Abstract
Forced swimming is a behavioural stress model increasingly used to investigate the neurocircuitry of stress responses. Although forced swim stress clearly is a psychological stressor (anxiety, panic), its physical aspects are often neglected. There are indications that behavioural and neurochemical responses to swim stress depend on the water temperature. Thus, we investigated the responsiveness of hippocampal serotonergic neurotransmission (important in the coordination of stress responses), and of behaviour and core body temperature to forced swimming at different water temperatures (19, 25 and 35°C). In vivo microdialysis and biotelemetry in freely-behaving rats were used. Dialysates were analysed for serotonin (5-HT) and its metabolite 5-HIAA (5-hydroxyindoleacetic acid) by HPLC with electrochemical detection. Forced swimming in water at 25 and 19°C decreased core body temperature by 8 and 12°C, respectively. A rapid and pronounced increase in hippocampal 5-HT and 5-HIAA was found in rats that swam at 35°C, whereas biphasic responses in 5-HT and 5-HIAA were observed at 25 and 19°C. Also swim stress behaviour and post-stress home cage behaviour depended on the water temperature. Comparing the serotonergic and core body temperature changes revealed that a combination of two different 5-HT and 5-HIAA responses seems to shape the neurotransmitter response. Swimming-induced increases in hippocampal extracellular concentrations of 5-HT and 5-HIAA occurred at all water temperatures, but these increases were temporarily quenched, or concentrations were transistently decreased, when core body temperature fell below ∼31°C in water at 25 or 19°C. These data demonstrate that water temperature is a key factor determining the impact of forced swim stress on behaviour and neurochemistry, and underscore that changes in these parameters should be interpreted in the light of the autonomic responses induced by this stressor.
| Abbreviations | ||
| 5-HIAA | = | 5-hydroxyindoleacetic acid |
| 5-HT | = | serotonin |
| ANOVA | = | analysis of variance |
| DRN | = | dorsal raphé nucleus |
| MRN | = | median raphé nucleus |
Introduction
During the past decade, our and other laboratories have demonstrated that the brain serotonin (5-hydroxytryptamine, 5-HT) system plays an important role in the coordination of neuroendocrine, autonomic and behavioural responses to stress (Lucki Citation1998; Van de Kar and Blair Citation1999; Maier and Watkins Citation2005; Linthorst Citation2005b). Stress-induced changes in serotonergic neurotransmission are not only stressor-specific but also vary depending on the brain region under investigation (Linthorst Citation2005b). An important brain structure in the coordination of the stress response is the hippocampus. Importantly, hippocampal serotonergic neurotransmission in rodents responds to stressful challenges such as immune stress, exposure to a predator, novelty and forced swim stress (Kirby et al. Citation1995; Linthorst et al. Citation1995; Rueter and Jacobs Citation1996; Beekman et al. Citation2005; Linthorst Citation2005a,Citationb).
Swim stress, as in the original Porsolt forced swim paradigm (Porsolt et al. Citation1977), has been (and still is) extensively used by neuropharmacologists to discover drug leads with putative antidepressant properties (Cryan et al. Citation2002). However, it has become increasingly evident that forced swim stress is also an important tool to investigate the neurocircuitry of the stress response and to understand stress-related learning and memory processes (De Pablo et al. Citation1989; Cullinan et al. Citation1995; Bilang-Bleuel et al. Citation2002; Citation2005); here we focus on the use of forced swimming as a stress model. Forcing rats or mice to swim in a container from which they cannot escape is an anxiogenic life-threatening situation for the animal as evidenced by vigorous attempts to escape from the container (“flight” response) and activation of the hypothalamic–pituitary–adrenocortical axis (Porsolt et al. Citation1977; Cryan et al. Citation2002; Bilang-Bleuel et al. Citation2002). Because of its anxiogenic and putatively panicogenic properties, forced swim stress is often regarded as a psychological stressor. However, forced swimming for an extensive period (often 10–15 min, or longer) in relatively cold water (normally 25°C or lower) causes profound changes in the physical homeostasis of the animal, such as increased motor activity, cardiovascular changes and marked decreases in body temperature (Stone Citation1970b; Porsolt et al. Citation1979). Often, these physical aspects are barely taken into account when interpreting the outcome of forced swim stress, and the Porsolt forced swim paradigm.
There are several indications that water temperature has a pronounced impact on the behaviour of the animal during forced swim stress and on the elicited molecular and neuroendocrine responses (Abel Citation1993; Taltavull et al. Citation2003; Bilang-Bleuel et al. Citation2005; Drugan et al. Citation2005). Moreover, post-mortem studies suggest that changes in monoamine turnover may depend on the water temperature (Stone Citation1970a,Citationb). Importantly, there is also evidence showing that the water temperature impacts on the outcome of learning and memory testing in water mazes and in re-testing in the Porsolt forced swim paradigm (Sandi et al. Citation1997; Bilang-Bleuel et al. Citation2005). Given that the applied water temperature plays an important role in the swim stress-induced responses, we hypothesised that water temperature may also be a key determinant for the serotonergic response to this stressor, since 5-HT is involved in the behavioural response to forced swimming (Borsini Citation1995; Cryan et al. Citation2005). Furthermore, serotonergic cell groups receive autonomic and viscero-sensory information from the periphery indirectly via various brain regions (e.g. nucleus tractus solitarii, parabrachial nucleus, hypothalamus, prefrontal cortex) (Jacobs and Azmitia Citation1992; Saper Citation2002). Therefore, the aim of the present study was to investigate the responsiveness of hippocampal serotonergic neurotransmission to forced swim stress at different water temperatures (19, 25 and 35°C) by in vivo microdialysis and to relate these responses to changes in core body temperature and forced swimming-induced behaviour. Despite the longstanding use of the forced swim test this is, to our knowledge, the first comprehensive study on the relationship between water temperature, core body temperature, different aspects of behaviour and the hippocampal 5-HT response to this form of stress.
