Abstract
Attachment insecurity, as assessed via the adult attachment interview (AAI), may be expected to relate to basal hypothalamic–pituitary–adrenal (HPA) activity because it is retrodictive of stressful early experiences, which may influence HPA development. In addition, because AAI insecurity may reflect limitations on concurrent cognitive, emotional, and behavioral strategies for managing interpersonal distress, insecurity may also relate to cortisol reactivity specifically during inter-personal challenges. Nevertheless, only two studies have examined associations between AAI insecurity and cortisol, and in total only eight non-clinical men were included. To expand upon past research, the current study focused on college aged men and examined relations between attachment status (via categories and continuous scores) and cortisol levels during daily life and during interpersonal laboratory challenges, wherein subjects were asked to visualize and respond to hypothetical situations concerning loss, separation, and abandonment. Unlike prior research, salivary cortisol was measured during cognitive challenges (e.g. non-autobiographical memory tests), so as to inform questions concerning the specificity of effects. Contrary to expectations, only limited evidence suggested a relation between insecurity and basal HPA functioning. However, in keeping with expectations, associations between insecurity, and in particular dismissing idealization, and comparatively higher cortisol values following interpersonal challenges were observed.
Introduction
Attachment relationships are long-enduring affectional bonds wherein an attached individual tries to achieve the perception of closeness with a unique figure during times of alarm (Ainsworth Citation1989). Infants form attachment relationships with their primary caregivers and these relationships may be internally represented, contributing to expectations in other relationships (Bowlby Citation1969). A primary means of assessing individual differences in mental representations of attachment is the Adult Attachment Interview (AAI; George et al. Citation1984–1996; see also, Hesse Citation1999; Main et al. Citation1984–2003). Important to stress research, AAI status relates to constructs affecting HPA activity. Nevertheless, only two studies have examined relations between AAI status and hypothalamic–pituitary–adrenal (HPA) activity (Scheidt et al. Citation2000; Adam and Gunnar Citation2001). The overarching goal of this research was to further explore these associations in college age males—an understudied group in attachment and cortisol research (i.e. in combination, a total of eight non-clinical men were included in past research). Two hypotheses were investigated: the first concerned overall HPA regulation, the second focused upon responses to specific challenges.
Adult attachment and HPA regulation
First, as animal research demonstrates that early and chronic exposure to cortisol influences adult HPA regulation (Plotsky et al. Citation2005), it was hypothesized that insecure adult attachment would predict daily cortisol values; however, because stress may relate to higher or lower daily values (Heim et al. Citation2000), the direction of effects was not predicted. This first hypothesis additionally relies on findings that those considered secure (roughly 50% of low risk groups; for meta-analytic percentages see van IJzendoorn and Bakermans-Kranenburg Citation1996) are less likely to have experienced exposure to heightened cortisol levels beginning in infancy than those considered insecure (i.e. [1] insecure-dismissing; [2] insecure-preoccupied; and [3] insecure-disorganized, which includes unresolved and cannot classify). This is because AAI status has been found retrodictive of mother–infant attachment relationships (Hamilton Citation2000; Waters et al. Citation2000; Weinfield et al. Citation2004; Main et al. Citation2005; but see Zimmermann et al. Citation2000). These mother–infant relationships are associated with the quality of maternal care an infant has received (Ainsworth et al. Citation1978; van IJzendoorn Citation1995; van IJzendoorn et al. Citation1999; Madigan et al. Citation2006), the infant's subsequent socio-emotional development including relationships with peers (Shulman et al. Citation1994; van IJzendoorn et al. Citation1999), and the likelihood an infant will experience comfort upon distress (Ainsworth et al. Citation1978).
Thus, adults who are secure on the AAI may have been the least likely to have experienced parenting and peer behaviors that cause increases in cortisol levels, and most likely to have had parents who regulated their child's HPA activity when other stressors were encountered (Ahnert et al. Citation2004). Indeed, research demonstrates that infant attachment security is linked to less pronounced and/or prolonged cortisol responses to challenging situations (Spangler and Grossmann Citation1993).
Adult attachment and challenges
Second, because situations perceived as uncontrollable and/or likely to result in a failure or a diminution of respect result in higher cortisol levels (Dickerson and Kemeney Citation2004), and because AAI status reflects strategies (or breakdowns of strategy), used to manage interpersonal situations, which may incorporate expectations of failure and/or social rejection (Main et al. Citation1985; see also Maier et al. Citation2004, Citation2005), it was hypothesized that AAI insecurity would relate to comparatively higher levels of cortisol following a separate attachment related interpersonal challenge. In addition, the specificity of effects was further examined by assessing cortisol values following cognitive challenges.
AAI security is marked by mental flexibility in discussions of both positive and negative scenarios, forgiveness, a valuing of emotions and an appreciation of both connectedness and autonomy (Main et al. Citation1984–2003); thus, in a variety of settings, those who are considered secure may be expected to show an openness that implies a sense of control, to hold beliefs that displays of vulnerability must not necessarily lead to rejection, and a sense of self-worth that is not dependent upon relatively distorted views of one-self and/or relationships (Main Citation2000). Thus, when rejection is not eminent, those considered secure should meet challenges with expectations of successful management and acceptance.
In contrast, those considered dismissing complete the AAI by avoiding, denying, and/or minimizing discussions of negative parent–child experiences and emotions, despite the fact that, to a trained observer, the content of their reports suggests frequent parental rejection and unloving behavior (Main et al. Citation1984–2003). Thus, it appears that dismissing speakers' past displays of vulnerability were met with unloving and/or rejecting behavior, that dismissing individuals expect concurrent displays to be met with similar rejection, and that they are therefore, attempting to avoid displays of vulnerability in order to defend against concurrent rejection (Main Citation2000; see also Van Emmichoven et al. Citation2003; Maier et al. Citation2004). Hence, when dismissing individuals are forced to focus upon negative affect and/or display vulnerability, they may expect their emotional vulnerability to be met with social rejection and so may exhibit increases in cortisol.
Also in contrast to security, the lack of incisiveness associated with preoccupation may indicate that feelings of self-respect can only be maintained when an incomplete exploration of emotions and memories is undertaken (Main Citation2000). Therefore, when preoccupied individuals encounter challenges requiring both autonomy and a full exploration of emotions or memories, increases in cortisol release may be expected.
Finally, the speech used by those considered disorganized during the AAI may also suggest difficulties managing other situations. In response to the AAI, those considered insecure-disorganized (i.e. unresolved or cannot classify) appear unable to display one coherent discourse pattern across the entirety of the interview. Regardless of whether the marked incoherence is limited to discussions of loss or trauma (as in the case of those considered unresolved) or more global (as in the case of those considered cannot classify) it is considered to reflect a collapse in strategy (Hesse Citation1996; Main et al. Citation1984–2003). Because a coherent strategy may be an important means of controlling any unfolding event, those considered disorganized (unresolved or cannot classify) may be expected to exhibit relatively high cortisol levels when encountering challenges that include reference to trauma, loss, and/or more generally, attachment.
