Abstract
Exposure to an acute naturalistic stressor induces both psychological and physiological changes in humans. The two studies reported here explored the impact of exposure to an acute naturalistic stressor on state anxiety, working memory and HPA axis activation (salivary cortisol). In both experiments, ten healthy male participants were exposed to an acute naturalistic stressor, helicopter underwater evacuation training (HUET), and their physiological and behavioural responses before (first study) and after (second study) the stressor were compared to ten non-stressed controls. The results of both experiments showed that working memory performance was preserved during anticipation of an acute stressor, but impairments were observed immediately after stress exposure. Participants reported significantly higher state anxiety levels during anticipation and following stress exposure, whereas significant elevations in cortisol levels were only observed 25 min post exposure to stress, but not before or immediately after stress exposure. The results of both experiments demonstrated a dissociation between behavioural and biochemical measures and provided evidence for a dissociation of the effects of stress on cognitive and physiological measures depending on the time of testing, with cognitive impairments most evident following stress exposure.
Introduction
Exposure to a short-term acute stressor, such as the threat of psychological or physical harm, has been shown to cause cognitive impairment (Lazarus Citation1966) leading to a reduced ability to produce useful behaviours in response to that threat (Mileti and Peek Citation2000). In more extreme cases, such impairment can lead to cognitive paralysis or “freezing” in the face of danger (Leach Citation2005). Anecdotal reports (Leach Citation1994) and the limited empirical research that has been completed (Morgan et al. Citation2006) suggest that working memory is particularly vulnerable to demanding, time pressured, stressful situations. According to Funahashi (Citation2001, p. 147) working memory allows us to, “…monitor the external world continuously, pay attention to necessary information, input wanted information, retrieve related information from long-term memory, manipulate and integrate information and then output appropriate information to particular brain areas”. Working memory is, therefore, critical when dealing with novel and threatening environmental situations (Miyake and Shah Citation1999).
Working memory function is considered to be governed by the prefrontal cortex (PFC) (Braver et al. Citation1997) with physical damage to the PFC resulting in an inability to inhibit distracting stimuli along with poor judgment, impaired planning and impaired decision-making (Drewe Citation1975; Stuss and Benson Citation1986; Woods and Knights Citation1986; Thompson-Schill et al. Citation2002). However, impairment in working memory function can also occur as a result of changes in neurochemistry or increased cognitive demands without physical damage to the PFC.
At a biological level, it is well established that exposure to threats or stressors results in activation of two major endocrine systems, the hypothalamic–anterior pituitary–adrenocortical axis (HPA) and the sympatho-adrenomedullary axis (SAM axis). Activation of the HPA axis is associated with the release of glucocorticoids from the adrenal cortex (corticosterone in rodents and cortisol in humans) and activation of the SAM axis leads to a release of adrenaline from the adrenal medulla. A major physiological role of activation of both endocrine systems is considered to be a temporary increase in energy production at the expense of processes that not required for immediate survival (Sapolsky Citation1992).
Increases in circulating cortisol levels have been observed in response to both naturalistic and laboratory stressors with a response peak occurring 20–40 min after exposure (Ring Citation2002; Dickerson and Kemeny Citation2004). Some studies have also suggested that cortisol levels may increase in anticipation of threat. For example, increases in cortisol levels have been observed prior to public speaking (Lupien and McEwen Citation1997; Garcia-Leal et al. Citation2005) and in novice parachutists before their first jump (Deinzer et al. Citation1997).
Although the physiological response to stress is overall highly adaptive since it allows the individual to cope with an emergency energetic crisis (“fight or flight” response, Cannon Citation1929), previous research has shown that even relatively brief stressors do not have a uniformly positive influence on behaviour, and more specifically on cognition and memory. For example, it has been shown that increased glucocorticoid levels through stress exposure or pharmacological manipulation (administration of hydrocortisone) can have impairing as well as enhancing effects on cognition. In individual studies glucocorticoid elevation has been associated with impairments in declarative memory (Newcomer et al. Citation1994, Citation1999; Lupien et al. Citation1999; Tops et al. Citation2004), enhanced short-term memory performance (Vedhara et al. Citation2000), deficits in working memory (Kirschbaum et al. Citation1996), impairments in autobiographical memory (Buss et al. Citation2004), and impairments in attentional processing (Bohnen et al. Citation1990). However, most studies in human populations observe no clear cognitive or memory effects after exposure to an acute psychological stressor (Kirschbaum et al. Citation1996; Domes et al. Citation2002; Takahashi et al. Citation2004) and currently there is no consensus as to the impact that stress has on cognition.
Glucocorticoids are lipophilic hormones and can readily pass the blood–brain barrier, where they have been shown to influence multiple regions of the brain. Glucocorticoid receptors have been found in multiple areas of the brain including those that are relevant to cognition and memory, namely, the hippocampus (important in declarative and spatial memory), the amygdala (important in emotional memory) and the PFC (important in working memory) (e.g. McEwen et al. Citation1986; Cahill and McGaugh Citation1998; Aggleton and Brown Citation1999). Consequently, it is not surprising that variations in glucocorticoid levels should affect cognitive performance pertaining to these brain areas and that some stress hormones might act as endogenous modulators of cognitive or more specifically memory processes. Since the frontal cortex contains densely packed glucocorticoid receptors (Lupien et al. Citation1999) it follows that any changes in cortisol levels could result in corresponding impairments in those cognitive functions associated with the PFC, such as working memory. This suggestion is supported by recent studies that found elevations in cortisol correlated with working memory dysfunction (Lupien et al. Citation1999; Elzinga and Roelofs Citation2005; Oei et al. Citation2006).