Material and methods
Animals
Experiments were performed on male Wistar rats purchased from Charles River Wiga (Sulzfeld, Germany). Rats were housed four per cage under standard housing conditions (lights on between 07:30 and 19:30 h; temperature 22–23°C; relative humidity 40–60%) and had free access to food and water. Body weight was approximately 250 g on the day of surgery. Rats were handled once per day (5 min per rat) starting 1 week before surgery and continuing until the day before the experiment to reduce non-specific stress. The experimental protocols were approved by the Ethical Committee on Animal Care and Use of the Government of Bavaria, Germany.
Surgical procedures
Ten days before the start of the forced swim stress experiment, rats were surgically prepared under halothane anaesthesia (0.5–2% halothane, flow rate 60–100 ml/min) either for microdialysis of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) in the hippocampus or for biotelemetric recording of core body temperature and motor activity. After surgery, rats were moved to the experimental room (with environmental conditions similar to the holding facility).
For microdialysis a guide cannula (CMA/12, CMA Microdialysis AB, Stockholm, Sweden) was implanted to just enter the hippocampus dorsally as described previously (Linthorst et al. Citation1994; Citation1995). A metal peg for connection to a liquid swivel system was fixed to the skull with dental cement. After surgery, rats were housed individually in Plexiglas cages designed for microdialysis (length × width × height = 25 × 25 × 35 cm) with food and water ad libitum. Rats could see, hear and smell one another.
For biotelemetry, rats were implanted with a battery-powered transmitter (TA-F40; Data Sciences International, St Paul, MN, USA) in the peritoneal cavity as described previously (Linthorst et al. Citation1997). After surgery, rats were housed individually (but could see, hear and smell one another) in standard Makrolon cages (length × width × height = 42.5 × 26.6 × 18.5 cm) with food and water ad libitum, and biotelemetric recording was started immediately (see below).
Protocol A: Microdialysis
A microdialysis probe was inserted slowly into the hippocampus via the guide cannula under brief, light halothane anaesthesia (0.5–2% halothane, flow rate 60–100 ml/min) 8 days after surgery. At the same time, rats were connected to a liquid swivel and a counterbalancing arm via a 40-cm wire attached to the peg on the head. Rats could move freely in all three dimensions and were able to reach the bottom of the glass beaker when diving during the forced swim test. Microdialysis probes were perfused with sterile, pyrogen-free Ringer solution at a flow rate of 2 μl/min. Fluoroethylenepolymer tubing was used for all connections and samples were collected in vials on top of the swivel. The dead volume of the system was taken into account when synchronising behavioural and neurochemical data.
Two days after insertion of the probe, nine 15-min samples were collected between 09:00 and 11:15 h for the measurement of baseline concentrations of 5-HT and 5-HIAA. Next, rats were forced to swim for 15 min (11:15–11:30 h; as described below) during which another dialysate sample was collected (swim stress sample). After completion of the swim procedure fourteen 15-min samples were collected to determine post-stress concentrations (11:30–15:00 h). Experiments were always performed on four rats simultaneously. Microdialysis samples (30 μl) were stabilised with 20 μl acetic acid (final concentration 0.01 M) and stored at − 80°C for later analysis. Storage at − 80°C had no detrimental effects on the dialysate concentrations of 5-HT and 5-HIAA.
Protocol B: Biotelemetry
Experiments were performed on six rats simultaneously. Core body temperature (°C) and motor activity (expressed as arbitrary units) were monitored in 2-min intervals starting immediately after surgery. The signal from the radiotelemetric implant reached a receiver positioned underneath the rat's cage. Signals were processed by a specialist computer programme (Dataquest IV; Data Sciences International, St Paul, MN, USA). Ten days after surgery, rats were forced to swim for 15 min between 11:15 and 11:30 h, after which monitoring of core body temperature and activity continued for another 3.5 h (until 15:00 h).
Forced swim stress: Procedure and assessment of behaviour
Rats were forced to swim for 15 min in a glass beaker (internal diameter 18.5 cm) containing water at 19, 25 or 35°C. The water depth was 20 cm, which permitted the rats to touch the bottom of the glass beaker with the tip of their tail only. After completion of the swim procedure, rats were carefully removed from the water and dried with a towel before transfer to their home cage.
Video camera recordings during forced swimming were used to score the behaviour of the rat in 15 s intervals. The behaviour was scored as (1) climbing: active movements of the forepaws in and out of the water and resulting in an upward movement of the body usually directed towards the wall of the glass beaker; (2) swimming: active swimming motions more than necessary to keep the head above the water surface (often a fast paddling movement of the hind limbs); (3) floating: immobile position during which only small, occasional movements to keep the head above the water surface were allowed; (4) diving (all diving events were scored, also when they occurred between two scoring intervals); and (5) grooming: rapid wiping of the head usually with both forepaws.
Assessment of home cage behaviour during the microdialysis experiments (Protocol A)
The behaviour of the animals displayed in the home cage was scored by visual observation as described previously (Linthorst et al. Citation2002; De Groote et al. Citation2005). Briefly, behaviour was scored every 30 s (maximal number of counts per 15-min microdialysate sample is 30) and categorised as follows: (1) resting (sleeping, sitting, lying); (2) grooming (including scratching); (3) exploration (locomotion, sniffing, rearing, or digging); (4) eating or drinking; and (5) chewing (repetitive movements of the mouth or jaws not directed to an object or food). Total behavioural activity during sample collection is the sum of the behavioural categories 2–5 as described above.
5-HT and 5-HIAA measurement
Concentrations of 5-HT and 5-HIAA in dialysate samples were assessed using HPLC with electrochemical detection essentially as described previously (Peñalva et al. Citation2002; but with a Waters 460 pump). The detection limits for 5-HT and 5-HIAA were 0.3 and 1.0 fmol/injection on the column, respectively, (signal to noise ratio is 3). In one rat (swim stress at 35°C), 5-HT concentrations could not be measured due to a chromatographic problem (5-HIAA could be measured in this rat).
