Abstract
Chronic subordinate colony housing (CSC) is a relevant chronic psycho-social stressor for male mice. Here, we investigated effects of CSC on the severity of dextran sulphate sodium (DSS)-induced colitis and the involvement of adrenal mechanisms.
After 19 days of CSC, male C57BL/6 mice were treated with 1% DSS (8 days). After 8 days, inflammatory shortening of the colon and the histological inflammation score were increased in CSC mice. Additionally, the increased secretion of pro-inflammatory cytokines by mesenteric lymph node cells found on day 2 and 4 of DSS treatment was down-regulated in CSC mice on day 8 of DSS treatment, paralleled by an increase in plasma corticosterone. In contrast, in unstressed controls, elevation of cytokine secretion was delayed and only found on day 8 of DSS treatment, associated with a prompt rise in plasma corticosterone.
To reveal adrenal mechanisms in CSC-induced effects on colitis, mice were adrenalectomized, exposed to CSC and treated with DSS (8 days). In adrenalectomized CSC mice, the severity of DSS-induced colitis was reduced, as body weight loss, shortening of colon, histological damage score, and cytokine secretion from mesenteric lymph node cells were diminished compared with sham-operated CSC mice.
In conclusion, exposure to chronic psycho-social stress increases the severity of acute DSS colitis, an effect which is, at least partly, mediated by adrenal mechanisms.
Introduction
After first reports in the 1970s (Salem and Shubair Citation1967), stress has been recognized as one of the key factors modulating the onset and the severity of spontaneous colitis in humans (Mendeloff et al. Citation1970; Mitchell and Drossman Citation1987; Robertson et al. Citation1989; Riley et al. Citation1990; North et al. Citation1991) and nonhuman primates (Drossman Citation1985; Gozalo and Montoya Citation1992). As a consequence, there is a growing number of animal studies investigating the relationship between exposure to acute, intermediate or chronic stress and immune parameters including effects on both spontaneous colonic inflammation (Reber et al. Citation2007) and experimentally-induced colitis (Qiu et al. Citation1999; Pfeiffer et al. Citation2001; Milde and Murison Citation2002; Reber et al. Citation2006). The described stress effects strongly depend on the quality and duration of the stressor and are, therefore, partially controversial (Gue et al. Citation1997; Million et al. Citation1999; Cakir et al. Citation2004; Gulpinar et al. Citation2004). Recently, we showed that chronic exposure of male mice to subordinate colony housing (CSC-housing) for 19 consecutive days is a clinically relevant animal model for chronic psycho-social stress. Exposure to CSC alters not only behavioural parameters (increased anxiety), but also results in the development of spontaneous colonic inflammation (Reber et al. Citation2007). In CSC mice, an increased cytokine secretion by mesenteric lymph node cells and an increased histological score were found. Interestingly, in chronically stressed mice, an adrenal insufficiency could be revealed at the end of stressor exposure both in vivo and in vitro (Reber et al. Citation2007). There is evidence that a blunted responsiveness of the hypothalamo–pituitary–adrenal (HPA) axis makes the animals also more prone to a chemically induced inflammation (Gue et al. Citation1997; Million et al. Citation1999; Cakir et al. Citation2004; Gulpinar et al. Citation2004). This is in line with the increased severity of DSS-induced colitis described recently by our group after a 19-day exposure to the social defeat/overcrowding (SD/OC) paradigm, another model of chronic psycho-social stress resulting in adrenal insufficiency (Reber et al. Citation2006). An adequate secretion of immunosuppressive glucocorticoids might be important for the suppression of the immune response and prevention of overshooting (Besedovsky et al. Citation1986; Suzuki et al. Citation1986). This hypothesis was tested in mice exposed to CSC and subsequently treated with dextran sulphate sodium (DSS) to induce colonic inflammation (Okayasu et al. Citation1990; Obermeier et al. Citation2002). In detail, we aimed to reveal whether the CSC-induced blunted responsiveness of the adrenals (Reber et al. Citation2007) persists during subsequent DSS treatment and contributes to the chronic stress-induced increase in colitis severity.