However, it has been demonstrated that both storage and processing capacities of working memory can become restricted during exposure to an acute stressor without apparent changes occurring at the neurochemical level (Engle Citation1996). It has been argued that this might be due to cognitive overload due to the increase in information that arises from the novelty of the stressor (Matthews Citation1996; Miyake and Shah Citation1999). In addition, stressors can result in increased anxiety levels, and a corresponding increase in affect-evoked intrusive thoughts has previously been shown to reduce both the storage and processing capacities of working memory (Eysenck and Calvo Citation1992; Matthews Citation1996; Dutke and Stoebber Citation2001; Ashcraft Citation2002; Somer et al. Citation2005). Overall, despite a wide variety of research avenues it is still unclear which aspects of cognitive performance are affected by an acute stressor and to what extent. The failure to clearly delineate the effects of stress on cognition could be explained by limitations in experimental design used in previous research. For example, the majority of the investigations into the relationship between stress, stress hormones and cognition have been either anecdotal (Cohen and Raghavulu Citation1979; Leach Citation1994) or largely experimental (i.e. using laboratory stress paradigms such as the TSST or the DISS or pharmacologically induced changes: Newcomer et al. Citation1994).
Administration of synthetic cortisol out of the context of stressful experience appears to alter the behavioural and neuronal response, for example impairments in retrieval were observed compared to impaired encoding and recall following stress-induced cortisol elevations in humans (De Quervain et al. Citation2000, Citation2003; Buss et al. Citation2004). In addition, the majority of work in this area has focused on the period after the event (Bryant and Panasetis Citation2001; Yehuda et al. Citation2004). However, five major stages of stress have been identified: pre-impact (time before stressor onset), impact, recoil (immediately after impact), rescue and post trauma (Leach Citation1994) and anecdotal reports and previous research suggest that different patterns of behavioural and physiological responses can be observed during different stages of a survival situation or brief naturalistic stressor (Leach Citation1994; Cassuto and Tarnow Citation2003). Consequently, the aim of this study was to examine the effect of exposure to a naturalistic acute stressor on cognition, mood and cortisol levels at two different stages. Experiment 1 examined the effect of anticipation (pre-exposure) to an acute stressor on working memory, HPA axis activation (cortisol increases) and state anxiety whilst experiment 2 examined the same factors immediately following exposure to the stressor.
In summary, the aims of this study were to examine changes in HPA axis activation (salivary cortisol concentrations) and working memory performance immediately prior to and immediately after exposure to an acute stressor, namely, escaping from a submerged and inverted helicopter body. Based on previous research and anecdotal reports we expected that anticipation and exposure to a naturalistic stressor (HUET) would result in decrements in working memory performance and increases in cortisol and anxiety levels compared to non-stressed controls and baseline.
Experiment 1
In experiment 1 the effect of anticipation (pre-exposure) to an acute stressor on working memory, state anxiety and HPA axis activation (cortisol increases) was examined.
Method
Participants
Participants in the experimental condition comprised 10 healthy male volunteers, aged between 25 and 50 years (mean = 35 years), recruited via opportunity sampling from individuals attending sea survival training at Fleetwood Nautical College (UK). Ten male, age-matched control participants, of a similar educational level, were recruited from staff and students at the University of Lancaster via opportunity sampling.
A modified version of the Blood Services screening questionnaire and a confidential medical questionnaire were used for exclusion criteria. These comprised: (i) active infections, jaundice within the last year, hepatitis, haemophilia or HIV antibody positive; (ii) any history of neurological or psychiatric illness; (iii) participants who awoke earlier than 6:30 am or later than 8 am to reduce the impact of cortisol diurnal patterns (Edwards et al. Citation2001); (iv) participants who consumed food or drink (apart from water) within 1 h before testing and (v) participants taking medication known to affect cortisol levels, such as anti-depressants (Kirschbaum et al. Citation1996). All participants gave written consent and were tested in accordance with the national and local ethics guidelines according to the Declaration of Helsinki.
Materials and apparatus
Helicopter underwater evacuation training (HUET) simulator
The naturalistic acute stressor used in both experiments was the HUET task. HUET is mandatory for UK military and civilian personnel (e.g. oil rig workers) who fly over water in helicopters. During this training students are strapped into a helicopter simulator that is suspended over an environmental pool. On command the students adopt the crash position whereupon the helicopter undergoes a rapid but controlled ditching into the water, submerges and rotates 180°. The students are required to remain seated upside down in the submerged helicopter until it has ceased all movement then they release their seatbelts, evacuate the aircraft, inflate their lifejackets and ascend to the surface. The HUET procedure takes approximately 5 min. After surfacing from the helicopter simulator the students then swim to and board a life raft where they remain for 20 min before exiting the pool and report for testing.
Assessment of anxiety
Anxiety levels in both experiments were measured using Form Y of the State-Trait Anxiety Inventory (Spielberger Citation1966). The inventory comprises 40 statements, 20 of which assess state anxiety and 20 assess trait anxiety. For example, “I feel at ease” or “I am a steady person”. Participants circle one of four options relating to how much they agree with each statement (ranging from “Not at all” to “Almost Always”). The answers circled gave a total score for state and trait anxiety, with a high final score indicating a high level of anxiety.