Methods
This research involved three phases: (1) an AAI (George et al. Citation1984–1996) session, from which attachment classifications and scores were derived; (2) a home session, where samples for cortisol measurement and mood data were obtained; (3) a laboratory session, where cortisol and mood data were collected. The research was approved by the University's Committee for the Protection of Human Subjects. All participants gave written consent at the time of the AAI, and, for those who participated in the other measures, prior to follow-up research.
Participants
Seventy-three male students (mean age = 19.23 years, SD = 1.08) were recruited from a university's research participation pool for the AAI (George et al. Citation1984–1996). They were asked to participate only if they were willing to consider returning for further course credit and/or monetary compensation. To limit extraneous influences on variables of interest, they were asked not to participate if they had: (1) a diagnosis of clinical depression or PTSD; (2) taken medications for psychological disorders; (3) exposure to high levels of corticosteroids; (4) currently taken any hormones; (5) been born premature; (6) a mother who had diabetes when pregnant with them; (7) cystic fibrosis, multiple sclerosis or immune system deficiencies; or (8) suffered any major traumas within the year prior to the AAI.
Fifty-one of the 73 returned for followup sessions components, occurring roughly one year later (70% retention rate). Forty-six participants completed the AAI, and the laboratory and home sessions. Forty-seven participants completed both the AAI and the entire laboratory session, and 50 participants took part in both the AAI and home cortisol collection. Audio recording difficulties resulted in missing AAI data for two participants.
Though participants had been asked, at the time of recruitment, not to participate if they had certain risk factors, as an extra control, during follow-up components, participants were asked about experience with factors likely to influence cortisol measurements (i.e. life-time corticosteroid exposure, current taking of any hormones, exposure to medications between visits, history of psychological disease, and intervening medical or psychological diagnosis). Participants who did not answer one or more of these follow-up questions were removed from analysis. Of the remaining participants, those who indicated a history of and/or current experience with psychiatric illness or psychological diagnosis were removed from analysis. In keeping with Main et al. (Citation1984–2003), two individuals classified as unresolved-disorganized solely due to text surrounding loss within a year prior to the interview were removed from analysis. In total, all data from 12 individuals were removed.
In sum, then, data from 37 participants were included. This included data from one participant who had briefly taken medication between visits, as well as four participants who indicated having taken corticosteroids “once/only a little” earlier in their lives.
The distribution of ethnicity/race and socio-economic status amongst the 37 participants retained for analysis was similar to that of the 51 person group. (Race/ethnicity for the 37 person group: Caucasian = 11 [30%]; Chinese = 11 [30%]; Korean = 4 [11%]; Mexican = 4 [11%]; African American = 1 [3%]; Japanese = 1 [3%]; Filipino = 1 [3%]; other = 1 [3%]; unknown = 3 [8%]) (SES as indicated by highest level of education completed by participant's mother for the 37 person group: elementary or junior high school = 4 [10%]; high school = 9 [24%]; college and/or some form of professional licensure = 16 [43%]; graduate school = 7 [19%]; unknown = 1 [3%]).
Adult attachment interview (AAI) session
Interview overview and classifications
The AAI has good predictive validity and test–retest reliability with adequate observed stability of up to 18 months (reviewed in Hesse Citation1999). In this study, the 1996 version of the AAI (George et al. Citation1984–1996) was used. This is a 20 question semi-structured interview that lasts roughly 45–90 min. During the interview participants are asked about childhood filial relationships, current relationships, and trauma, including loss throughout life. The AAI was transcribed verbatim and coded according to the 2002–2003 version of Main and colleagues' scoring system. Based on this system participants may be considered “secure” or one of three forms of “insecure,” which include “dismissing,” “preoccupied,” and disorganized (i.e. “Unresolved” and/or “Cannot Classify”). When a disorganized classification is assigned, a secondary classification (i.e. secure, dismissing or preoccupied) is also assigned. In other words, there are two dimensions to the AAI: one that distinguishes between secure and dismissing/preoccupied; and one that distinguishes between disorganized and not disorganized. However, to be considered primarily secure, one must be both secure and not disorganized.
When deciding between a secure, dismissing, or preoccupied classification, the coder must ensure that the transcript meets a number of global characteristics (e.g. as described above, security is associated with valuing; dismissal with minimization of distress; and preoccupation with vagueness or excessive anger). In addition, the transcript must have received appropriate scores on AAI scales, which range from 1 to 9. If these global characteristics are not adhered to, or scale scores do not comply with expectations, the coder will consider assigning a disorganized (i.e. cannot classify) category.
Scale scores
There are two sets of 1–9 point scales. “Likely Experience” scales concern the coder's estimation of the speaker's likely childhood experiences. “State-of-Mind” scales concern the ways narratives are presented. “State of Mind” scales are associated with classifications. As would be expected, secure texts receive relatively low scores (e.g. 1–3) on “insecure” “State of Mind” scales. This study focused upon three of the numerous “State of Mind” scales: “Dismissing Idealization,” which is associated with the dismissing category, “Passivity-of-Thought,” which is associated with the preoccupied category, and “Unresolved Slips,” which is related to the (unresolved) disorganized category.
“Dismissing Idealization” (hereafter “Ds Idealization”) is linked to discrepancies between the coder's estimation of speakers' likely childhood experiences and the impression being created by speakers; for example, a speaker who claims that their mother was “loving, caring, beautiful, sweet, and protective” and then often discusses memories indicative of maternal rejection or neglect might receive a high score on Ds idealization.
“Passivity-of-Thought” is associated with relatively confusing speech; for example a speaker who repeatedly uses unclear speech (e.g. “She would berate me or something and that. I thought her yelling was unfair to her”) might receive a high score on this scale.
Finally, “Unresolved Slips” (hereafter “U/d Slips”) is a scale associated with a speaker's inability to discuss loss or trauma without making statements indicative of lapses in reasoning or a failure to monitor their conversations and thoughts; for example, relatively extreme confusion about the age at which a major loss or abuse was experienced might result in a high score on the U/d slips scale. In this study, when U/d slips scores were at or above a 5.0, placement in the disorganized category occurred. In addition, speakers may also be placed in the disorganized category (i.e., specifically as “Cannot Classify”) when their transcripts are markedly incoherent, even without evidencing high scores on U/d slips.
Interview administration and reliability
All interviews were administered and coded (approximately 2 years later) by the author, who had received over a year of training in AAI interviewing, and was considered reliable with two of the AAI scoring system's authors (i.e. M. Main and E. Hesse) across 32 standard reliability transcripts. In addition, within the 51 person sample collected for this research, there was good inter-coder reliability between the current study's author and M. Main (83% agreement on case classification [i.e. secure, dismissing, preoccupied or disorganized]). These six cases chosen for reliability were purposefully difficult. Portions of other difficult cases in the current sample were discussed with Hesse and Main.
Home session
The home session occurred within roughly one month of the laboratory visit (described below), and both the home and laboratory sessions utilized nearly identical salivary collection procedures. Saliva samples were refrigerated shortly after sampling. Within approximately 48 h samples were stored in a − 80°C freezer. Samples were shipped to Dr Andrea Gierens at the University of Trier for analysis, where time-resolved fluorescence immunoassays were utilized. Intra and inter assay coefficients were deemed acceptable (i.e. falling within 4.0–6.7% and 7.1–9.0%, respectively).