Moreover, activation of the adrenal glands as reflected by increased plasma corticosterone levels during the acute phase of CSC exposure is likely to contribute to induction of spontaneous colonic inflammation (Reber et al. Citation2007). Therefore, we aimed to study whether the initial stress-induced activation of the adrenal glands (Reber et al. Citation2007) is also involved in the effects of CSC on the severity of a DSS-induced colitis.
Overall, the present study was designed to determine whether exposure to chronic psycho-social stress prior to colitis induced by DSS treatment affects the severity of the chemically-induced intestinal inflammation and to what extendt these effects are mediated by adrenal mechanisms.
Materials and methods
Animals
Male C57BL/6 mice (Charles River, Sulzfeld, Germany) weighing 19–22 g (experimental mice) or 30–35 g (dominant mice) were individually housed in standard polycarbonate mouse cages (16 × 22 × 14 cm) for at least one week before the experimental procedure started. All mice were kept under standard laboratory conditions (12-h light/dark cycle, lights on at 06:00 h, 22°C, 60% humidity) and had free access to tap water and standard mouse diet. All experimental protocols were approved by the Committee on Animal Health and Care of the local government, and performed according to international guidelines on the ethical use of animals. All efforts were made to minimise the number of animals used and their suffering.
Chronic subordinate colony (CSC) housing
One week after arrival, experimental mice were randomly assigned to the control or the CSC group. Controls were singly housed and remained undisturbed in their home cages except for change of bedding once a week. As described before (Reber et al. Citation2007), four experimental mice were housed together with a larger dominant male in a polycarbonate observation cage (38 × 22 × 35 cm) for 19 consecutive days. To avoid habituation, each dominant male was replaced by a novel dominant male on days 8 and 15. During the first 30 min of formation of the colonies on day 1, 8, and 15, the mice were videotaped for behavioural analyses. In all colonies, the larger male mouse established a “dominant” status by chasing and attacking all four experimental mice. The four experimental mice were considered as “subordinates” based on their defensive behaviour, including flight, retreat and submissive upright posture, as described before (Reber et al. Citation2007) and according to a recent report in rats (Stefanski et al. Citation2001).
Experimental procedures
Experiment 1
In order to investigate whether chronic psycho-social stress influences the severity of subsequent DSS colitis and whether adrenal insufficiency persists during DSS treatment, mice were randomly assigned to the control and the CSC group. At 6 p.m. on day 19 of CSC exposure all mice were singly housed. On the next day (day 20), CSC and unstressed control mice were tested on the elevated plus-maze to confirm the effects of chronic psycho-social stress on anxiety-related behaviour (Reber et al. Citation2007). Mice were then treated with 1% DSS in their drinking water from day 20 to 27, and were killed on either day 21, 23 or 27, i.e. on day 2, 4 or 8 of DSS treatment for quantification of colon length, histological assessment of the colon (histological score, see below), and plasma corticosterone concentrations. Additionally, cytokine secretion by mesenteric lymph node cells was assessed as described before (Obermeier et al. Citation1999; Reber et al. Citation2006; Reber et al. Citation2007). On day 27, additional groups of CSC and control mice, which were not treated with DSS (no DSS) were killed for assessment of direct effects of CSC exposure on colonic inflammatory parameters.
Experiment 2
In order to investigate the role of the adrenals in mediating the effects of CSC-exposure on the severity of the DSS-induced colitis, mice underwent either adrenalectomy (ADX) or were sham operated (SHAM) one week before the CSC procedure started. Respective ADX and SHAM unstressed controls remained singly housed. At 6 p.m. on day 19, CSC mice were singly housed and on the next day all mice were treated with 1% DSS from day 20 to 27. On day 8 of DSS treatment, mice were killed for quantification of colon length, histological assessment of the colon (histological score, see below), and cytokine secretion from mesenteric lymph node cells.
Elevated plus-maze test
To assess the effect of CSC on anxiety-related behaviour, both controls and CSC mice were transported to the plus-maze test room in the evening of day 19 of CSC exposure. The next day, they were tested for 5 min between 8 and 11 a.m. (Pellow et al. Citation1985; Lister Citation1987). The elevated plus-maze adapted for mice consisted of two open (6 × 30 cm) and two closed (6 × 30 × 17 cm) arms radiating from a central platform (6 × 6 cm) to form a plus-shaped figure elevated 130 cm above the floor. The open arm edges were 0.3 cm in height to avoid mice falling. Each mouse was placed on the central platform facing a closed arm. The number of entries onto the open and closed arms, and the time spent on the respective arms were recorded by means using video/computer setup to allow calculation of the percentage of time spent on, and the percentage of entries performed onto the open arms of the maze. The maze was cleaned thoroughly before each test.