Histology
After completion of the experiments rats were killed by an overdose of pentobarbital (200 mg/kg body weight, intraperitoneal). The brains of rats used for microdialysis were collected in a 4% formalin solution for histological verification of probe placement (Linthorst et al. Citation1995). Only data from rats with probes in the hippocampus (see Linthorst et al. Citation1994 for illustration) were used for further analysis.
Materials
All chemicals (analytical or HPLC grade) used were obtained from Merck (Darmstadt, Germany) or Sigma–Aldrich Chemie GmbH (Taufkirchen, Germany).
Data analysis
Protocol A
For each rat basal 5-HT and 5-HIAA concentrations were calculated by averaging values of the baseline samples during which the animal was largely inactive ( ≤ 10% activity during the 15-min sample period). All concentrations were then expressed as percentage of this basal value (no samples were discarded). This method of calculation of basal concentrations is based on our extensive previous work demonstrating a positive correlation between hippocampal 5-HT and 5-HIAA concentrations and behavioural activity during ongoing (i.e. pre-treatment or pre-challenge) conditions (Linthorst et al. Citation1994; Citation1995; Citation1996; see also Linthorst Citation2005b). Analysis of variance (ANOVA) with repeated measures design was used to estimate the effects of time (within subject factor) and water temperature (between subject factor) on hippocampal 5-HT and 5-HIAA and on post-stress home cage behaviour. For the data on hippocampal extracellular 5-HT and 5-HIAA, these analyses were followed by post hoc simple contrasts to seek significant changes compared to baseline concentrations (Bonferroni corrected for multiple comparisons and using six time-levels to decrease the probability of type 1 errors; see also Peñalva et al. Citation2002; Linthorst et al. Citation2002; Oshima et al. Citation2003). Moreover, post hoc Scheffé comparisons were used to further statistically analyse differences among the three water temperatures. The time-levels used were (1) average of all baseline samples (09:00–11:15 h), (2) swim stress sample, (3) post-stress period 1 (11:30–12:15 h), (4) post-stress period 2 (12:15–13:00 h), (5) post-stress period 3 (13:00–13:45 h) and (6) post-stress period 4 (13:45–15:00 h). For the data on post-stress behaviour in the home cage, we assessed whether differences existed between data collected after swimming at different water temperatures by using post hoc Scheffé analyses in appropriate cases. Behavioural data were collapsed into 15-min intervals to reflect the sampling time for the collection of microdialysates.
Protocol B
The raw 2-min interval measures collected during the experiment are depicted in . Next, the core body temperature measurements were averaged over 15-min intervals reflecting the sampling time used in Protocol A to check for possible relationships between mean core body temperature and 5-HT and 5-HIAA concentrations in the hippocampus and behavioural activity ().
Behaviour during forced swim stress
The effect of water temperature on behaviours displayed during forced swim stress was investigated using ANOVA (with water temperature as the between subject factor), followed by the post hoc Scheffé multiple comparison test in appropriate cases.
Data in the figures are presented as group mean ± SEM. The SPSS software package (version 11.5, SPSS Inc., Chicago, USA) was used for statistical analysis. The level of significance was set at P < 0.05. For clarity, results of ANOVA are not depicted in the figures, but described in the text.
Results
Effects of forced swim stress at different water temperatures on hippocampal extracellular concentrations of 5-HT and 5-HIAA (Protocol A)
In Protocol A, rats were equipped for microdialysis in the hippocampus. During baseline, i.e. pre-stress, conditions the hippocampal concentrations of 5-HT and 5-HIAA showed small fluctuations that were related to the ongoing behavioural activity of the animals, as previously published (Linthorst Citation2005b; ). At 11:15 h, rats were forced to swim for 15 min. This procedure caused a significant change in the extracellular concentrations of 5-HT (effect of time: F(14,210) = 16.87, P ≤ 0.0005; ). The effects of forced swim stress on hippocampal 5-HT were strongly water temperature-dependent (interaction time × water temperature: F(28,210) = 4.37, P ≤ 0.0005; ). Thus, swimming at 35°C resulted in a marked increase in extracellular concentrations of 5-HT during and after swimming (maximum concentration 219 ± 37% of baseline), returning to baseline concentrations 75–90 min after return to the home cage. In contrast, swimming in water at 25°C caused a rise in 5-HT during the swim procedure (224 ± 27% of baseline) which was followed by an immediate decrease towards baseline concentrations in the first 15 min after completion of the swim procedure. Then 5-HT concentrations started to increase again gradually, reaching a second peak (213 ± 15% of baseline) about 75–90 min after the start of the stressor. Importantly, when rats swam in water at 19°C, hippocampal 5-HT concentrations did not change from baseline during and immediately after the swim stress procedure (a small, non-significant dip in 5-HT was seen in the first sample collected after swim stress). However, 5-HT concentrations started to increase gradually from 15 to 30 min after completion of swimming at 19°C and reaching a maximum another 30–45 min later (208 ± 41% of baseline; ).
Figure 1 Effects of forced swim stress at different water temperatures on hippocampal extracellular concentrations of 5-HT (A) and 5-HIAA (B; % of baseline) as assessed by in vivo microdialysis. After collection of nine 15-min baseline samples, rats were forced to swim for 15 min (swim sample is indicated by the arrow) in water at 19, 25 or 35°C. After return to the home cage another fourteen 15-min samples were collected. Time points on the x-axis indicate time of day at which collection of the sample was started. *, significantly different from baseline for 35°C; +, significantly different from baseline for 25°C; #, significantly different from baseline for 19°C (Bonferroni corrected simple contrasts). a, 19°C significantly different from 25 and 35°C; b, 19°C significantly different from 35°C; c, 25°C significantly different from 35°C (Scheffé post hoc comparisons). For ANOVA analyses see text under “Results”. 5-HT basal values were 6.70 ± 0.62 fmol/15 min sample (n = 18); 5-HIAA basal values were 5.5 ± 3.4 pmol/15 min sample (n = 19).