Saliva collection
Participants were asked to provide eight saliva samples (at four discreet times) during two weekdays that they thought would be “non-stressful”. Participants were reminded to sample through calls to mobile phones and/or pagers. At the time of sampling participants were asked to indicate whether: (1) they felt anxious; (2) anything stressful had happened prior to the sample; and (3) (for all non-morning samples) whether anything stressful had occurred since the time of the last sample. They were also asked to report on their mood. Participants were asked to circle one of five pictures indicating their current feelings (1 = positive) and one of five pictures indicating current arousal (1 = calm), as well as to rate how angry, sad, and afraid they were on 1–7 point scales.
Prior to saliva collection participants were asked to rinse out their mouths with water and then to swallow multiple times. Salivary samples were collected via plain Stardstadt polyester salivettes. Participants were asked to adhere to conditions prior to giving a saliva sample including: (1) refraining from eating and chewing gum, as well as drinking milk, coffee, alcohol or beverages high in citric acid content for at least 30 min; (2) not engaging in strenuous physical activity or teeth brushing for at least 60 min; and (3) for the few who smoked, refraining from smoking for at least 1 h. Participants were asked to report the time they awoke and the exact time each sample actually occurred.
Participants were asked about compliance. When participants were noncompliant, or when the actual timing of the sample fell outside of the expected time frame (i.e.+1 h for non-morning samples; >10 min past the moment of wake-up) the associated sample was discarded from analysis. Twenty-three (8%) of the samples were discarded (13 wake-up samples; 4 14:30 h samples; 2 17:30 h samples; 4 21:00 h samples); in addition, all of one individual's first day samples were excluded, as his values were so high that they either indicated contamination by blood or treatment with glucocorticoids, or cross reactivity with antibodies or other substances in the assays (personal communication A. Gierens). In sum, only 4 subjects failed to provide useable data at any one time point on both days (i.e two at wake-up; one at wake-up and 17:30; and one at 21:00 h). When available, cortisol data were averaged across days to increase power.
Wake-up sample
The first sample was purposefully chosen to be at “wake-up” rather than at a set time as the most informative morning samples are obtained when individuals wake up according to their normal schedules (John D. and Catherine T. MacArthur Research Network on socioeconomic status and health, 2000); hence reminder calls for the morning sample occurred on evenings prior to cortisol collection. A wake-up value was included since morning values may reflect stable individual differences in HPA functioning, as opposed to later day values, which may be influenced by daily stressors and experiences (Kirschbaum and Hellhammer Citation1989; Smyth et al. Citation1998; Schreiber et al. Citation2006).
14:30 h sample
The timing of the afternoon saliva sample corresponded to the time the laboratory session began. Values obtained from this time of day not only served as criterion variables in analyses aimed at understanding attachment's relation to daily afternoon cortisol level, but also served as important controls in analyses aimed at understanding whether, for insecure young men, simply entering the laboratory was associated with increased cortisol secretion.
In addition, in conjunction with the 17:30 h sample, the 14:30 h sample allowed for the calculation of the total amount of non-laboratory cortisol expressed between 14:30 h and 17:30 h (i.e. BASALAUC_AFTERNOON) using Pruessner et al. (Citation2003) “Area Under the Curve, Ground” formula. This represents cortisol release expressed during the same time of day as the overall laboratory (LABAUC) value. Thus, BASALAUC_AFTERNOON served as a control to ensure that any attachment related differences in LABAUC were unique to the laboratory context, as opposed to being a consequence of more general differences in HPA activity between groups.
17:30 h Sample
The timing of the evening cortisol sample corresponded to the ending of the laboratory session.
21:00 h Sample
Nighttime cortisol samples were obtained at 21:00 h. This value was included as alterations in evening values have been reported amongst individuals who have conditions associated with HPA dysregulation (Yehuda et al. Citation1996; Keller et al. Citation2006).
Laboratory session
The laboratory session was held between 14:30 and 17:30 h. Each session was comprised of small groups, generally ranging from 3 to 7 participants. Six salivary samples per individual were collected, and are referred to as LAB1, LAB2, LAB3, LAB4, LAB5, and LAB6. Shortly following entry to the laboratory participants were also asked whether they had engaged in any of the above prohibited activities that would preclude analyzing their first cortisol samples. LAB1 data from one subject was excluded on this basis. In addition, as had been the case with the extraordinarily high cortisol samples obtained from one participant during one of the non-laboratory days, all laboratory samples from this same participant were removed for this same reason.
Because of the time course between threatening situations and cortisol secretion, salivary samples likely reflect changes induced by experiences occurring roughly 20 min prior, though individual variation in peak responses may range from roughly 15 to 30 min (Ramsay and Lewis Citation2003). Within the current study, cortisol values also may have reflected a continuation of responses to earlier challenges. At the time of each sample, individuals were also asked about their mood via the scales described above.
Laboratory entry and the collection of LAB1
Upon arrival at the laboratory, participants were shown to their seats by a professionally dressed undergraduate research assistant (i.e. the “experimenter”). After participants signed informed consent forms, at approximately 14:45 h, the experimenter showed subjects how to take a saliva sample, and their first sample (LAB1) was obtained. This first laboratory sample was utilized in two different ways. First, as some people may be intimidated about taking part in (any) experiment, LAB1 values served as a criterion variable in regressions where attachment variables served as predictor variables, and 14:30 h non-laboratory cortisol served as control variables. Second, because a goal of this research was to determine whether cortisol reactivity to certain forms of challenges varied as a function of adult attachment status, LAB1 values also served as control variables in regressions where LAB2 values served as criterion variables.
The interpersonal challenges and collection of LAB2
The interpersonal challenges began at about 14:45 h and lasted roughly 45 min. Upon their ending (around 15:30 h) a second cortisol sample (LAB2) was collected.
LAB2 served as a criterion variable in regressions where attachment variables served as predictor variables, and LAB1 cortisol served as control variables. In addition, because LAB2 always proceeded LAB3- LAB6, LAB2 was also used as a control variable in analyses investigating the total amount of cortisol expressed during cognitive challenges.
Prior to the interpersonal challenges, the lights were dimmed and participants donned headphones in order to assist their ability to comply with visualization instructions during the interpersonal challenges. For the same reason, individual computer monitors, set to a blue and white cloud like screen, were positioned in front of all participants during the interpersonal challenges. Participants listened to the interpersonal challenge instructions (adapted from Mendoza-Denton et al. Citation2001), which were spoken by the author, via headphones and were asked to “close their eyes, relax, and live the (subsequent) experience.” Subsequent vignettes were read by an unfamiliar male voice, and involved themes associated with attachment: death, separation and abandonment ().
Table I. Example of attachment challenge vignette (potential abandonment).
Following each vignette, participants were asked to actively respond by writing about their feelings and coping strategies as if they were continuing to “live the event.” For each vignette, participants were consistently given three minutes to respond to these requests. The inclusion of these questions is in keeping with Kaplan's assessment of attachment strategies in six year olds (Main et al. Citation1985), and with the supposition that ego-involvement is necessary to induce increases in cortisol secretion (Kirschbaum and Hellhammer Citation1994).