Saliva collection and biochemical analysis
Saliva samples were taken using a salivette saliva sampling device (Sarstedt Ltd, Leicester, UK). Participants were instructed to give unstimulated saliva samples by placing a salivette under their tongue for a timed 2 min period. Samples were stored at − 40°C until analysis. For analysis saliva was recovered by thawing the salivette at room temperature for 15 min then centrifuging (1500 rpm) for 15 min. Cortisol concentration (nmol/l) in saliva was then determined by a high sensitivity salivary cortisol enzyme immunoassay kit (Salimetrics, USA) as per the manufacturer's instructions. Intra-assay variation is very low with a coefficient of variation of less than 4%.
Working memory test
Working memory performance was assessed using the operation-word span task (Turner and Engle Citation1989; Cantor and Engle Citation1993) since it assesses both working memory storage and processing capacity. In this test participants were presented with a simple arithmetic operation, such as “2 × 3+4 = 10”. They read aloud the operation presented and then responded whether the answer given was correct or incorrect. Responses were made via a computer keyboard (Toshiba Satellite 4030 CDT PC laptop) with “c” for correct and “i” for incorrect. After responding, a high frequency, one-syllable word was presented on screen, e.g. “dog” which the participants read aloud. After a pre-set number of operation-word combinations (between 2 and 7) participants recalled as many words as possible from the list. A total of 15 trials were presented. Storage was determined by the number of words correctly recalled whilst processing was determined by the number of correct true/false responses given for the arithmetic operations.
Procedure
Participants were requested not to consume food or drink (apart from water) for at least 1 h before testing because glucose, carbohydrate and caffeine intake has been shown to affect cortisol reactivity (Nehlig et al. Citation2003). Also, the effects of the cortisol diurnal pattern (Edwards et al. Citation2001) were controlled for by testing both experimental and control participants at the same time of day. Testing was carried out over a 5 day sea survival training course with data collected on days 1, 3 and 5 of the training course.
Day 1 (10:30 am): Participants provided a saliva sample and completed the state-trait anxiety questionnaire and the operation-word span task.
Day 3 (10:30 am): Participants completed the state anxiety questionnaire, the operation-word span task and provided a saliva sample prior to task exposure. Participants in the experimental condition then undertook the HUET training exercise that lasted 25 min. Participants in the control condition completed light office work. Twenty-five minutes after exposure to the stressor or office work participants completed a further state anxiety questionnaire and provided another saliva sample.
Day 5 (10:30 am): All participants completed the state anxiety questionnaire, the operation-word span task and provided one saliva sample.
Statistical analyses
Trait anxiety levels were analysed using a one-way analysis of variance (ANOVA). The between subject factor was condition (HUET vs. control). State anxiety levels were assessed on day 1, 3 (prior to task and post-task) and on day 5. State anxiety levels were examined using a two-way ANOVA. The between subject factor was condition (HUET vs. control), the within subject factor was time (i.e. when anxiety levels were assessed). Saliva samples were taken at four time points during the 5-day course: on day 1, day 3 (prior to task and post-task) and on day 5. Cortisol levels were examined using a two way ANOVA with repeated measures on 1 factor (time). The two different factors were condition (HUET vs. control) and time (when cortisol levels were measured). Working memory performance was measured at three time points during the 5-day course: on day 1, day 3 (prior to task) and on day 5. The results were analysed using a two way ANOVA. The between subject factor was condition (HUET vs. controls), the within subject factor was time (when working memory was assessed).
Results
Trait anxiety
There was no significant difference in trait anxiety across condition [F(1,18) = 1.50, p = 0.24, ns] indicating that participants in the stress group (HUET) and the control group had similar trait anxiety levels. Participants taking part in the HUET task had a mean trait anxiety level of 37 (SD = 11.41) and those in the control group had a mean level of 32 (SD = 4.80).
State anxiety
Analysis of state anxiety revealed a main effect of time [F(3,54) = 5.35, p < 0.01], and a significant (condition × time) interaction [F(3,54) = 5.46, p < 0.01]. No main effect of condition was observed [F(1,18) = 0.43, p = 0.52, ns]. Descriptive statistics are given in .
Figure 1 State anxiety levels (mean and standard deviations) as a function of condition and time (n = 10 per experimental condition). Single asterisks indicate a significant difference between times or groups (p < 0.05).
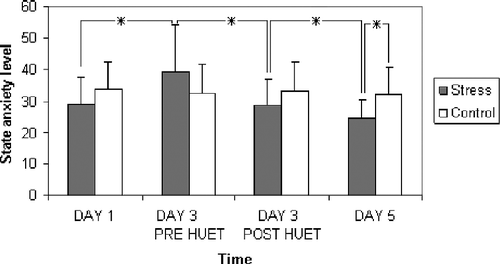
Planned comparisons revealed a significantly higher level of state anxiety for the stress group on day 3 (pre-HUET) compared to day 1 [F(1,18) = 12.02, p < 0.01], day 3 (post-HUET) [F(1,18) = 12.36, p < 0.01 and day 5 [F(1,18) = 18.42, p < 0.01]. For the control group there was no difference in state anxiety between day 1 and 3 (pre-task) [F(1,18) = 0.22, p = 0.64, ns]; nor between day 3 pre-task vs. post-task [F(1,18) = 0.4, p = 0.84, ns]; nor between day 3 (pre-task) and day 5 [F(1,18) = 0.01, p = 0.91, ns]. Furthermore, no significant difference was found between the control group and the stress group in state anxiety levels on day 1, [F(1,18) = 1.51, p = 0.24, ns], day 3 (pre) F(1,18) = 1.65, p = 0.22, ns] and day 3 (post) [F(1,18) = 1.18, p = 0.29, ns]. However, there was a significant difference between these groups on day 5 [F(1,18) = 5.08, p < 0.05] with lower levels of state anxiety reported in the stress group.