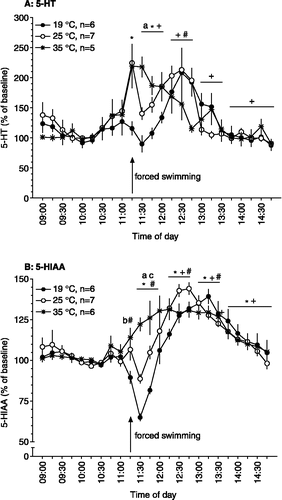
Various studies, applying pharmacological manipulations of the synthesis, release and metabolism of 5-HT, suggest that the metabolite 5-HIAA is largely derived from the metabolism of newly synthesised, unreleased 5-HT (Grahame-Smith Citation1974; Kuhn et al. Citation1986). Thus, extracellular concentrations of 5-HIAA are a useful indicator for the synthesis of 5-HT in the brain (Peñalva et al. Citation2002), and therefore, we also determined the concentrations of this metabolite during the swim stress paradigm. Forcing rats to swim caused significant changes in hippocampal extracellular concentrations of 5-HIAA (effect of time: F(14,224) = 43.35, P ≤ 0.0005; ). The effects of forced swimming on extracellular 5-HIAA were, similar to those on 5-HT, also clearly water temperature-dependent (interaction time × water temperature: F(28,224) = 8.74, P ≤ 0.0005; ). Hippocampal 5-HIAA concentrations increased when rats were forced to swim at 35°C, with a peak concentration (131 ± 6% of baseline) observed 45–60 min after the start of the swimming. In contrast, a significant decrease from baseline 5-HIAA concentrations was observed during swimming at 19°C, reaching, 15–30 min after the start of the stressor, a minimum concentration of 65 ± 2% of baseline. A small dip in extracellular concentrations of 5-HIAA (89 ± 3% of baseline) was also observed in the first sample collected after swimming at 25°C. Hereafter, hippocampal 5-HIAA concentrations increased gradually, reaching a maximum of 144 ± 4 and 139 ± 4% of baseline, 60 min and 90–105 min later, for 25 and 19°C, respectively, (). For all temperatures, 5-HIAA concentrations had returned to baseline at the end of the experiment (i.e. 3 h 45 min after start of the swim session). It should be noted that in vitro tests have shown that swimming at different water temperatures has no effect on dialysis efficiency (De Groote and Linthorst unpublished).
Forced swim stress-induced changes in behaviour: Effect of water temperature (Protocol A)
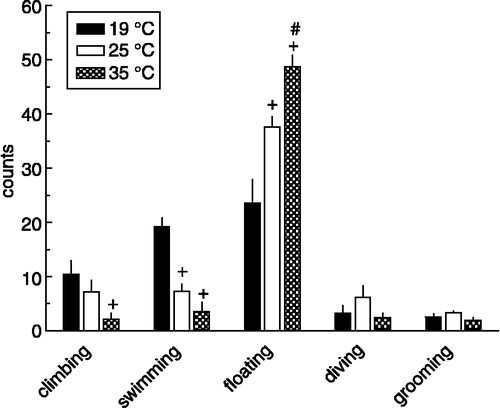
summarises the effects of water temperature on behaviour displayed by the rats during the 15-min forced swim session. Most experiments by us and other groups are routinely carried out at 25°C. At this water temperature rats first vigorously try to escape by climbing against the wall of the glass beaker and by diving towards the bottom of the beaker to investigate other escape possibilities (about 50% of rats dive during the forced swim session). After this phase, rats become less active and will start floating interspersed with some bouts of swimming. This characteristic behavioural profile is dependent on the water temperature used. For climbing, swimming and floating, but not for diving and grooming, significant effects of water temperature were found (climbing: F(2,16) = 3.76, P < 0.05; swimming: F(2,16) = 26.99, P ≤ 0.0005; floating: F(2,16) = 17.80, P ≤ 0.0005; diving: F(2,16) = 1.60, P>0.05; grooming: F(2,16) = 2.13, P>0.05). Thus, the amount of floating behaviour (immobility) decreased when colder water temperatures were applied, whereas, in contrast, active behaviours such as climbing and swimming increased (), probably in an attempt to counteract the drop in body temperature caused by these lower water temperatures (see below). It is important to note that similar results were obtained in rats during Protocol B (biotelemetry; data not shown), indicating that the swivel/microdialysis system did not influence the behaviour of the rats during the forced swim session.
Figure 2 Behaviour displayed by rats during a 15-min forced swim session at different water temperatures (19 (n = 6), 25 (n = 7) or 35 (n = 6) °C; behaviour counts). Rats were equipped with a microdialysis probe for simultaneous assessment of hippocampal 5-HT and 5-HIAA concentrations (). +, significantly different from 19°C; #, significantly different from 25°C (post hoc Scheffé analyses). For ANOVA analyses see text under “Results”. For definition of the behaviours displayed see Materials and Methods.
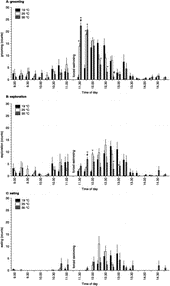
shows the effects of forced swimming at different water temperatures on post-stress (i.e. after return to the home cage) behaviour. A distinct succession of behaviours was observed, starting with grooming (drying of the fur), which was followed by exploratory behaviours and some bouts of eating food pellets. For grooming, eating and exploratory behaviour significant effects of water temperature were found (interactions between time and water temperature for grooming: F(26,208) = 9.65, P ≤ 0.0005; for eating F(26,208) = 2.25, P = 0.001; for exploration F(26,208) = 4.64, P ≤ 0.0005). Thus, whereas rats that had swum at 25 and 35°C started grooming immediately after return to the home cage, after swimming at 19°C rats remained largely immobile and showed mainly shivering behaviour during a period of about 30 min ().