During the interpersonal challenge participants were also repeatedly asked self-report questions about their feelings of closeness to others, their own identities, and their moods. There were fifty-five mood questions over the half-hour attachment challenge. In sum, in order to complete the task, participants needed to attend and respond to negative scenarios, while also focusing on the feelings these scenarios produced.
Cognitive challenges and the collection of LAB3, LAB4, & LAB5 saliva samples
The cognitive challenges began at about 15:30 h and lasted approximately an hour and a half. They consisted of three sets of tests (Rifkin Citation2006). Each set was followed by saliva collection and the completion of questions about mood. After the first set (i.e. short term memory tests) LAB3 was obtained at roughly 16:00 h. After the second (i.e. the working memory and executive functioning tests) LAB4 was obtained at roughly 16:35 h. Finally, after the last set (i.e. the long term memory tests) LAB5 was obtained at roughly 17:00 h.
The total amount of cortisol released during the cognitive tasks was calculated with the “Area Under the Curve, Ground” formula (i.e. LABAUC_Cognitive) (Pruessner et al. Citation2003), and utilized LAB2, LAB3, LAB4 and LAB5, as well as the time elapsed between samples.
In contrast to the individualized atmosphere created earlier, the cognitive challenge portion mimicked an atmosphere encountered during standardized testing procedures. The cognitive challenges began when, following the attachment-challenge, the lights were turned on. As in many standardized testing settings, the experimenter, following a rigid thirty-plus page protocol, reminded participants of the strict schedule and told them that there was no time for questions. Sufficient time for completion was not always provided.
In sum, to complete the cognitive challenges, participants needed to answer the questions as quickly and accurately as possible, which was, by design, not always possible.
Laboratory exit and collection of LAB6 saliva sample
The last laboratory sample (LAB6) was collected at around 17:30 h, roughly 30 min after the end of the cognitive tasks. This sample, in combination with the five samples obtained earlier in the laboratory, was used to determine the overall amount of cortisol expressed during the lab session, and was calculated via Pruessner et al (Citation2003) “Area Under the Curve, Ground” formula (i.e. LABAUC).
Between the ending of the cognitive tests and LAB6 participants took part in a test expected to tap procedural memory, completed additional forms, and learned about home collection procedures. Experimenters were instructed to create a more relaxed setting.
Overview of statistical procedures
Relations between attachment variables and cortisol values were determined via multiple regressions. Attachment variables (i.e. attachment insecurity as contrast coded, as well as scale scores on Ds idealization, passivity-of-thought, and U/d slips) served separately as predictor variables and particular cortisol values served as criterion variables. In addition, when appropriate, empirically related scale scores (see Results) were also entered into equations in order to better estimate the unique variance explained by any particular scale score. Likewise, to better determine relations between attachment and specific laboratory values, in some cases prior laboratory values or corresponding home values were also entered into equations. More complete explanations of the analytic strategies used to investigate relations between attachment and both non-laboratory and laboratory values appear below in the corresponding Results sections.
For both non-laboratory and laboratory analyses, marginal significance refers to p ≤ 0.10 and significance to p ≤ 0.05. Bonferroni corrections were not applied, to avoid Type II errors. Nevertheless, in keeping with theoretically driven smaller scale research (de Haan et al. Citation1998), to avoid conclusions based upon Type I errors, the reader is encouraged to consider the pattern of results as well as individual correlations.
Results
AAI classifications and scale scores
AAI classifications
When the disorganized-not-disorganized dimension was collapsed, the distribution was: 54% (20) secure, 43% (16) dismissing, and 3% (1) preoccupied. When the disorganized-not-disorganized dimension was also considered the distribution was 32% (12) secure; 35% (13) dismissing; 3% (1) preoccupied; and 30% (11) disorganized. In both cases these rates were similar to that found for the 50 person sample as a whole (i.e. respectively, 52% secure; 42% dismissing; and 6% preoccupied; and, 32% secure; 28% dismissing; 4% preoccupied; and 36% disorganized).
AAI scale scores
After collapsing the disorganized-not-disorganized dimension, as expected, Ds idealization scores differentiated secure (n = 20, M = 3.58, SD = 1.05) from dismissing (n = 16, M = 6.20, SD = 0.83) groups, t(1,34) = 8.17, p < 0.001, one-tailed. As few individuals received a preoccupied classification, similar comparisons using scores on the preoccupied passivity-of-thought scale were not conducted. Nevertheless, passivity-of-thought scores ranged from 1.0 to 5.0, with 16 individuals receiving above 3.0. Finally, U/d slip scores were significantly higher amongst the disorganized (i.e. unresolved/disorganized and/or cannot classify) (n = 11, M = 4.86, SD = 1.48) than the not disorganized group (n = 26, M = 2.56, SD = 1.18), t(1,35) = 5.03, p < 0.001, one-tailed.
U/d slip scores significantly negatively related to Ds idealization scores (r = − 0.34, p = 0.04, n = 37), and marginally positively related to passivity-of-thought scores (r = 0.28, p = 0.09, n = 37). Ds idealization and passivity-of-thought were not significantly inter-related (r = − 0.06, p = 0.71, n = 37).
Non-laboratory values
Salivary cortisol concentrations
Means and SDs for non-laboratory cortisol values for all subjects (concentrations in nmol/l) were as follows: wake-up: M = 12.32, SD = 4.83; 14:30 h = 6.22, SD = 2.84; 17:30 h: M = 5.44, SD = 2.40; 21:00 h M = 3.56, SD = 1.84; AUC afternoon M = 1051.85, SD = 388.24. Cortisol data were skewed or leptokurtotic and so were analysed after logarithmic transformation.
Attachment and non-laboratory cortisol analyses
Attachment variables served as predictors in regressions where cortisol values served as criterion variables. To evaluate general effects of attachment security vs. insecurity, attachment security was coded as − 1 and attachment insecurity as 1.
To better examine the effects of specific forms of insecurity, continuous state-of-mind scores associated with each type of insecurity served as predictors in similar regression equations. When Ds idealization served as a predictor, U/d slips served as a control variable, since these two scales were significantly correlated. Likewise, when passivity-of-thought served as a predictor, U/d slips served as a control variable. Finally, when U/d slips served as a predictor, both Ds idealization and passivity-of-thought served as control variables. Thus, in analyses examining the state-of-mind scale scores in relation to daily cortisol, R2Δ are from the final step in equations examining a specific scale after controlling for other, empirically related scale scores, and significance levels are in reference to these R2Δ values and accompanying β's ().
Table II. Relations between attachment classifications/scale scores and non-laboratory salivary cortisol values at four times of day.
Table III. Relations between attachment classifications/scale scores and laboratory salivary cortisol values at four times of day.
Wake-up
None of the attachment variables significantly or marginally related to non-laboratory cortisol values upon waking ().
14:30 h
Insecurity was positively associated with 14:30 h cortisol values. No other attachment variable significantly or marginally related to 14:30 h values ().
17.30 h
Neither insecurity nor Ds idealization related to 17:30 h cortisol values. However, there was a trend for passivity-of-thought to be positively associated with 17:30 h cortisol. There was also a trend for U/d slip scores to be negatively associated with 17:30 h cortisol ().