Induction of acute colitis
Acute colitis was induced by administering 1% DSS (36–50 kDa; ICN Biomedicals, cat.no. 160110, Eschwege, Germany) in the drinking fluid (experiment 1: water, experiment 2: 0.9% saline, see below), ad libitum from day 20 to 27, as previously described (Obermeier et al. Citation1999).
Blood sampling and radioimmunoassay for corticosterone
To determine the effect of CSC exposure on plasma corticosterone concentrations, mice were rapidly killed by decapitation under CO2 anaesthesia within 3 min after entering the animal room. Trunk blood was collected in EDTA-coated tubes on ice (Sarstedt, Nümbrecht, Germany) containing 10 μl aprotinin (Trasylol, Bayer Corp. AG, Leverkusen, Germany) and centrifuged at 4°C (5000 rpm, 10 min). Plasma samples were stored at − 20°C until assayed using a commercially available radioimmunoassay for corticosterone (MP Biomedicals GmbH, Eschwege, Germany; detection limit: 10 ng/ml). The intra- and interassay coefficients of variation were below 10%.
Determination of colonic length and histological score
Reduction of colonic length was used as a parameter to assess colonic inflammation (Kojouharoff et al. Citation1997; Axelsson et al. Citation1998). The colon was removed, the length measured to 0.1 cm precision, and mechanically cleaned of faeces. Afterwards, 1 cm of the distal third of the colon was cut longitudinally, laid on a filter paper and fixed in 10% formalin overnight. The next day, the fixed tissue was embedded in paraffin and cut longitudinally. Three 3-μm haematoxylin–eosin stained sections taken 100-μm apart were evaluated by histological scoring performed by an investigator blinded to treatment. For statistics, each individual score represented the mean of the three sections. Histology was scored as follows after (Steidler et al. Citation2000; Obermeier et al. Citation2003):
Epithelium. 0: normal morphology; 1: loss of goblet cells; 2: loss of goblet cells in large areas; 3: loss of crypts; 4: loss of crypts in large areas
Infiltration. 0: no infiltration; 1: infiltrate around crypt basis; 2: infiltrate reaching to lamina muscularis mucosae; 3: extensive infiltration reaching the lamina muscularis mucosae and thickening of the mucosa with abundant oedema; 4: infiltration of the lamina submucosa.
The total histological score represents the sum of the epithelium and infiltration score and ranges from 0 to 8.
Isolation and incubation of mesenteric lymph node cells
Mesenteric lymph nodes (pooled from each experimental group) were harvested under sterile conditions and collected on ice in cell culture medium [RPMI-1640 supplemented with 10% fetal calf serum (Biochrom, Berlin, Germany), 100 U/ml penicillin and 100 μg/ml streptomycin (GIBCO-BRL, Eggenstein, Germany) and 3 × 10− 5 M β-mercaptoethanol (Sigma, Deisenhofen, Germany)]. Lymph nodes were mechanically disrupted and filtered through a cell strainer (70-μm Nylon, Falcon™, Becton Dickinson, Heidelberg, Germany). Afterwards cells were washed three times in cell culture medium and adjusted to a concentration of 106 cells/ml. Then 2 × 105 (200 μl) lymph node cells were transferred to wells of a 96-well plate; to stimulate the cells, the wells were pre-coated with 200 μl of 2.5 μg/ml anti-CD3 antibody in the presence of IL-2 (final concentration 100 U/ml). Eight wells were loaded with the respective number of cells for each experimental group. After incubation for 24 h (37°C, 5% CO2), cytokine concentrations were measured in the supernatants by ELISA (all from Endogen, Woburn, MA) according to the respective protocols, using four wells per experimental group.