Operation-word span task
Storage capacity (word span)
There was a significant main effect of time [F(2,36) = 5.43, p < 0.01] and a significant interaction (time × condition) [F(2,36) = 4.35, p < 0.05] but there was no main effect for condition [F(1,18) = 0.20, p = 0.66, ns]. Descriptive statistics are given in .
Table I. Mean (with standard deviation) operation-word span scores for HUET and control group over three test sessions (n=10 per experimental condition).
Planned comparisons revealed no significant difference in storage capacity in the stress group between day 1 and 3 [F(1,18) = 0.037, p = 0.85, ns]; nor between day 3 and 5 [F(1,18) = 0.01, p = 0.91, ns]; nor between day 1 and 5 [F(1,18) = 0.09, p = 0.76, ns]. Planned comparisons within the control group revealed significantly higher storage capacity on day 5 [F(1,18) = 14.81, p < 0.01] and day 3 [F(1,18) = 11.48, p < 0.01] compared to day 1. No significant difference in storage capacity was found between day 1 and 3 [F(1,18) = 0.01, p = 0.91, ns]. No significant differences in storage capacity were found between the control and stress groups on day 1, [F(1,18) = 1.28, p = 0.27, ns], day 3 [F(1,18) = 2.79, p = 0.11, ns] or day 5 [F(1,18) = 1.29, p = 0.27, ns].
Processing capacity (operation span)
Accuracy in calculating the arithmetic equations was taken as a measure of processing capacity within working memory. No significant main effects were found for either time [F(2,36) = 3.01, p = 0.06, ns] or condition [F(1,18) = 0.66, p = 0.43, ns] and there was no significant interaction [F(2,36) = 1.41, p = 0.26, ns].
Processing rate
The time taken to complete the operation-word span task was analysed as a separate variable in order to assess possible speed-accuracy trade-off. No significant main effects were found for either time [F(2,36) = 2.36, p = 0.11, ns] or condition [F(1,18) = 0.01, p = 0.97, ns] and there was no significant interaction [F(2,36) = 0.61, p = 0.55, ns].
Salivary cortisol concentration
There was a significant main effect of time [F(3,54) = 3.44, p < 0.05] and a significant condition × time interaction [F(3,54) = 4.08, p < 0.01]. No main effect of condition was observed [F(1,18) = 2.02, p = 0.17, ns]. Descriptive statistics are given in .
Figure 2 Salivary cortisol concentrations (mean and standard error) as a function of time and condition (n = 10 per experimental condition). Post-HUET is 25 min after the stressor. Single asterisks indicate a significant difference between time points (p < 0.05) and a single diamond indicates a significant difference between groups (p < 0.05).
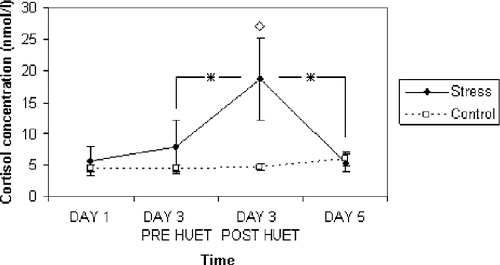
Planned comparisons for the experimental group revealed no significant difference in salivary cortisol concentrations between day 1 and 3 (pre-HUET) [F(1,18) = 2.08, p = 0.17, ns]. However, cortisol concentrations for post-HUET (day 3) were found to be significantly elevated compared to both pre-HUET levels (day 3) [F(1,18) = 7.82, p < 0.01] and day 5 levels [F(1,18) = 8.20, p < 0.01]. Planned comparisons for the control group revealed no significant difference in cortisol concentrations between day 1 and 3 (pre-task) [F(1,18) = 0.01, p = 0.91, ns]; nor between pre- and post-task (day 3) [F(1,18) = 0.01, p = 0.99, ns]; nor between day 3 (post-task) and day 5 [F(1,18) = 0.25, p = 0.62, ns].
Planned comparisons revealed no significant difference between the control group and the stress group in cortisol concentrations on day 1, [F(1,18) = 0.23, p = 0.63, ns], day 3 (pre-HUET) [F(1,18) = 0.55, p = 0.47, ns] or day 5 [F(1,18) = 0.15, p = 0.70, ns]. However, cortisol concentrations were significantly higher in the stress group compared to controls on day 3 (post-HUET) [F(1,10) = 4.56, p < 0.05].
Experiment 2
In experiment 2 the effect of an acute stressor on working memory performance, anxiety and HPA axis activation (cortisol) was examined immediately following exposure to the stressor.
Method
Participants
For the experimental condition, ten healthy male participants, were recruited via opportunity sampling from individuals attending a sea survival training course at Fleetwood Nautical College (UK). Age-matched control participants, of a similar educational level, were recruited from staff and students at the University of Lancaster via opportunity sampling. Participants' ages ranged from 24 to 52 years with a mean age of 41 years. The same exclusion criteria as used in experiment 1 were adopted. Written consent was given by all participants who were tested in accordance with national and local ethics guidelines according to the Declaration of Helsinki.
Materials and apparatus
As in experiment 1, helicopter underwater evacuation training was used as the naturalistic stressor. Anxiety levels were again assessed by the State-Trait Anxiety Inventory (Form Y; Spielberger Citation1966).