21:00 h
Insecurity, Ds idealization, and U/d slips were not related to 21:00 h cortisol values. However, passivity-of-thought was positively associated with 21:00 h values ().
Self reported stress and mood data
Although subjects were asked to pick “non stressful” days, some reported encountering stressors and/or anxiety near the time of sampling. At a descriptive level, frequency data suggested that stress/anxiety may have been perceived as higher at 14:30 h than at other times of day (i.e., over the course of two days there were 19 reports of stress/anxiety at 14:30 h, while there were 9–10 at the other three sampling times). However, t-tests did not demonstrate relations between cortisol levels and reports of stress or anxiety.
A repeated measures ANOVA using mood data averaged across days yielded significant results for the effects of time-of-day upon feelings (F(3,108) = 8.34, p ≤ 0.01) and arousal (F(3,108) = 10.90, p ≤ 0.01), though answers concerning sadness, anger, and fear did not vary by time-of-day. The general pattern of results suggested that overall negative feelings were highest at morning wake-up while arousal was lowest at this time. Pearson correlations provided little evidence that mood related to cortisol levels. Out of 40 potential relations (i.e. 20 on day 1; 20 on day 2), only a total of seven were significant (n = 3) or marginal (n = 4). Five of these occurred between mood and cortisol obtained at morning wake-up, with more negative emotion and arousal relating to higher salivary cortisol level.
Cortisol and mood daily values: Summary
In sum, few correlations were observed between the AAI variables and daily values. Overall insecurity only related to mid-afternoon values (), and of the scale scores, only preoccupation significantly related to higher later day values. Furthermore, though self-reported mood varied across the day, conscious changes in mood were unlikely to explain the limited associations between AAI variables and daily values, since, with the possible exception of early morning values, self-reported mood was not predictive of salivary cortisol levels.
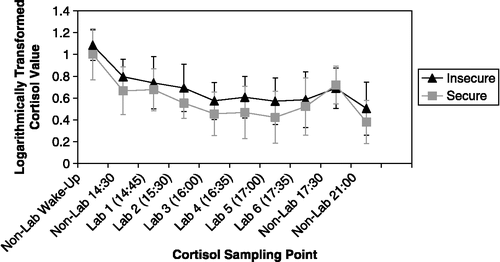
Figure 1 Attachment security and logarithmically transformed salivary cortisol concentrations. This figure reflects logarithmically transformed cortisol values at varying sample points, as a function of attachment status (insecure, secure). Importantly, analyses with laboratory variables control for relevant times of day, whereas values in the figure only represent attachment groups' average salivary cortisol concentration at each time point (and do not reflect the portion of variance explained after accounting for other times-of-day). Values are means + SD. See Tables and for statistics and significance values. Lab1 follows laboratory entry; Lab 2 follows the interpersonal challenges; Lab 3–5 follow sets of explicit cognitive tasks; Lab 6 follows a procedural memory task and laboratory session wrap-up.
Laboratory values
Salivary cortisol during the laboratory session
Means and SDs for laboratory cortisol values for all subjects (concentrations in nmol/l) were as follows: LAB1 M = 6.01, SD = 3.31, LAB2 M = 5.05, SD = 3.02; LAB3 M = 3.77, SD = 1.65; LAB4 M = 4.13, SD = 2.14; LAB5 M = 3.82, SD = 1.98, LAB6 M = 4.32, SD = 2.45. Cortisol data were skewed or leptokurtotic, and so were analysed after logarithmic transformation.
For the group as a whole, cortisol concentration in the laboratory (LABAUC) (M = 2.83, SD = 1.70) was lower than cortisol level obtained at the same time of day outside the laboratory context (BASALAUC_Afternoon) (M = 3.00, SD = 1.4), t(1,34) = 6.37, p ≤ 0.001. Likewise, at a trend level, salivary cortisol level at laboratory entry (LAB1, M = 0.72, SD = 0.23) was lower than cortisol level obtained at the same time outside of the laboratory (BASAl14:30, M = 0.78, SD = 0.17), t(1,34) = 1.68, p ≤ 0.10. Furthermore, for the group as a whole, cortisol levels declined across the laboratory session with LAB1 values (M = 0.72, SD = 0.23) being higher than LAB2 values (M = 0.65, SD = 0.21), t(1,34) = 2.51, p ≤ 0.05; as well as being higher than LAB6 values (M = 0.58, SD = 0.25), t(1,34) = 3.08, ≤ 0.01.
Thus, the laboratory session did not prevent normative afternoon cortisol declines (Kirschbaum and Hellhammer Citation1989). Nevertheless, in keeping with other investigations of individual differences in salivary cortisol (Schlotz et al. Citation2006), analyses focusing upon post-challenge cortisol level were still performed. This is because between-subject differences in post-challenge levels may reflect (relative) increases in cortisol associated with challenges, which are less pronounced than increases occurring in daily life and/or the normative decreases associated with changing time-of-day. Thus, within this study, attachment related cortisol reactivity was still expected to systematically limit the degree of decline.
Attachment and salivary cortisol in the laboratory
Laboratory values served as criterion variables in regressions that, excepting the inclusion of additional cortisol control variables, were identical to those performed when non-laboratory values served as criterion variables. That is, within analyses examining overall security vs. insecurity, R2Δ and β's (reported in ) indicate the relation between attachment and laboratory values in the final step of equations also accounting for other relevant cortisol variables (described below); in analyses examining the state-of-mind scale scores, R2Δ and β's are from the final step in analyses assessing relations between attachment and laboratory values after accounting for other relevant cortisol variables (described below) as well as other empirically related state-of-mind scores.
Laboratory entry (LAB1)
When predicting LAB1, cortisol level collected at 14:30 h in the non-laboratory context served as a control variable. None of the attachment variables related to LAB1 values ().
Inter-personal challenge (LAB2)
When predicting LAB2, LAB1 served as an additional control variable. Insecurity marginally positively predicted LAB2 values. Similarly, Ds idealization was positively associated with higher LAB2 values. Neither passivity-of-thought nor U/d slip scores were significantly or marginally associated with LAB2 ().
Cognitive Challenges (Labauc_cognitive)
When LAB2 values served as a control, no significant relations were observed between LABAUC_Cognitive and security, Ds idealization, passivity-of-thought, or U/d slips ().
Overall laboratory cortisol level (LABAUC)
When predicting overall laboratory values (LABAUC), non-laboratory afternoon cortisol (BASALAUC_Afternoon) served as a control variable. Insecurity marginally positively predicted LABAUC values. Similarly, Ds idealization positively related to LABAUC values. Neither passivity-of-thought nor U/d slips related to LABAUC ().
Self reported mood data
Eight subjects reported having experienced something stressful before their first laboratory cortisol sample.