ADX procedure
ADX was performed under isoflurane anaesthesia. A 2-cm skin incision was performed on the back of the mice at the level of the kidneys (midline), and the adrenals were removed bilaterally via two peritoneal incisions performed on the left and right side of the abdomen of the mouse. Sham-operated mice underwent the same procedure except removal of the adrenals. Following surgery, ADX and SHAM mice received 0.9% saline in drinking water (until they were killed) and were housed singly for one week until the CSC procedure started. Saline allowed ADX mice to compensate for loss of mineralocorticoids.
Statistics
For statistical comparisons, the software package SPSS (version 12) was used. Data of two experimental groups were compared by Mann-Whitney U-tests. All other comparisons were done using a two-way ANOVA (experiment 1:factor CSC, factor DSS; experiment 2:factor ADX, factor CSC) and following post hoc Tukey-HSD test. Data are presented as means +/ − SEM. Significance was taken at p < 0.05.
Results
Effects of CSC exposure on body weight and anxiety-related behaviour (experiment 1)
CSC mice gained significantly less body weight during the 19 days of CSC compared with controls (). In addition, exposure to CSC significantly increased anxiety-related behaviour as reflected by a decrease in the percentage of time spent on the open arms, and in the percentage of open arm entries compared with control mice (). The number of entries onto the closed arms, indicative of locomotor activity, was not altered by CSC exposure ().
Table I. Effects of a chronic psycho-social stressor (CSC) on body weight and anxiety-related behaviour on the elevated plus-maze.
Effects of CSC exposure on the severity of DSS-induced colitis and on plasma corticosterone concentrations (experiment 1)
Prior exposure to CSC significantly increased the severity of acute DSS-induced colitis, as indicated by a greater body weight loss, shorter colon length, higher histological damage score of the colon, and increased secretion of pro- and anti-inflammatory cytokines by draining mesenteric lymph node cells compared with non-stressed mice.
Body weight
The body weight change of CSC mice did not differ from non-stressed mice between days 1 and 2, and between days 1 and 4 of DSS treatment, but between days 1 and 8 of DSS treatment, the body weight change was found to depend on prior CSC exposure and DSS treatment (factor CSC × DSS:F1,33 = 21.5; p < 0.001). DSS treatment resulted in a decrease in body weight in both CSC and control groups, but this effect was more pronounced in CSC mice compared with unstressed controls ().
Figure 1 Effects of prior exposure to CSC on body weight changes from day 1 to 2 [control/DSS; CSC/DSS], day 1 to 4 [control/DSS; CSC/DSS], and day 1 to 8 [control/noDSS; CSC/noDSS; control/DSS; CSC/DSS] of DSS treatment. CSC exposure prior to DSS treatment had no effect on body weight changes from day 1 to 2 and day 1 to 4 of DSS administration. Enhanced body weight loss in CSC mice was found from day 1 to 8 of DSS treatment. Numbers in parenthesis indicate number of mice per group. Data represent mean ± SEM; *** p < 0.001 vs. respective controls; ### p < 0.001 vs. respective group without DSS.
![Figure 1 Effects of prior exposure to CSC on body weight changes from day 1 to 2 [control/DSS; CSC/DSS], day 1 to 4 [control/DSS; CSC/DSS], and day 1 to 8 [control/noDSS; CSC/noDSS; control/DSS; CSC/DSS] of DSS treatment. CSC exposure prior to DSS treatment had no effect on body weight changes from day 1 to 2 and day 1 to 4 of DSS administration. Enhanced body weight loss in CSC mice was found from day 1 to 8 of DSS treatment. Numbers in parenthesis indicate number of mice per group. Data represent mean ± SEM; *** p < 0.001 vs. respective controls; ### p < 0.001 vs. respective group without DSS.](/cms/asset/b378b492-ebc2-4c51-9d68-19bafc58caef/ists_a_273275_f0001_b.gif)
Colon length
The colon length of CSC and control mice was not statistically different on day 2 or 4 of DSS treatment, but on day 8 of DSS treatment, a significant interaction between CSC and DSS treatment was found (factor stress × DSS: F1,33 = 7.51; p = 0.01). Specifically, colon length was significantly reduced by DSS administration, in both control and CSC mice, compared with respective mice given tap water. However, the effect of DSS was more severe in CSC compared with control mice ().