Saliva samples were collected with the salivette sampling device (Sarstedt Ltd, Leicester, UK) and analysed for cortisol concentrations using the method described above.
A non-computerised version of Hayman's (Citation1942) serial sevens task was used to measure working memory performance. This measure of working memory was chosen for this second experiment as it was unsafe for participants, who were wet as a result of the underwater escape, to touch electrical equipment. For this task participants were required to compute and report out loud a running subtraction of seven, starting from a randomly generated number between 700 and 900. The task took 120 s to complete and was scored for the number of correct subtractions.
Procedure
Day 1 (11:30 am): Participants (experimental: HUET; control: office work) completed the state/trait anxiety questionnaire (Spielberger Citation1966) and provided a saliva sample that was later analysed for cortisol concentration.
Day 1 (1:30 pm): Participants in the experimental condition undertook the HUET task whereas those in the control condition carried out light office duties. Immediately post-task, participants again completed the state anxiety questionnaire and were asked to provide a further saliva sample. Next, working memory performance on the serial sevens task was assessed. Upon completion of the experiment, participants were debriefed and thanked for their participation.
As in experiment 1, participants were requested not to consume food or drink (apart from water) for at least 1 h prior to the task, and testing for both HUET and control participants took place at the same time of day.
Statistical analyses
Trait anxiety levels were examined using a one-way ANOVA with condition (HUET vs. control) as the between factor. Levels of state anxiety were assessed prior to and immediately after exposure (HUET and control) and analysed using a two-way ANOVA with condition (HUET vs. control) as the between factor and time (i.e. when anxiety levels were assessed) as the within factor. Cortisol concentrations were examined using a two-way ANOVA with 2 factors: condition (HUET vs. control) and time (pre-and post-exposure). The serial sevens task results were analysed using a one-way ANOVA with condition (HUET vs. control) as the between-participant factor.
Results
Trait anxiety
No significant difference in trait anxiety levels were observed between the stress (HUET) and control group [F(1,18) = 0.09; p = 0.77, ns]. The HUET group had a mean level of trait anxiety of 33.80 ( ± 5.39, SD) compared to 35 ( ± 11.07, SD) in the control group.
State anxiety
Descriptive statistics are given in .
Figure 3 State anxiety levels (mean and standard deviations) as a function of condition and time (n = 10 per experimental condition). A single diamond indicates a significant difference between groups (p < 0.05).
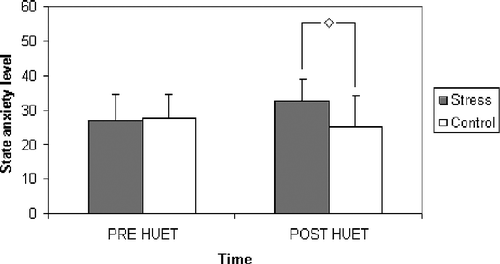
There was a significant condition × time interaction [F(1,18) = 5.09; p < 0.05], but no main effect of time [F(1,18) = 0.67; p = 0.42, ns] or condition [F(1,18) = 1.57; p = 0.23, ns]. Planned comparisons revealed no significant difference between state anxiety levels of participants between the groups prior to the task [F(1,18) = 0.03; p = 0.86, ns], however, the stress group reported significantly higher state anxiety levels immediately after exposure to HUET compared to the control group [F(1,18) = 4.81; p < 0.05].
Cortisol
Descriptive statistics are given in .
Figure 4 Salivary cortisol concentrations (mean and standard error) as a function of time and condition (n = 10 per experimental condition). Post-HUET is immediately after the stressor.
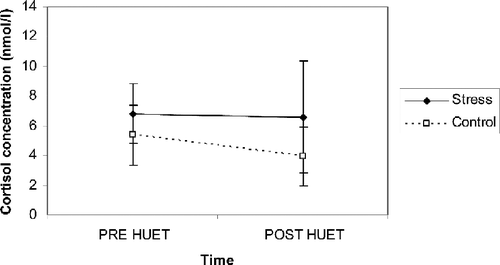
Analyses revealed no significant main effect of time [F(1,18) = 0.16; p = 0.69, ns] or condition [F(1,18) = 0.47; p = 0.50, ns] and there was no significant condition × time interaction [F(1,18) = 0.08; p = 0.77, ns]. These results indicated that cortisol concentrations for both conditions were similar.
Working memory performance
Analysis revealed a significant difference between the stress and control groups (post HUET) in the number of correct subtractions [F(1,18) = 6.24; p < 0.05]. Mean working memory scores for the HUET group was 19.40 ( ± 10.38, SD) and 28.90 ( ± 6.06, SD) for the control group.
Discussion
The two experiments reported here examined the psychophysiological responses in individuals exposed to an acute, naturalistic stressor, namely undergoing an evacuation from a submerged and inverted helicopter. Working memory, state anxiety and salivary cortisol concentrations were assessed during anticipation of, and immediately after exposure to underwater evacuation, with the results being compared to a non-stressed control group.
The results of both studies showed that working memory performance was preserved during anticipation of an acute stressor, but impairments were observed following stress exposure. Significantly higher state anxiety levels were observed during anticipation and immediately following stress exposure, whereas significant elevations in cortisol levels were only observed 25 min post exposure to stress, but not before or immediately after stress exposure.