Self-reported mood significantly to marginally significantly differed across the laboratory session for each mood variable (feelings: F(5,175) = 2.75, p = 0.02; arousal: F(5,180) = 4.43, p = 0.001; anger: F(5,180) = 2.02, p = 0.08; sad: (5,175) = 8.58, p ≤ 0.001; scared = F(5,175) = 3.86, p ≤ 0.01). In general, followup analyses comparing baseline (LAB1) values to subsequent values suggested two patterns: overall feelings became more negative and arousal increased as compared to baseline, while individual negative emotions decreased. Pearson correlations revealed no relations between any of the self-reported measures of mood and laboratory cortisol values obtained at corresponding time points.
Values in the laboratory: Summary
In sum, overall insecurity as well as DS idealization related to comparatively higher salivary cortisol values during the laboratory session (). These relations were likely due to the inter-personal challenge, and were not accounted for by differences in daily values or cortisol levels at laboratory entry. Furthermore, while self-reported mood data suggested that participants may have noticed some changes in their mood across the laboratory session, conscious changes in mood are unlikely to account for the relations between attachment and cortisol, as self-reported mood was not related to simultaneously assessed salivary cortisol concentration.
Discussion
In keeping with past work, relations between attachment classifications/scores and salivary cortisol levels were observed. Contrary to expectations, overall findings concerning daily values did not indicate a relation between attachment and HPA system regulation in low-risk young men, though it remains possible that rarer forms of insecurity influence system regulation. Still, as predicted, the pattern of results indicated that attachment status, and dismissing idealization, influence salivary cortisol reactivity to specific interpersonal challenges, by limiting the rate of expectable decline associated with circadian rhythms.
Adult attachment and HPA regulation
Associations between adult attachment and cortisol levels may be expected due to the relatively greater amount of past life stress likely experienced by insecure individuals (Beckwith et al. Citation1999), and its toll on neural circuitry essential to the HPA axis (Heim et al. Citation1997). Alterations in cortisol values obtained during non-stressful times (e.g. at the moment of wake-up) can mark HPA axis dysregulation, since any differences are not easily attributable to varying daily experience (Kirschbaum and Hellhammer Citation1989; Smyth et al. Citation1998), while later day values may indicate concurrent variation in daily life experience (Schreiber et al. Citation2006). Nevertheless, the majority of this study's findings did not indicate correlations between AAI status and HPA system functioning, though limited data suggest that more rare forms of AAI status might be associated with HPA system dysregulation.
That is, insecurity was associated only with daily cortisol values occurring in the mid-afternoon and not with morning or evening values. This implies that the observed difference in 14:30 h cortisol levels does not reflect system dysfunction, but rather indicates differential experience and/or perceptions occurring within a typical school day. This interpretation is in keeping with findings demonstrating negative impacts of attachment insecurity on college students' beliefs, emotional reactions, and behaviors in learning situations (Larose et al. Citation2005).
In contrast to the findings regarding insecurity, in general, scales associated with rarer forms of attachment insecurity (i.e. unresolved slips and passivity) displayed limited relations to nighttime cortisol values. This is potentially important as comparatively low (Yehuda et al. Citation1996) and comparatively high (Keller et al. Citation2006) evening cortisol level may indicate HPA system dysregulation. Indeed, though past work has not reported a similar relation between U/d slips and lowered cortisol, daily cortisol secretion has been found to be related to preoccupation (Adam and Gunnar Citation2001).
Still, given the current study's small sample size and as these results would not have been/approached significance were Bonferroni corrections applied, these results are best considered as a spring-board for future research. For example, HPA system dysregulation could be better investigated through measures such as the dexamethasone (DEX) suppression test (Caroll Citation1972) and by the inclusion of additional samples aimed at assessing the degree of change following wake-up (Wust et al. Citation2000), while the examination of the influence of daily stress would be aided by the inclusion of diary measures (Smyth et al. Citation1998; Adam Citation2005); however, such a study should not rely solely on subjective appraisals, as self-reported mood was not related to cortisol values within this study. In addition, longitudinal research would greatly aid in our understanding of the effects of early attachment on adult HPA regulation.
Adult attachment and the management of challenges
Beyond expectations concerning HPA system dysregulation, AAI status can be expected to relate to cortisol levels because of its influence on the concurrent management of stressful situations. As noted, increases in cortisol may be expected when challenges are perceived as likely to lead to social rejection, either because the challenges are likely to end in failure or because they are likely to provoke rejection via some other means (Dickerson and Kemeny Citation2004). Thus, regardless of an individual's attachment representation, he may be motivated to evade failing in the presence of others. However, his particular attachment strategy may influence the ways in which he evades failure during interpersonal challenges, and his expectations concerning the ways in which others will view his responses. If an individual is not able to use his normative strategy, he may expect to fail, and thus the HPA axis may become more engaged. Similar increased HPA activity is also likely if an individual feels he is being required to engage in a task that will lead to rejection. Since those higher on DS idealization are expected to: (1) use avoidance to maintain controllability during interpersonal tasks, and (2) to be more likely to think emotional vulnerability will lead to rejection (Main Citation2000), during this study's interpersonal tasks those higher on DS idealization may have been struggling against competing pressures (i.e. the avoidance of failure and hence social rejection, as well as the avoidance of social rejection itself).
This perceived struggle may be one explanation for the relations between insecurity/Ds idealization and comparatively higher post-inter-personal challenge cortisol values. First, those high on Ds idealization may have found it difficult to utilize their (avoidant) strategies because the interpersonal tasks: (a) required much emotional reflection; and (b) occurred within a situational context that may have limited their ability to ignore or deny the suggested experiences (i.e. a dimmed room may prevent attention from being focused elsewhere, and an automated task may prevent the subject from denying occurrences and thus, changing the task's content). Therefore, while attempting to complete the task (and avoid failure and maintain control) those high on Ds idealization may have felt deprived of their normative distress management strategy, and likewise an ability to exert control.
Second, because those higher on Ds idealization may be more likely to assume emotional vulnerability will lead to social rejection, they may have found the interpersonal tasks, which took place in the presence of others and for an experimenter with whom they were relatively highly familiar, to be especially distressing. Indeed, despite their frequent statements indicating the appropriateness of limiting feelings of vulnerability and rejecting others when others exhibit such feelings (Main et al. Citation1984–2003), dismissing individuals: pay increased attention to social stimuli (Maier et al. Citation2005); exhibit physiological markers of conflict when discussing rejection (Dozier and Kobak Citation1992); and show a lack of association between physiological and facial cues of distress (Roisman et al. Citation2004). Thus, during this study's interpersonal challenges those high on Ds idealization may have both felt that displays of vulnerability would be cause for social rejection and also felt feelings of vulnerability. That is, they may have felt the need to constrain emotional expressions, and this may have caused internal physical changes (Roisman et al. Citation2004).
This does not, however, imply that Ds idealization is likely associated with relatively higher cortisol in all contexts. In fact, once cortisol values associated with the inter-personal challenge were controlled, there were no associations with attachment and cortisol levels during the more general (i.e. cognitive) laboratory tasks, nor were there associations between cortisol values and DS idealization during a “normal” day. Likewise, prior research investigating cortisol responses to the AAI, itself, which allows for the denial and avoidance of negative experience, did not find relations between insecurity and higher cortisol values amongst members of low-risk groups (Adam Citation1999; Scheidt et al. Citation2000). Similarly, and in keeping with Adam's (Citation1999) report of marginal relations between preoccupation and laboratory cortisol, these findings do not imply that other types of challenges would not be uniquely stressful to preoccupied and/or disorganized individuals.