Figure 2 Effects of prior exposure to CSC on the length (A) and the histological damage score (B) of the colon estimated on day 2, day 4, and day 8 of DSS treatment. CSC exposure prior to DSS treatment further reduced colon length on day 8 of DSS treatment. The DSS-induced increase in the histological damage score was more pronounced in CSC mice on day and 8 of DSS treatment. (C) and (D) show two representative colonic haematoxylin and eosin-stained sections [a: Lamina mucosae; b: Lamina muscularis mucosae; c: Lamina submucosae; d: Lamina muscularis (circular muscle); e: Lamina muscularis (longitudinal muscle)] from: (C) non-stressed control mice receiving tap water without DSS for 8 days, with normal colon histology, and (D) non-stressed controls treated with DSS for 8 days, showing crypt loss in large areas; thickening of the mucosa with abundant oedema; infiltration reaching the lamina submucosa. Numbers in parenthesis indicate number of mice per group. Data represent group mean+/ − SEM * p < 0.05 vs. respective controls; ### p < 0.001 vs. respective group without DSS.
![Figure 2 Effects of prior exposure to CSC on the length (A) and the histological damage score (B) of the colon estimated on day 2, day 4, and day 8 of DSS treatment. CSC exposure prior to DSS treatment further reduced colon length on day 8 of DSS treatment. The DSS-induced increase in the histological damage score was more pronounced in CSC mice on day and 8 of DSS treatment. (C) and (D) show two representative colonic haematoxylin and eosin-stained sections [a: Lamina mucosae; b: Lamina muscularis mucosae; c: Lamina submucosae; d: Lamina muscularis (circular muscle); e: Lamina muscularis (longitudinal muscle)] from: (C) non-stressed control mice receiving tap water without DSS for 8 days, with normal colon histology, and (D) non-stressed controls treated with DSS for 8 days, showing crypt loss in large areas; thickening of the mucosa with abundant oedema; infiltration reaching the lamina submucosa. Numbers in parenthesis indicate number of mice per group. Data represent group mean+/ − SEM * p < 0.05 vs. respective controls; ### p < 0.001 vs. respective group without DSS.](/cms/asset/67370e4f-86de-4cd1-87d8-58f95a630f11/ists_a_273275_f0002_b.gif)
Histological score
A trend towards an increased histological score was detected in CSC mice compared with controls already on day 2 (p = 0.064) of DSS treatment. On day 8 of DSS treatment the histological score was dependent on prior CSC exposure and DSS treatment (factor CSC × DSS: F1,32 = 14.2; p = 0.001). The low histological score of CSC and control () mice receiving no DSS indicated no colonic inflammation. In contrast, DSS treatment increased the histological score in both CSC and control () mice. Importantly, CSC mice receiving DSS showed a significantly increased histological score compared with respective controls, reflecting a more severe inflammatory infiltration and increased epithelial damage of the colon ().
Secretion of cytokines from draining mesenteric lymph node cells
In DSS-treated mice, the secretion of the pro-inflammatory cytokines TNF-α, IFN-γ, IL-6, and the anti-inflammatory cytokine IL-10 from mesenteric lymph node cells was significantly higher in CSC compared with control mice both on days 2 and 4 of DSS treatment. On day 8 of DSS treatment, an interaction between CSC and DSS treatment was found for all pro-inflammatory cytokines (TNF-α: F1,12 = 38.7; p = 0.001; IFN-γ: F1,12 = 8.03; p = 0.015; IL-6: F1,12 = 10.1; p = 0.008). In detail, DSS-treatment of control mice for 8 consecutive days resulted in an increased secretion of TNF-α, IFN-γ, and IL-6 compared with controls given tap water. DSS treatment also resulted in an increased secretion of IL-6 within the CSC group. Moreover, in CSC mice not treated with DSS after stressor exposure an increased secretion of pro-inflammatory cytokines was found compared with controls 8 days after termination of the CSC procedure ().