The results of experiment 1 indicate that no difference in state and trait anxiety was observed at baseline between the control and experimental groups. However, during anticipation of an acute stressor, the experimental group showed significantly higher state anxiety levels. The control group, in contrast, showed no significant change in state anxiety scores across the sessions. It can be taken, therefore, that the HUET task is a valid acute stressor, and this supports previous findings that anticipation of an acute stressor can induce changes in an individual's psychological state (Leach Citation1994; Eriksen et al. Citation1999).
It has been suggested that exposure to anticipation of an acute stressor results in biological changes such as elevated cortisol levels (Eriksen et al. Citation1999). However, we found no significant increase in salivary cortisol levels during anticipation of the HUET task. This finding was unexpected, as activation of the HPA axis in response to anticipation of threat is well documented (Deinzer et al. Citation1997; Lupien and McEwen Citation1997 and Garcia-Leal et al. Citation2005).
It could be argued that the lack of increase in cortisol levels is due to the fact that participants did not perceive the HUET task to be sufficiently stressful to induce HPA activation. However, anticipation of the HUET task did lead to significant elevations in state anxiety scores and mean levels of cortisol were slightly higher in HUET participants prior to HUET exposure than at baseline. Although this increase in cortisol was not statistically significant at the time, it does lend some support to the idea that HPA axis activation may have occurred. Consequently, the observed dissociation between behavioural and cortisol response might be due to sampling before an increase in cortisol could be measured in saliva. The observation that 25 min post-HUET, participants in the stress group exhibited significantly increased cortisol concentrations demonstrates that HUET exposure did induce HPA activation as expected (Kirschbaum and Hellhammer Citation2000; Dickerson and Kemeny Citation2004) but that this increase in cortisol did not occur until after the pre-HUET saliva sample had been taken. This finding highlights the critical nature of timing within experiments when observing psychophysiological responses to acute stressors (Eriksen et al. Citation1999). It is also worth noting that, whilst cortisol concentrations were significantly elevated 25 min post-HUET, self-reported anxiety levels had by then returned to baseline.
The effect of anticipation of a threat task on processing and storage capacities of working memory was examined through the operation-word-span task (Turner and Engle Citation1989; Cantor and Engle Citation1993) since previous research has suggested that activities which are dependent upon working memory can become impaired prior to threat (Lazarus Citation1966; Mileti and Peek Citation2000; Leach Citation2005). Contrary to our expectations, working memory function remained preserved with participants showing no significant reduction in storage or processing capacities prior to the HUET task compared either to their own baseline measure or to the control group. One possible reason for preserved working memory function could be that cortisol levels, which have previously been associated with working memory impairments (Lupien et al. Citation1999), were not significantly elevated prior to HUET exposure. However, previous research has also suggested that working memory performance can be impaired as a result of high state anxiety levels (Matthews Citation1996; Dutke and Stoebber Citation2001; Ashcraft Citation2002; Somer et al. Citation2005), yet although high levels of state anxiety were found in the pre-HUET group there was no corresponding impairment in working memory.
Even though working memory performance was preserved during anticipation of the stressor, impairments were observed immediately following exposure to the HUET task in experiment 2. This finding supports previous research which has suggested that working memory is impaired during or immediately following exposure to an acute stressor (Lazarus Citation1966; Mileti and Peek Citation2000; Leach Citation2005; CitationLeach and Griffith in press). Although this is a small scale study and due to health and safety reasons different working memory tasks were used in each experiment, it is interesting to note that working memory may be impaired only at certain stages of exposure to an acute stressor.
There are a number of possible causes for impairment in working memory function immediately following exposure to an acute stressor. One possibility is that elevated levels of circulating cortisol may impair those aspects of cognition associated with PFC function (e.g. working memory) via the high density of glucocorticoid receptors in this region (Lupien et al. Citation1999). However, in experiment 2 working memory was found to be impaired immediately post exposure to the HUET task in the absence of significant cortisol increases. Dissociation between behavioural and biochemical changes in response to stress has previously been observed, for example, in infants (Gunnar and Barr Citation1998), adults (Schommer et al. Citation2003) and non-human primates (Elder and Menzel Citation2001). As mentioned earlier, in the current studies the dissociation between cortisol and behavioural responses might be due to the timing of saliva sampling.
However, it is important to note that HPA activation (and concomitant cortisol release) is only one of many biochemical changes occurring as a response to stress exposure. Stress triggers the activation of many different hormonal and neurotransmitter systems which may result in behavioural changes. For example, it has been suggested that high levels of norepinephrine (Arnsten Citation1998; Ramos and Arnsten Citation2007) and dopamine (Arnsten et al. Citation2000) in primates exposed to acute stressors, can lead to marked changes in working memory tasks which pertain to the PFC. Dissociation between HPA activation and catecholamine response (Malarkey et al. Citation1995), as well as differential stress habituation patterns between these endocrine systems have previously been observed (Schommer et al. Citation2003). Consequently, the observed decrements in working memory might have been mediated by a catecholamine response or activation of other neurotransmitter systems. Parallel assessment of multiple hormonal systems and neurotransmitters influenced by stress rather than concentrating on only one system is important in order to further our understanding of how stress influences behaviour and cognition.
Another possible reason for working memory impairment following exposure to the HUET stressor is the increase in state anxiety (Rosen and Engle Citation1998; Ashcraft Citation2002; Somer et al. Citation2005). The results of experiment 2 indicate that, although state anxiety was the same in both conditions at baseline, participants in the stress condition exhibited significantly higher levels of state anxiety immediately following HUET exposure. Therefore, it is possible that the cognitive correlates of state anxiety consume working memory capacity thus denying resources to the processing of other task relevant operations. Indeed, this is the basis of resource depletion models of cognitive function (e.g. Eysenck and Calvo Citation1992) and threat-induced restriction in working memory has also been found in another naturalistic stressor, namely parachuting (CitationLeach and Griffith in press).