Thus, there are a number of issues that remain to be explored. For example, studies with larger populations may be able to recruit individuals with more rare forms of attachment insecurity, and to employ a variety of counterbalanced challenges. This would allow for a better discernment of whether relations between cortisol reactivity and forms of attachment insecurity are moderated by challenge type. In addition, within ethical constraints, future studies may wish to incorporate more direct interpersonal challenges wherein participants are required to engage in emotional discussions within contexts that could likely lead to social rejection; unlike the current stressor, such direct interpersonal challenges might be likely to result in robust increases in cortisol across all (even secure) participants, and to elicit relatively extreme increases amongst those high on DS Idealization. In addition, it is possible that such direct challenges would also lead to more consistent changes in self-reported mood data across the laboratory session, as well as relations between self-reported mood and cortisol values. Likewise, larger studies could directly test mediational paths involving distress management techniques by also assessing relations between attachment and performance on implicit tests of memory, attention, and defensive processing (Van Emmichoven et al. Citation2003; Maier et al. Citation2004; Maier et al. Citation2005; see also Bar-Haim et al. Citation2007). Finally, larger studies should examine the potential moderating effects of variables such as gender, age, clinical sample and genetic make-up. This is important as, for example, participant sex may be related to physiological vulnerability and distress (McEwen Citation2000; Stroud et al. Citation2002; Slotten et al. Citation2006), and genetic make-up may influence the degree to which individuals are influenced by their attachment experiences (van IJzendoorn and Bakermans-Kranenburg Citation2006).
Summary
Given the administrative and financial demands of AAI research, it is not surprising that initial investigations of adult attachment status and cortisol have been small scale. Nevertheless, three studies have now reported associations between attachment and cortisol. In addition, though partially reliant upon marginal associations, the results of the current study demonstrated that amongst low-risk young men, one index of attachment insecurity—DS idealization—accounted for variance in overall laboratory cortisol (R2Δ = 0.16) and post-interpersonal challenge cortisol (R2Δ = 0.11) comparable to that explained by depressive symptomatology on young men's cortisol response-to-awakening (r2 = 0.09–0.12, Pruessner et al. Citation2003) and extraversion on men's cortisol responses to a robust laboratory challenge (r2 = 0.10, Oswald et al. Citation2006). Given the variance explained, the widespread nature of insecurity (e.g. ∼50% of low risk groups are considered insecure, van IJzendoorn and Bakermans-Kranenburg Citation1996), as well as the consequences of cortisol on mental, cognitive and physical health (see for e.g. Sapolsky et al. Citation2000), it may now be time for a larger scale examination of associations between AAI insecurity and cortisol secretion.
Acknowledgements
This work was partially funded by NIH R0I DA 14410, Elizabeth Roboz Einstein Fellowships and a Dissertation grant from the University of California at Berkeley. Support for the author during the write-up of this manuscript was via 5T32 MH016259-27. The research was made possible through contributions of dedicated research participants and research assistants (Lanni Anakosa, Lucy Chi, Heidi Hasbun, Dhara Thakar, Susan Maioriano, and Duane Vickeroy), as well as generous advice during planning stages from a number of researchers including Dr Thomas W. Boyce, Dr Rudolfo Mendoza-Denton, Dr Ozlem Ayduk, Dr Emma Adam, Dr Mary Dozier, Dr Andrea Gierens, and Dr Clemens Kirschbaum. Dr Mary Main and Dr Erik Hesse provided much appreciated expertise, both in general and with regards to the coding of AAI's and Dr Mary Main provided invaluable mentorship. Finally, Dr Sarah Whitton, Dr Pehr Granqvist, Dr Mary Main, Dr Judith Crowell and Dr Stuart Hauser provided especially important feedback on prior manuscript drafts.
Notes
† This work was completed as part of a doctoral dissertation at the University of California, Berkeley, submitted to Dr Mary Main, and much of the time spent writing-up this manuscript occurred during the author's postdoctoral fellowship at Harvard Medical School, Judge Baker Children's Center.
† The mean U/d slip score for individuals considered part of the disorganized group, only due to Unresolved/disorganized slips (and not because of cannot classify status) was 5.25, SD = 0.79.
References
- Adam EK. Emotional and physiological stress in mothers of toddlers: An adult attachment model. Proquest information and learning. 1999
- Adam EK. Momentary emotion and cortisol levels in the everyday lives of working parents. Being together, working apart: Dual-career families and the work-life balance, B Schneider, LJ Waite. Cambridge University Press, New York, NY 2005; 105–133
- Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology 2001; 26(2)189–208
- Ahnert L, Gunnar MR, Lamb ME, Barthel M. Transition to child care: Associations with infant–mother attachment, infant negative emotion, and cortisol elevations. Child Dev 2004; 75(3)639–650
- Ainsworth MS. Attachments beyond infancy. Am Psychol 1989; 44(4)709–716
- Ainsworth MS, Blehar MC, Waters E, Wall S. Patterns of attachment: A psychological study of the strange situation. Lawrence Erlbaum, Mahwah 1978
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol Bull 2007; 133(1)1–24
- Beckwith L, Cohen SE, Hamilton CE. Maternal sensitivity during infancy and subsequent life events relate to attachment representation at early adulthood. Dev Psychol 1999; 35(3)693–700
- Bowlby J. Attachment, separation and loss, volume one. Basic Books, New York 1969
- Caroll B. Stimulation tests in depression. Depressive illnesses: Some research studies, B Carrol. Pergamon, London 1972; 151–153
- de Haan M, Gunnar MR, Tout K, Hart J, Stansbury K. Familiar and novel contexts yield different associations between cortisol and behavior among 2-year-old children. Dev Psychobiol 1998; 33(1)93–101
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull 2004; 130(3)355–391
- Dozier M, Kobak RR. Psychophysiology in attachment interviews: Converging evidence for deactivating strategies. Child Dev 1992; 63(6)1473–1480
- George C, Kaplan N, Main M. Adult attachment interview protocol3rd ed. Department of Psychology, University of California, Berkeley 1984–1996
- Hamilton CE. Continuity and discontinuity of attachment from infancy through adolescence. Child Dev 2000; 71(3)690–694
- Heim C, Owens MJ, Plotsky PM, Nemeroff CB. Persistent changes in corticotropin-releasing factor systems due to early life stress: Relationship to the pathophysiology of major depression and post-traumatic stress disorder. Psychopharmacol Bull 1997; 33(2)185–192
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 2000; 25(1)1–35
- Hesse E. Discourse, memory, and the adult attachment interview: A note with emphasis on the emerging cannot classify category. Infant Ment Health J 1996; 17(1)4–11