Figure 3 Effects of prior exposure to CSC on the secretion of pro-inflammatory [TNF-α (A), IFN-γ (B), IL-6 (C)], and anti-inflammatory [IL-10 (D)] cytokines by mesenteric lymph node cells on day 2, day 4, and day 8 of DSS treatment. CSC exposure prior to DSS treatment increased secretion of all cytokines on day 2 and 4 of DSS administration. On day 8 after CSC exposure cytokine secretion was also increased in CSC mice given tap water only. Numbers in parenthesis indicate number of mice per group. Data represent mean +SEM * p < 0.05; ** p < 0.01; *** p < 0.001 vs. respective controls; ## p < 0.01; ### p < 0.001 vs. respective group without DSS.
![Figure 3 Effects of prior exposure to CSC on the secretion of pro-inflammatory [TNF-α (A), IFN-γ (B), IL-6 (C)], and anti-inflammatory [IL-10 (D)] cytokines by mesenteric lymph node cells on day 2, day 4, and day 8 of DSS treatment. CSC exposure prior to DSS treatment increased secretion of all cytokines on day 2 and 4 of DSS administration. On day 8 after CSC exposure cytokine secretion was also increased in CSC mice given tap water only. Numbers in parenthesis indicate number of mice per group. Data represent mean +SEM * p < 0.05; ** p < 0.01; *** p < 0.001 vs. respective controls; ## p < 0.01; ### p < 0.001 vs. respective group without DSS.](/cms/asset/32d0167d-8dc3-4672-a774-32e235c6c6da/ists_a_273275_f0003_b.gif)
Finally, on day 8 of DSS treatment, a CSC effect (F1,12 = 123.0; p < 0.001) and a DSS treatment effect (F1,12 = 83.8; p < 0.001) were found for the anti-inflammatory cytokine IL-10. In detail, IL-10 secretion was increased by prior CSC exposure in both mice receiving DSS and tap water. Additionally, DSS treatment resulted in an increased secretion of IL-10 in CSC and control mice.
Effects of CSC exposure on plasma corticosterone concentrations during subsequent DSS-treatment (experiment)
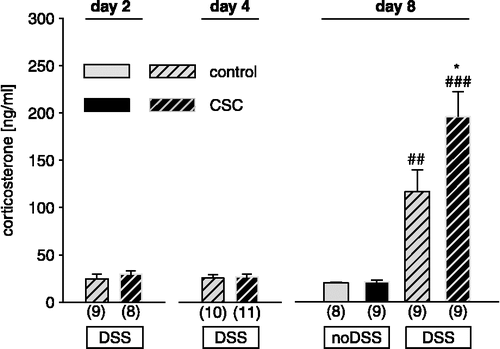
Plasma corticosterone concentrations were similar between CSC and control mice on day 2 and 4 of DSS treatment. On day 8, corticosterone concentrations were dependent on prior CSC exposure and DSS-treatment (factor CSC × DSS: F1,31 = 4.67; p < 0.038). Plasma corticosterone was similar in CSC and control mice, which did not receive DSS, 8 days after termination of the chronic stressor. In contrast, DSS treatment significantly increased plasma corticosterone concentrations in CSC and control mice, with the effect being more pronounced in CSC mice ().
Figure 4 Effects of prior exposure to CSC on plasma corticosterone concentrations on day 2, day 4, and day 8 of DSS treatment. Corticosterone concentrations of controls and CSC mice were increased on day 8 of DSS treatment, with the effect being more greater in CSC mice. Numbers in parenthesis indicate number of mice per group. Data represent mean +SEM * p < 0.05 vs. respective controls; ## p < 0.01; ### p < 0.001 vs. respective group without DSS.
Effects of ADX on CSC-induced aggravation of DSS-colitis (experiment 2)
ADX, performed one week before CSC exposure, significantly reduced the CSC-induced exacerbation of an acute DSS-colitis, as indicated by reduced body weight loss, less reduction in colon length, and a lower histological damage score of the colon on day 8 of DSS treatment in CSC mice of the ADX compared with respective mice of the SHAM group.
Body weight change
The body weight change during the 7 days of DSS treatment was dependent on ADX and CSC exposure (factor ADX × CSC: F1,25 = 7.23; p = 0.013). DSS treatment resulted in a loss of body weight in CSC mice of both the ADX and SHAM group. However, this effect was less pronounced in ADX compared with SHAM mice ().