Figure 3 Behaviour displayed by rats in their home cage before and after a 15-min forced swim session at different water temperatures (19 (n = 6), 25 (n = 7) or 35 (n = 6) °C; counts collapsed into 15-min intervals). Rats were equipped with a microdialysis probe for simultaneous assessment of hippocampal 5-HT and 5-HIAA concentrations (). Time points on the x-axis indicate time of day at which collection of the 15-min dialysate sample was started. +, significantly different from 19°C; #, significantly different from 25°C (post hoc Scheffé analyses). For ANOVA analyses see text under “Results”. For definition of the behaviours displayed see Materials and Methods.
Effects of forced swim stress at different water temperatures on core body temperature and motor activity (Protocol B)
For Protocol B, rats were equipped with an intraperitoneal radiotelemetric implant to monitor core body temperature and general motor activity. Forcing rats to swim for 15 min had profound effects on core body temperature (). In particular, swimming at 25 and 19°C caused dramatic decreases in core body temperature reaching minimum levels at the end of the stressor of 29.20 ± 0.32 and 25.21 ± 0.35°C, respectively. Only a small decrease in core body temperature (about 1.2°C from baseline) was observed in rats that were exposed to water at 35°C. After return to the home cage, core body temperature increased again reaching pre-stress levels after about 25, 60 and 80 min for 35, 25 and 19°C water temperature, respectively.
Figure 4 Effects of forced swim stress at different water temperatures on core body temperature (A; °C) and general motor activity (B; arbitrary units) as assessed by in vivo biotelemetry. After measuring baseline core body temperature and motor activity between 09:00 and 11:15 h, rats were forced to swim for 15 min (as indicated by the grey bar and arrow) in water of 19, 25 or 35°C (n = 6 for all groups). After return to the home cage recording continued until 15:00 h. Time points on the x-axis indicate time of day at which the measurement was made. For sake of clarity error bars are not depicted in .
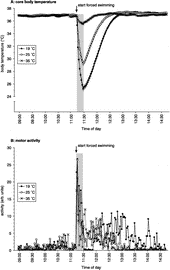
The effects of water temperature on motor activity during and after swim stress are depicted in . The radiotelemetric measurements mimicked and hence confirmed our visual observations as presented in and . Thus, rats were less active during swimming in water at 35°C than at the lower temperatures. Furthermore, as can be clearly seen in , rats that had been exposed to water at 19°C showed a period of no motor activity during the first 20 min after return into their home cage.
Relationship between the effects of water temperature on core body temperature and hippocampal extracellular concentrations of 5-HT and 5-HIAA
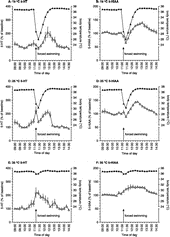
To investigate the relationship between the effects of forced swimming on core body temperature and hippocampal serotonergic neurotransmission, the body temperature data (i.e. the 2-min interval data as shown in ) were averaged over 15-min intervals to match the dialysate sample duration (). Averaging the core body temperature data over 15-min intervals revealed that, in the case of swimming at 19 or 25°C, the lowest average core body temperature was found during the first dialysate sample collected after return of the rats into the home cage (–D). As can seen from , there is a striking (i.e. water temperature-dependent) relationship between the effects of forced swimming at different water temperatures on 5-HT and 5-HIAA on the one hand and core body temperature on the other hand. Thus, swimming in water of 35°C caused only a slight decrease in core body temperature together with a rapid and profound increase in both hippocampal extracellular concentrations of 5-HT and 5-HIAA ( and F). In contrast, when the core body temperature of the animals was severely reduced by swimming at low water temperatures, 5-HT and 5-HIAA concentrations in the hippocampus show bimodal changes (–D). At 19°C, the concentrations of 5-HT and 5-HIAA remained at baseline or decreased below baseline concentrations during the phase when the core body temperature of the rats decreased ( and B). After returning to the home cage, both core body temperature and concentrations of 5-HT and 5-HIAA started to increase again. The situation at 25°C was more complex. During and after forced swimming in water at 25°C, 5-HIAA concentrations seemed to follow the same pattern as core body temperature, namely a decrease followed by an increase (). Yet, 5-HT concentrations displayed two peaks in response to forced swim stress at 25°C (). A first peak was observed during the forced swim session. This peak was followed by a rapid decrease towards baseline 5-HT concentrations after which both core body temperature and 5-HT concentrations again started to increase gradually.
Figure 5 Relationship between the effects of forced swim stress at different water temperatures (19°C: A and B; 25°C: C and D; 35°C: E and F) on core body temperature (closed triangles) and hippocampal extracellular concentrations of 5-HT and 5-HIAA (open triangles). The 2-min body temperature data as depicted in were averaged over 15-min intervals to match the 15-min dialysate sample duration and are here depicted as means ± SEM. For further details, including n-values, see legends to and .
Discussion
Here we demonstrate that forced swim stress produces distinct effects on hippocampal extracellular concentrations of 5-HT and 5-HIAA, and on stress-induced behaviour, which are clearly dependent on the water temperature. We hypothesise that the changes in body temperature caused by forced swimming at different water temperatures shape the profiles of the hippocampal serotonergic neurotransmission and the behavioural responses to swim stress.
We used in vivo biotelemetry to measure the effects of swim stress on core body temperature and motor activity with a high time resolution (i.e. 2 min intervals). The advantages of biotelemetry, as compared to measurements of rectal temperature, clearly are (1) the stress-free nature of the recording, (2) the above-mentioned high time resolution and (3) the possibility to record during a swim stress procedure. Forced swim stress caused water temperature-dependent effects on core body temperature. Whereas swimming for 15 min at 35°C hardly had an effect, swim stress at 19 and 25°C caused rapidly substantial decreases in core body temperature. Our observations are in accordance with early studies by Eric Stone (Stone Citation1970b) demonstrating that a 20-min swim session at 14.5°C decreased rectal temperature to 21–22°C, and by Porsolt et al. (Citation1979) describing that rat rectal temperature drops about 7°C during swimming for 15 min at 25°C. In the latter study, a relatively small decrease in rectal temperature (about 2.5°C) was found in rats that had swum at 35°C. In a recent study on stressor controllability, water temperature-dependent decreases in core body temperature were found during a 80-trial intermittent swim stress paradigm (Drugan et al. Citation2005). Also in mice, forced swim stress at 25°C, but not at 35°C, resulted in a profound decrease in rectal temperature (Arai et al. Citation2000). Interestingly, not only core body temperature but also brain temperature has been found to decrease in mice during swimming at 25°C, but not at 37°C (Taltavull et al. Citation2003).