In conclusion, the two experiments reported here examined the effect of exposure to a novel acute stressor, the HUET task, on working memory function, state anxiety and salivary cortisol concentrations immediately prior to and immediately post stress exposure. The HUET task does induce both a psychological and physiological stress response, with increases observed in both state anxiety and cortisol concentrations. However, the effect of exposure to an acute stressor is complex, with an individual's psychophysiological responses differing over the duration of the stressor exposure.
Identification of the particular types of cognitive dysfunctions, physiological responses and the stages at which they occur in relation to acute stress, are crucial. Further investigations on the time-course and clear delineation of the aspects of cognitive performance affected at various stages are necessary to specify the nature and extent of cognitive deficits induced by stress. In addition, parallel assessment of multiple hormonal systems and neurotransmitters influenced by stress rather than concentrating on only one system is important in order to further our understanding of how stress influences behaviour and cognition.
Acknowledgements
We would like to thank Mr Joe Bottomley and all the staff and students at Fleetwood Nautical College (UK) for all their help and support in this study.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia and the hippocampal–anterior thalamic axis. Behav Brain Sci 1999; 22: 425–444
- Arnsten AFT. Catecholamine modulation of prefrontal cortical cognitive function. Trends Cogn Sci 1998; 2(11)436–447
- Arnsten AFT, Murphy B, Merchant K. The selective dopamine D4 receptor antagonist, PNU-101387G, prevents stress-induced cognitive deficits in monkeys. Neuropsychopharmacology 2000; 23(4)405–410
- Ashcraft MH. Math anxiety: Personal, educational and cognitive consequences. Curr Dir Psychol Sci 2002; 11: 181–185
- Bohnen N, Houx P, Nicolson N, Jolles J. Cortisol reactivity and cognitive performance in a continuous mental task paradigm. Biol Psychol 1990; 31(2)107–116
- Braver TS, Choen JD, Nystorm LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 1997; 5: 49–62
- Bryant RA, Panasetis P. Panic symptoms during trauma and acute stress. Behav Res Ther 2001; 39: 961–996
- Buss C, Wolf OT, Witt J, Hellhammer DH. Autobiographic memory impairment following acute cortisol administration. Psychoneuroendocrinology 2004; 29: 1093–1096
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci 1998; 21: 294–299
- Cannon WB. Bodily changes in pain, hunger, fear and rage. Branford, Boston 1929
- Cantor J, Engle RW. Working memory capacity as long-term memory activation: An individual differences approach. J Exp Psychol Learn Mem Cogn 1993; 19(5)1101–1114
- Cassuto J, Tarnow P. The discotheque fire in Gothenburg 1998. A tragedy among teenagers. Burns 2003; 29: 405–416
- Cohen SP, Raghavulu CV. The Andhra cyclone of 1977: Individual and institutional responses to mass death. Vikas Publishing House PVT Ltd, New Delhi 1979
- De Quervain DJF, Roozendall B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Natl Neurosci 2000; 3: 313–314
- De Quervain DJF, Henke K, Aemi A, Treyer V, McGaugh JL, Berthold T, Nitsch RM, Buck A, Roozendaal B, Hock C. Glucocorticoid-induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. Eur J Neurosci 2003; 17(6)1269
- Deinzer R, Kirschbaum C, Gresele C, Hellhammer DH. Adrenocortical responses to repeated parachute jumping and subsequent h-CRH challenge in inexperienced healthy subjects. Physiol Behav 1997; 61(4)507–511
- Dickerson SS, Kemeny ME. Acute stressors and cortisol reactivity: A meta analytic review. Psychosom Med 2004; 64: 85–174
- Domes G, Heinrichs M, Reichwald U, Hautzinger M. Hypothalamic pituitary–adrenal axis reactivity to psychological stress and memory in middle-aged women: High responders exhibit enhanced declarative memory performance. Psychoneuroendocrinology 2002; 27(7)843–853
- Drewe EA. Go-no-go learning after frontal lobe lesions in humans. Cortex 1975; 11: 8–16
- Dutke S, Stoebber J. Test anxiety, working memory, and cognitive performance. Cogn Emotion 2001; 15: 381–389
- Edwards S, Evans P, Hucklebridge F, Clow A. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology 2001; 6: 613–622
- Elder CM, Menzel CR. Dissociation of cortisol and behavioural indicators of stress in an orangutan (pongo Pygmaeous) during a computerized task. Primates 2001; 42(4)345–357
- Elzinga BM, Roelofs K. Cortisol-induced impairments of working memory require acute sympathetic activation. Behav Neurosci 2005; 119: 98–103
- Engle RW. Working memory and human cognition, JTE Richardson, RW Engle, L Hasher, RH Logie, ER Stoltzfus, RT Zacks. Oxford University Press, New York and Oxford 1996
- Eriksen HR, Olff M, Murisin R, Ursin H. The time dimension in stress responses: Relevance for survival and health. Psychiatry Res 1999; 85: 39–50
- Eysenck MW, Calvo MG. Anxiety and performance: The processing efficiency theory. Cogn Emotion 1992; 6: 409–434
- Funahashi S. Neuronal mechanisms of executive control by the prefrontal cortex. Neurosci Res 2001; 39: 147–165
