurons involved in the sympathetic outflow in response to unilateral physiological or pathological alterations either at the spinal or supraspinal levels may need exact knowledge of the pathways that interconnect neurons in these brain regions.
Acknowledgements
The authors thank Judit Helfferich for her skilful technical assistance. This work was supported by grants from the Hungarian National Science Foundation, OTKA TS49861 and Hungarian Ministry of Welfare, ETT 554/2000.
References
- Boldogköi Zs, Sik A, Dénes Á, Reichart A, Toldi J, Gerendai I, Kovács KK, Palkovits M. Novel tracing paradigms—genetically engineered herpesviruses as tools for mapping functional circuits within the CNS: Present status and future prospects. Prog Neurobiol 2004; 72: 417–445
- Cechetto DF, Saper C. Neurochemical organization of the hypothalamic projection to the spinal cord in the rat. J Comp Neurol 1988; 272: 579–604
- Chiba T, Masuko S. Direct synaptic contacts of catecholamine axons on the preganglionic sympathetic neurons in the rat thoracic spinal cord. Brain Res 1986; 380: 405–408
- Clark FM, Proudfit HK. The projection of locus coeruleus to the spinal cord in the rat determined by anterograde tracing combined with immunohistochemistry. Brain Res 1991a; 538: 231–245
- Clark FM, Proudfit HK. The projection of noradrenergic neurons in the A7 catecholamine cell group to the spinal cord in the rat demonstrated by anterograde tracing combined with immunocytochemistry. Brain Res 1991b; 547: 279–288
- Clark FM, Proudfit HK. The projections of noradrenergic neurons in the A5 catecholamine cell group to the spinal cord in the rat: Anatomical evidence that A5 neurons modulate nociception. Brain Res 1993; 616: 200–210
- Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 1994; 74: 323–364
- Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RC, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 1998; 21: 1375–1385
- Fields HI, Basbaum AI. Brainstem control of spinal pain-transmission neurons. Annu Rev Physiol 1978; 40: 217–248
- Fields HI, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci 1991; 14: 219–245
- Hosoya Y, Sugiura Y, Okado N, Loewy AD, Kohno K. Descending input from the hypothalamic paraventricular nucleus to sympathetic preganglionic neurons in the rat. Exp Brain Res 1991; 85: 10–20
- Loewy AD, McKellar S, Saper CB. Direct projections from the A5 catecholamine cell group to the intermediolateral column. Brain Res 1979; 174: 309–314
- Loughlin SE, Foote SL, Grzanna R. Efferent projections of the nucleus locus coeruleus: Morphologic subpopulations have different efferent targets. Neuroscience 1986; 18: 307–319
- Luiten PG, ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res 1985; 329: 374–378
- Milner TA, Morrison SF, Abate C, Reis D. Phenylethanolamine N-methyltransferase-containing terminals synapse directly on sympathetic preganglionic neurons in the rat. Brain Res 1988; 448: 205–222
- Moon EA, Goodchild AK, Pilowsky PM. Lateralization of projections from the rostral ventrolateral medulla to sympathetic preganglionic neurons in the rat. Brain Res 2002; 929: 181–190
- Nygren L-G, Olson L. A new major projection from locus coeruleus: The main source of noradrenergic nerve terminals in the ventral and dorsal columns of the spinal cord. Brain Res 1977; 132: 85–93
- Palkovits M. Interconnections between the neuroendocrine hypothalamus and the central autonomic system. Front Neuroendocrinol 1999; 20: 270–295
- Palkovits M, Baffi JS, Pacak K. Stress-induced Fos-like immunoreactivity in the pons and the medulla oblongata of rats. Stress 1977; 1: 155–168
- Palkovits M, Tapia-Arancibia T, Kordon C, Epelbaum J. Somatostatin connections between the hypothalamus and the limbic system of the rat brain. Brain Res 1982; 250: 223–228
- Palkovits M, Baffi JS, Pacak K. The role of ascending neuronal pathways in stress-induced release of noradrenaline in the hypothalamic paraventricular nucleus of rats. J Neuroendocrinol 1999; 11: 529–539
- Pyner S, Coote JH. Rostroventrolateral medulla neurons preferentially project to target-specific sympathetic preganglionic neurons. Neuroscience 1998; 83: 617–631
- Ross CA, Armstrong DM, Ruggiero DA, Pickel VM, Joh TH, Reis DJ. Adrenaline neurons in the rostral ventrolateral medulla innervate thoracic spinal cord: A combined immunocytochemical and retrograde transport demonstration. Neurosci Lett 1981; 25: 257–262
- Saper CB. The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci 2002; 25: 433–469
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol 1982; 205: 260–272
- Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res 1998; 801: 239–243
- Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res 1989a; 491: 156–162
- Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res 1989b; 491: 274–296
- Sun MK. Central neural organization and control of sympathetic nervous system in mammals. Prog Neurobiol 1995; 47: 157–233
- Tucker DC, Saper CB. Specificity of spinal projections from hypothalamic and brainstem areas which innervate sympathetic preganglionic neurons. Brain Res 1985; 360: 159–164
- Tóth ZE, Gallatz K, Fodor M, Palkovits M. Decussations of the descending paraventricular pathways to the brainstem and spinal cord autonomic centers. J Comp Neurol 1999; 414: 255–266
- Zagon A, Smith AD. Monosynaptic projections from the RVLM oblongata to identified sympathetic preganglionic neurons. Neuroscience 1993; 54: 729–743