The water temperature used in forced swim stress also determines behaviour in the swim container as well as post-stress behaviour in the home cage. We found that lowering the water temperature resulted in significantly less immobility and more climbing/struggling and swimming behaviour during the swim procedure, which is in agreement with results of other studies (Abel Citation1993; Bilang-Bleuel et al. Citation2005). The increased activity of the animals in the swim container at lower water temperatures may represent a means of heat production to counteract the rapid drop in core body temperature. After termination of the forced swim stress procedure, a distinct sequence of behaviours is displayed by the rats in their home cage, i.e. rats first groom to dry their fur and then start to explore the cage and to eat food pellets. The time course of these behaviours was, however, significantly dependent on the water temperature used in the preceding forced swim session. Interestingly, rats that had swum at 19°C remained largely immobile and mainly shivered for approximately 20 min before they started their behavioural sequence with grooming. Water temperature-dependent effects on post-swim (30 min at 14.5 or 37°C) behaviour have also been described by Stone (Stone Citation1970a). This author demonstrated that forced swimming at 14.5°C, as compared to 37°C, results in inhibition of post-test explorative behaviour in rats. The delayed increase reported here in behavioural activity of rats after swimming at 19°C may be related to the severe decrease in core body temperature in these rats; this notion is strengthened by Stone's observation that rapid re-warming of rats in warm, 38°C, water after exposure to cold water prevents the inhibition of explorative behaviour (Stone Citation1970a).
Apart from water temperature-dependent changes in core body temperature and behaviour, water temperature also had a profound influence on the effects of forced swim stress on hippocampal serotonergic neurotransmission. Swim stress at 35°C resulted in an immediate and prolonged increase in hippocampal extracellular concentrations of 5-HT and of its metabolite 5-HIAA. In contrast, swimming at lower water temperatures (19 and 25°C) resulted in a biphasic 5-HT response. Similar results were observed in a previous study on swim stress at 25°C and hippocampal 5-HT by our group (Linthorst et al. Citation2002). Also 5-HIAA concentrations showed a clear biphasic response to forced swim stress at lower water temperatures with a significant decrease below baseline concentrations during and immediately after swim stress at 19°C. In line with this, a 10-min swim session at 25°C causes a significant transient decrease in hippocampal 5-HIAA concentrations in mice with a 129/OLA-CD1 background (Peñalva et al. Citation2002), but no effect on 5-HIAA concentrations during forced swimming in C57BL6/N mice (Oshima et al. Citation2003), although a monophasic increase in hippocampal 5-HT was observed in these mouse strains.
To elucidate the putative relationship between the core body temperature and neurochemical responses to forced swim stress, body temperature measurements were averaged over 15-min intervals to match the dialysate sample duration and subsequently plotted together with 5-HT or 5-HIAA (). It appears from that two mechanisms govern the 5-HT and 5-HIAA responses, i.e. a forced swim stress-induced and/or an activity-induced increase, and a temporary quenching of this increase and/or a transient decrease in concentrations when core body temperature falls below ∼31°C. Thus, it is clear from our data that swimming at progressively lower water temperatures, thus leading to greater decreases in core body temperature, results in a progressive attenuation of the initial forced swimming-induced surge in 5-HT, and to a decrease even in 5-HIAA. A post-stress enhancement in 5-HT and 5-HIAA appears to be maintained at lower water temperatures albeit in a delayed fashion. This gradual increase in hippocampal extracellular 5-HT and 5-HIAA after swim stress at low water temperatures (i.e. 19 and 25°C) occurred simultaneously not only with the gradual increase in core body temperature towards normal, pre-stress levels but also with the (delayed) increased behavioural activity of the animals observed during this phase.
These new data are of particular interest as they give an explanation for the disparate results published in the literature on the effect of swim stress on hippocampal extracellular concentrations of 5-HT. Kirby et al. (Citation1995) compared the effects of a 30-min swim stress paradigm at 21–22°C on serotonergic neurotransmission in different brain regions using in vivo microdialysis in rats (Kirby et al. Citation1995). They found that a 30-min swim stress at this water temperature, which dramatically reduces core body temperature (Flachskamm and Linthorst, unpublished), had no effect on extracellular concentrations of 5-HT in the hippocampus, but caused a prolonged decrease in extracellular 5-HIAA. Forcing rats to swim for 5 min at 25°C was found to decrease ventral hippocampal 5-HT concentrations (5-HIAA was not measured in that study and a re-uptake inhibitor was added to the perfusion fluid (Adell et al. Citation1997)). In contrast, Rueter and Jacobs (Citation1996) demonstrated that 30-min forced swim stress in water at 30–35°C caused an increase in extracellular concentrations of both 5-HT and 5-HIAA in the rat hippocampus, which is in agreement with our results at 35°C. Thus, together with these literature data, our results clearly show that water temperature is a major determinant for the serotonergic response in the hippocampus to forced swim stress. Importantly, other factors may also shape the 5-HT response to this form of stress as we have found that rats wearing a plastic collar around their neck (instead of a skull-connected peg) to connect to a swivel system show a dramatic (about 1500%) increase in extracellular hippocampal 5-HT concentration, but only when they dive during the swim session; in the present study no plastic collars were used, all rats were connected to the swivel system via a peg on their head (Linthorst et al. Citation2002)].