- Hesse E. The adult attachment interview: Historical and current perspectives. Handbook of attachment: Theory, research, and clinical applications, J Cassidy, PR Shaver. Guilford Press, New York, NY 1999; 395–433
- Keller J, Flores B, Gomez RG, Solvason HB, Kenna H, Williams GH, et al. Cortisol circadian rhythm alterations in psychotic major depression. Biol Psychiatry 2006; 60(3)275–281
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: A overview. Neuropsychobiology 1989; 22(3)150–169
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 1994; 19(4)313–333
- Larose S, Bernier A, Tarabulsy GM. Attachment state of mind, learning dispositions, and academic performance during the college transition. Dev Psychol 2005; 41(1)281–289
- Madigan S, Bakermans-Kranenburg MJ, Van Ijzendoorn MH, Moran G, Pederson DR, Benoit D. Unresolved states of mind, anomalous parental behavior, and disorganized attachment: A review and meta-analysis of a transmission gap. Attach Hum Dev 2006; 8(2)89–111
- Maier MA, Bernier A, Perkrun R, Zimmermann P, Grossmann KE. Attachment working models as unconscious structures: An experimental test. Int J Behav Dev 2004; 28(2)180–189
- Maier MA, Bernier A, Pekrun R, Zimmermann P, Strasser K, Grossmann KE. Attachment state of mind and perceptual processing of emotional stimuli. Attach Hum Dev 2005; 7(1)67–81
- Main M. The organized categories of infant, child, and adult attachment: Flexible vs. inflexible attention under attachment-related stress. J Am Psychoanal Assoc 2000; 48(4)1055–1096
- Main M, Goldwyn R, Hesse E. Adult attachment scoring and classification system. Department of Psychology, University of California, Berkeley 1984–2003
- Main M, Kaplan N, Cassidy J. Security in infancy, childhood, and adulthood: A move to the level of representation. Monogr Soc Res Child Dev 1985; 50(1)66–104
- Main M, Hesse E, Kaplan N. Predictability of attachment behavior and representational processes at 1, 6, and 19 years of age: The berkeley longitudinal study. Attachment from infancy to adulthood: The major longitudinal studies, KE Grossmann, K Grossmann, E Waters. Guilford Publications, New York 2005; 245–304
- McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry 2000; 48(8)721–731
- Mendoza-Denton R, Ayduk O, Mischel W, Shoda Y, Testa A. Person × situation interactionism in self-encoding (Iam…when…): Implications for affect regulation and social information processing. J Pers Soc Psychol 2001; 80(4)533–544
- Oswald LM, Zandi P, Nestadt G, Potash JB, Kalaydjian AE, Wand GS. Relationship between cortisol responses to stress and personality. Neuropsychopharmacology 2006; 31(7)1583–1591
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology 2005; 30(12)2192–2204
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration vs. time-dependent change. Psychoneuroendocrinology 2003; 28(7)916–931
- Pruessner M, Hellhammer D, Pruessner J, Lupien S. Self-reported depressive symptoms and stress levels in healthy young men: Associations with the cortisol response to awakening. Psychosom Med 2003; 65(1)92–99
- Ramsay D, Lewis M. Reactivity and regulation in cortisol and behavioral responses to stress. Child Dev 2003; 74(2)456–464
- Rifkin A. 2006. Individual differences in responses to the adult attachment interview predict responses to neuropsychological testing, as well as both basal and laboratory cortisol, ProQuest Information and Learning.
- Roisman GI, Tsai JL, Chiang K-HS. The emotional integration of childhood experience: Physiological, facial expressive, and self-reported emotional response during the adult attachment interview. Dev Psychol 2004; 40(5)776–789
- Sapolsky R, Romero M, Munck A. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 2000; 21: 55–89
- Scheidt CE, Waller E, Malchow H, Ehlert U, Becker-Stoll F, Schulte-Monting J, Lucking C. Attachment representation and cortisol response to the adult attachment interview in idiopathic spasmodic torticollis. Psychother Psychosom 2000; 69(3)155–162
- Schlotz W, Schulz P, Hellhammer J, Stone AA, Hellhammer DH. Trait anxiety moderates the impact of performance pressure on salivary cortisol in everyday life. Psychoneuroendocrinology 2006; 31(4)459–472
- Schreiber JE, Shirtcliff E, Van Hulle C, Lemery-Chalfant K, Klein MH, Kalin NH, et al. Environmental influences on family similarity in afternoon cortisol levels: Twin and parent-offspring designs. Psychoneuroendocrinology 2006; 31(9)1131–1137
- Shulman S, Elicker J, Sroufe LA. Stages of friendship growth in preadolescence as related to attachment history. J Soc Pers Relation 1994; 11(3)341–361
- Slotten HA, Kalinichev M, Hagan JJ, Marsden CA, Fone KC. Long-lasting changes in behavioural and neuroendocrine indices in the rat following neonatal maternal separation: Gender-dependent effects. Brain Res 2006; 1097(1)123–132
- Smyth J, Ockenfels MC, Porter L, Kirschbaum C, Hellhammer DH, Stone AA. Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology 1998; 23(4)353–370
- Spangler G, Grossmann KE. Biobehavioral organization in securely and insecurely attached infants. Child Dev 1993; 64(5)1439–1450
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: Social rejection versus achievement stress. Biol Psychiatry 2002; 52(4)318–327
- Van Emmichoven IAZ, Van Ijzendoorn MH, De Ruiter C, Brosschot JF. Selective processing of threatening information: Effects of attachment representation and anxiety disorder on attention and memory. Dev Psychopathol 2003; 15(1)219–237
- van IJzendoorn MH. Adult attachment representations, parental responsiveness, and infant attachment: A meta-analysis on the predictive validity of the adult attachment interview. Psychol Bull 1995; 117(3)387–403
- van IJzendoorn MH, Bakermans-Kranenburg MJ. Attachment representations in mothers, fathers, adolescents, and clinical groups: A meta-analytic search for normative data. J Consult Clin Psychol 1996; 64(1)8–21
- Van Ijzendoorn MH, Bakermans-Kranenburg MJ. DRD47-repeat polymorphism moderates the association between maternal unresolved loss or trauma and infant disorganization. Attach Hum Dev 2006; 8(4)291–307
- van IJzendoorn MH, Schuengel C, Bakermans-Kranenburg MJ. Disorganized attachment in early childhood: Meta-analysis of precursors, concomitants, and sequelae. Dev Psychopathol 1999; 11(2)225–249
- Waters E, Merrick S, Treboux D, Crowell J, Albersheim L. Attachment security in infancy and early adulthood: A twenty-year longitudinal study. Child Dev 2000; 71(3)684–689
- Weinfield NS, Whaley GJL, Egeland B. Continuity, discontinuity, and coherence in attachment from infancy to late adolescence: Sequelae of organization and disorganization. Attach Hum Dev 2004; 6(1)73–97
- Wust S, Wolf J, Hellhammer D, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response—normal values and confounds. Noise Health 2000; 2(7)79–88
- Yehuda R, Teicher M, Trestman R, Levengood R, Siever L. Cortisol regulation in posttraumatic stress disorder and major depression: A chronobiological analysis. Biol Psychiatry 1996; 40: 79–88
- Zimmermann P, Becker-Stoll F, Grossmann K, Grossmann KE, Scheuerer-Englisch H, Wartner U. Longitudinal attachment development from infancy through adolescence. Psychologie in Erziehung and Unterricht 2000; 47(2)99–117