- Garcia-Leal C, Alexandre CBV, Parente C, Del-Ben CM, Guimarães FS, Moreira CA, Elias LLK, Graeff FG. Anxiety and salivary cortisol on symptomatic and nonsymptomatic panic patients and healthy volunteers performing simulated public speaking. Psychiatry Res 2005; 133: 239–252
- Gunnar MR, Barr RG. Stress, early brain development and behaviour. Infant Young Child 1998; 11(1)1–14
- Hayman M. Two minute clinical test for measurement of intellectual impairment in psychiatric disorders. Arch Neurol Psychiatry 1942; 47: 454–464
- Krischbaum C, Hellhammer DH. Salivary cortisol. Encyclopaedia of stress, G Fink. Academic Press, London 2000
- Krischbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci 1996; 58: 1475–1483
- Lazarus RS. Psychological stress and the coping process. McGraw-Hill, New York 1966
- Leach J. Survival psychology. MacMillan Press Ltd, London 1994
- Leach J. Cognitive paralysis in an emergency: The role of the supervisory attentional system. Aviat Space Environ Med 2005; 76(2)134–136
- Leach J, Griffith R. Restrictions in working memory capacity during parachuting: A possible cause of ‘no pull’ fatalities. Appl Cognit Psychol, In press
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Res Rev 1997; 24: 1–27
- Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: A dose-response study in humans. Behav Neurosci 1999; 113(3)420–430
- Malarkey WB, Pearl DK, Demers LM, Kiecolt-Glaser JK, Glaser R. Influence of academic stress and season on 24-hour mean concentrations of ACTH, cortisol and beta-endorphin. Psychoneuroendocrinology 1995; 22(5)499–508
- Matthews G. Extraversion, emotion and performance: A cognitive-adaptive model. Cognitive science perspectives on personality and emotion, G Matthews. Elsevier, Amsterdam 1996
- McEwen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev 1986; 66(4)1121–1188
- Mileti DS, Peek L. The social psychology of public response to warnings of a nuclear power plant accident. J Hazard Mater 2000; 75: 181–194
- Miyake A, Shah P. Models of working memory: Mechanisms of active maintenance and executive control. Cambridge University Press, New York 1999
- Morgan C, Doran A, Steffian G, Hazlett G, Southwick SM. Stress-induced deficits in working memory and visuo-constructive abilities in special operations soldiers. Biol Psychiatry 2006; 60(7)722–729
- Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Rev 2003; 17(2)139–170
- Newcomer JW, Craft S, Hershey T, Askins K, Bardgett ME. Glucocorticoid-induced impairment in declarative memory performance in adult humans. J Neurosci 1994; 14: 2047–2053
- Newcomer JW, Selke G, Melson AK, Hershey T, Craft H, Richards K, Alderson AL. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry 1999; 56: 527–533
- Oei NYL, Everaerd WTAM, Elzinga BM, Van Well S, Bermond B. Psychosocial stress impairs working memory at high loads: An association with cortisol levels and memory retrieval. Stress Int J Biol Stress 2006; 9(3)133–141
- Ramos BP, Arnsten AFT. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacol Ther 2007; 113: 523–536
- Ring C, Harrison LK, Winzer A, Carroll D, Drayson M, Kendall M. Secretory immunoglobulin A and cardiovascular reactions to mental arithmetic, cold pressor, and exercise: Effects of alpha-adrenergic blockade. Psychophysiology 2002; 37: 634–643
- Rosen W, Engle R. Working memory capacity and suppression. J Mem Lang 1998; 39: 418–436
- Sapolsky RM. Neuroendocrinology of the stress response. Behavioral endocrinology, JB Becker, SM Breedlove, D Crews. MIT Press, London 1992
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of hypothalamus–pituitary–adrenal axis and the sympathetic–adrenal–medullary system to repeated psychological stress. Psychosom Med 2003; 65: 450–460
- Somer E, Tamir E, Maguen S, Litz BT. Brief cognitive-behavioural phone-based intervention targeting anxiety about the threat of attack: A pilot study. Behav Res Ther 2005; 43: 669–679
- Spielberger CS. Theory and research on anxiety. Anxiety and behaviour, CS Spielberger. Academic Press, New York 1966
- Stuss DT, Benson DF. The frontal lobes. Raven Press, New York 1986
- Takahashi T, Ikeda K, Ishikawa M, Tsukasaki T, Nakama D, Tanida S, Kameda T. Social stress-induced cortisol elevation acutely impairs social memory in humans. Neurosci Lett 2004; 363(2)125–130
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc Natl Acad Sci 2002; 94: 14792–14797
- Tops M, van der Pompe G, Wijers AA, Den Boer JA, Meijman TF, Korf J. Free recall of pleasant words from recency positions is especially sensitive to acute administration of cortisol. Psychoneuroendocrinology 2004; 29: 327–338
- Turner ML, Engle RW. Is working memory capacity task dependent?. J Mem Lang 1989; 28: 127–154
- Vedhara K, Hyde J, Gilchrist ID, Tytherleigh M, Plummer S. Acute stress, memory, attention and cortisol. Psychoneuroendocrinology 2000; 25(6)535–549
- Wood DL, Knight RT. Electrophysiological evidence of increased distractibility after dorsolateral prefrontal lesions. Neurology 1986; 36: 212–216
- Yehuda R, Golier JA, Halligan SL, Harvey PD. Learning and memory in Holocaust survivors and posttraumatic stress disorder. Biol Psychiatry 2004; 55(3)291–295