It is well-known that 5-HT plays an important role in the regulation of body temperature. In this respect, most studies have concentrated on the effects of 5-HT within hypothalamic and preoptic structures. However, a series of studies from our laboratory has shown that hippocampal extracellular concentrations of 5-HT increase, in the absence of any motor activity, during hyperthermia caused by the administration of bacterial endotoxin and certain cytokines (Linthorst et al. Citation1995). Naturally, the question arises how body temperature can affect serotonergic neurotransmission at the level of a higher limbic brain structure such as the hippocampus? It is possible that changes locally within the hippocampus affect the extracellular concentrations of 5-HT. This possibility is underscored by electrophysiological studies demonstrating alterations in bio-electrical signals in the hippocampus when lowering body temperature and during re-warming (see Andersen and Moser Citation1995). Moreover, given that 5-HT and GABA interact at the level of the hippocampus, it is of interest to note that we have found water temperature-dependent changes also in extracellular concentrations of GABA in the hippocampus in response to forced swim stress, i.e. an increase at 35°C and a decrease at 25°C (De Groote and Linthorst Citation2007). However, the observation that also 5-HIAA concentrations show biphasic responses to forced swim stress indicates that water temperature-dependent changes in the firing rate of raphé neurons may be an alternative explanation. Extracellular concentrations of 5-HIAA are thought to reflect the metabolism of newly-synthesised 5-HT, rather than reflecting metabolism of released 5-HT, and thus can be taken as an index for the effects of manipulations on the synthesis of this indoleamine (Peñalva et al. Citation2002). The 4 mm long dialysis membranes used in this study were implanted such that they just entered the hippocampus from a dorsal approach and, thus, that dialysis would take place over the whole depth of the hippocampus (illustrated in Beekman et al. Citation2005). This part of the hippocampus receives serotonergic innervation from both the dorsal (DRN) and median raphé nucleus (MRN). Unfortunately, information on the effects of body temperature on the firing rate of the DRN and MRN is scarce and in part contradictory (Jacobs and Azmitia Citation1992), probably also due to variations in experimental protocol such as species, anaesthesia and magnitude and direction of the body temperature changes under study. Of particular interest, however, is the observation that infusion of tetrodotoxin (TTX) into the MRN, effectively decreasing the neuronal activity of this nucleus, induces hypothermia in conscious freely-moving rats (Ishiwata et al. Citation2001). Furthermore, recent electrophysiological studies have demonstrated that neurons of the more caudally located raphé nuclei (raphé pallidus, raphé magnus), responsible for descending serotonergic projections, respond to changes in body temperature (Rathner et al. Citation2001; Nason and Mason Citation2006). These are important observations as it is known that a cross-talk exists between these raphé nuclei and the raphé nuclei of the rostral serotonergic system, including the DRN and MRN. Given the hypothesis that the function of 5-HT is to coordinate behavioural and autonomic responses to environmental stimuli (Jacobs and Fornal Citation1999), the input from lower brainstem regions to the DRN and/or MRN may ultimately be translated into water temperature-dependent changes in serotonergic neurotransmission in higher brain structures such as the hippocampus. Alternatively or in addition, the raphé nuclei receive direct projections from the preoptic area and other hypothalamic areas (e.g. dorsomedial hypothalamus) known to play an important role in body temperature regulation. Thus, it is possible that these preoptic and hypothalamic structures play a modulatory role in the effects of forced swimming on extracellular 5-HT and 5-HIAA responses in the hippocampus.
Our data suggest that body temperature is a key determinant shaping the outcome of the effects of forced swim stress on serotonergic neurotransmission and behaviour. However, presently it cannot be excluded that changes in other stress-responsive systems (e.g. the hypothalamic–pituitary–adrenocortical axis, other neurotransmitter systems (e.g. GABA, noradrenaline), neuropeptide hormones (vasopressin, oxytocin) and blood chemicals (lactate, glucose) play a role (directly or indirectly) in forced swim stress-induced changes in hippocampal serotonergic neurotransmission (Stone Citation1970a; Abel Citation1993; Wotjak et al. Citation1998; De Groote and Linthorst Citation2007).
In conclusion, our results clearly demonstrate the impact of water temperature in the forced swimming test on serotonergic neurotransmission in the hippocampus; a brain structure of pivotal importance in the coordination of the stress response and in learning and memory. As such, these findings emphasise that the outcome of forced swim stress on parameters such as behaviour, neurochemistry and others should be carefully interpreted in the light of the autonomic responses induced by this procedure.
References
- Abel EL. Physiological correlates of the forced swim test in rats. Physiol Behav 1993; 54: 309–317
- Adell A, Casanovas JM, Artigas F. Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology 1997; 36: 735–741
- Andersen P, Moser EI. Brain temperature and hippocampal function. Hippocampus 1995; 5: 491–498
- Arai I, Tsuyuki Y, Shiomoto H, Satoh M, Otomo S. Decreased body temperature dependent appearance of behavioral despair in the forced swimming test in mice. Pharmacol Res 2000; 42: 171–176
- Beekman M, Flachskamm C, Linthorst ACE. Effects of exposure to a predator on behaviour and serotonergic neurotransmission in different brain regions of C57bl/6N mice. Eur J Neurosci 2005; 21: 2825–2836
- Bilang-Bleuel A, Rech J, De Carli S, Holsboer F, Reul JMHM. Forced swimming evokes a biphasic response in CREB phosphorylation in extrahypothalamic limbic and neocortical brain structures in the rat. Eur J Neurosci 2002; 15: 1048–1060
- Bilang-Bleuel A, Ulbricht S, Chandramohan Y, De Carli S, Droste SK, Reul JMHM. Psychological stress increases histone H3 phosphorylation in adult dentate gyrus granule neurons: Involvement in a glucocorticoid receptor-dependent behavioural response. Eur J Neurosci 2005; 22: 1691–1700
- Borsini F. Role of the serotonergic system in the forced swimming test. Neurosci Biobehav Rev 1995; 19: 377–395
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol Sci 2002; 23: 238–245
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 2005; 29: 547–569
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 1995; 64: 477–505
- De Groote L, Linthorst ACE. Exposure to novelty and forced swimming evoke stressor-dependent changes in extracellular GABA in the rat hippocampus. Neuroscience 2007, in press
- De Groote L, Penalva RG, Flachskamm C, Reul JMHM, Linthorst ACE. Differential monoaminergic, neuroendocrine and behavioural responses after central administration of corticotropin-releasing factor receptor type 1 and type 2 agonists. J Neurochem 2005; 94: 45–56
- De Pablo JM, Parra A, Segovia S, Guillamon A. Learned immobility explains the behavior of rats in the forced swimming test. Physiol Behav 1989; 46: 229–237
- Drugan RC, Eren S, Hazi A, Silva J, Christianson JP, Kent S. Impact of water temperature and stressor controllability on swim stress-induced changes in body temperature, serum corticosterone, and immobility in rats. Pharmacol Biochem Behav 2005; 82: 397–403
- Grahame-Smith DG. How important is the synthesis of brain 5-hydroxytryptamine in the physiological control of its central function?. Adv Biochem Psychopharmacol 1974; 10: 83–91
- Ishiwata T, Hasegawa H, Yasumatsu M, Akano F, Yazawa T, Otokawa M, Aihara Y. The role of preoptic area and anterior hypothalamus and median raphe nucleus on thermoregulatory system in freely moving rats. Neurosci Lett 2001; 306: 126–128
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev 1992; 72: 165–229
- Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology 1999; 21: S9–S15
- Kirby LG, Allen AR, Lucki I. Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res 1995; 682: 189–196
- Kuhn DM, Wolf WA, Youdim MB. Serotonin neurochemistry revisited: A new look at some old axioms. Neurochem Int 1986; 8: 141–154
- Linthorst ACE. Interactions between corticotropin-releasing hormone and serotonin: Implications for the aetiology and treatment of anxiety disorders. Handb Exp Pharmacol 2005a; 169: 181–204
- Linthorst ACE. Stress, corticotropin-releasing factor and serotonergic neurotransmission. Handbook of stress and the brain; part 1: The neurobiology of stress, T Steckler, NH Kalin, JMHM Reul. Elsevier, Amsterdam 2005b; 503–524
- Linthorst ACE, Flachskamm C, Holsboer F, Reul JMHM. Local administration of recombinant human interleukin-1 beta in the rat hippocampus increases serotonergic neurotransmission, hypothalamic–pituitary–adrenocortical axis activity, and body temperature. Endocrinology 1994; 135: 520–532
- Linthorst ACE, Flachskamm C, Müller-Preuss P, Holsboer F, Reul JMHM. Effect of bacterial endotoxin and interleukin-1 beta on hippocampal serotonergic neurotransmission, behavioral activity, and free corticosterone levels: An in vivo microdialysis study. J Neurosci 1995; 15: 2920–2934
- Linthorst ACE, Flachskamm C, Holsboer F, Reul JMHM. Activation of serotonergic and noradrenergic neurotransmission in the rat hippocampus after peripheral administration of bacterial endotoxin: Involvement of the cyclo-oxygenase pathway. Neuroscience 1996; 72: 989–997
- Linthorst ACE, Flachskamm C, Hopkins SJ, Hoadley ME, Labeur MS, Holsboer F, Reul JMHM. Long-term intracerebroventricular infusion of corticotropin-releasing hormone alters neuroendocrine, neurochemical, autonomic, behavioral, and cytokine responses to a systemic inflammatory challenge. J Neurosci 1997; 17: 4448–4460
- Linthorst ACE, Peñalva RG, Flachskamm C, Holsboer F, Reul JMHM. Forced swim stress activates rat hippocampal serotonergic neurotransmission involving a corticotropin-releasing hormone receptor-dependent mechanism. Eur J Neurosci 2002; 16: 2441–2452
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry 1998; 44: 151–162
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev 2005; 29: 829–841
- Nason MW, Jr., Mason P. Medullary raphe neurons facilitate brown adipose tissue activation. J Neurosci 2006; 26: 1190–1198
- Oshima A, Flachskamm C, Reul JMHM, Holsboer F, Linthorst ACE. Altered serotonergic neurotransmission but normal hypothalamic–pituitary–adrenocortical axis activity in mice chronically treated with the corticotropin-releasing hormone receptor type 1 antagonist NBI 30775. Neuropsychopharmacology 2003; 28: 2148–2159
- Peñalva RG, Flachskamm C, Zimmermann S, Wurst W, Holsboer F, Reul JMHM, Linthorst ACE. Corticotropin-releasing hormone receptor type 1-deficiency enhances hippocampal serotonergic neurotransmission: An in vivo microdialysis study in mutant mice. Neuroscience 2002; 109: 253–266
- Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977; 266: 730–732
- Porsolt RD, Deniel M, Jalfre M. Forced swimming in rats: Hypothermia, immobility and the effects of imipramine. Eur J Pharmacol 1979; 57: 431–436
- Rathner JA, Owens NC, Mcallen RM. Cold-activated raphe-spinal neurons in rats. J Physiol 2001; 535: 841–854
- Rueter LE, Jacobs BL. A microdialysis examination of serotonin release in the rat forebrain induced by behavioral environmental manipulations. Brain Res 1996; 739: 57–69
- Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci 1997; 9: 637–642
- Saper CB. The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci 2002; 25: 433–469
- Stone EA. Behavioral and neurochemical effects of acute swim stress are due to hypothermia. Life Sci Part 1 Physiol Pharmacol 1970a; 9: 877–888
- Stone EA. Swim-stress-induced inactivity: Relation to body temperature and brain norepinephrine, and effects of d-amphetamine. Psychosom Med 1970b; 32: 51–59
- Taltavull JF, Chefer VI, Shippenberg TS, Kiyatkin EA. Severe brain hypothermia as a factor underlying behavioral immobility during cold-water forced swim. Brain Res 2003; 975: 244–247
- Van de Kar LD, Blair ML. Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrinol 1999; 20: 1–48
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: New insights into the secretory capacities of peptidergic neurons. Neuroscience 1998; 85: 1209–1222