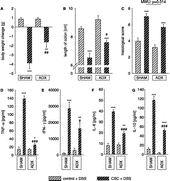
Figure 5 Effects of adrenalectomy (ADX) and CSC on the severity of acute colitis on day 8 of DSS treatment (SHAM/control: n = 8; SHAM/CSC: n = 7; ADX/control: n = 8; /ADX/CSC n = 6). ADX one week before exposure to 19 days of CSC reduced the severity of the DSS-induced colitis compared with SHAM mice as indicated by a diminished body weight loss (A), less inflammatory reduction of colonic length (B), a trend towards a lower histological damage score (C), and a reduced secretion of TNF-α (D), IFN-γ (E), IL-6 (F), and IL-10 (E) from mesenteric lymph node cells compared to CSC mice of the SHAM group. Data represent mean ± SEM; * p < 0.05; ** p < 0.01; *** p < 0.001 vs. respective controls; # p < 0.05; ## p < 0.01; ### p < 0.001 vs. respective SHAM group.
Colon length
A significant ADX (F1,25 = 7.40; p = 0.012) and CSC (F1,25 = 54.2; p < 0.001) effect was found for the length of the colon on day 8 of DSS treatment. More specifically, colon length was significantly reduced in CSC mice of both the ADX and SHAM groups compared with respective control mice. However, the effect of DSS was less severe in the ADX group compared with the SHAM group ().
Histological score
The histological damage score of colonic tissue was found to depend on ADX (F1,25 = 8.57; p = 0.007) and CSC exposure (F1,25 = 61.6; p < 0.001). Exposure to CSC increased the histological score compared with controls in both the ADX and SHAM groups on day 8 of DSS treatment. However, there was a trend towards a less severe CSC-induced increase in the histological score in the ADX compared to the SHAM mice (p = 0.087). This effect reached statistical significance when performing a Mann–Whitney U-comparison between the histological scores of these two groups (p = 0.014) ().
Secretion of cytokines from draining mesenteric lymph node cells
On day 8 of DSS treatment, an interaction between ADX and CSC exposure was found for all cytokines measured (TNF-α: F1,12 = 141.6; p < 0.001; IFN-γ: F1,12 = 7.78; p = 0.016; IL-6: F1,12 = 36.6; p < 0.001; IL-10: F1,12 = 39.0; p < 0.001). In both SHAM and ADX mice, the secretion of the pro-inflammatory cytokines TNF-α, IFN-γ, IL-6, and the anti-inflammatory cytokine IL-10 from mesenteric lymph node cells was significantly greater in CSC compared with control mice on day 8 of DSS treatment. However, this effect was significantly less pronounced in CSC mice of the ADX group for all measured cytokines (–G).
Discussion
The results of the present study indicate that exposure to CSC hastens the onset and increases the severity of subsequent DSS-induced colitis. Furthermore, there was no increase in plasma corticosterone in CSC mice at least until day 4 of DSS treatment, although the elevated cytokine secretion from mesenteric lymph node cells indicates an increased inflammatory state in these mice. Finally, ADX prior to CSC exposure attenuated the CSC-induced increase in the severity of DSS colitis suggesting that the initial activation of the adrenal glands during CSC exposure significantly contributes to the CSC-induced increase in the severity of DSS Colitis.
After exposure to 19 days of CSC, mice showed a reduced body weight gain and an increased anxiety-related behaviour as reflected by a decreased time spent on the open arms and a decreased number of entries onto the open arms of the elevated plus-maze compared with non-stressed controls. These findings, confirming our recent results on the effects of exposure to CSC (Reber et al. Citation2007), further indicate that CSC is a robust and reproducible chronic psycho-social stress model for male mice.
Within two days of subsequent DSS treatment, CSC mice showed an increased secretion of the pro-inflammatory Th1 (secreted by inflammatory CD4-T-cells) cytokines IFN-γ, TNF-α, and IL-6 and of the anti-inflammatory Th2 (secreted by CD4-T-cells) cytokine IL-10 by mesenteric lymph node cells. In contrast, in controls a comparable increase in the secretion of these cytokines was only found on day 8 of DSS treatment. Additionally, there was a trend towards an increased histological damage score in stressed mice already on day 2 of DSS treatment. These emerging signs of acute colitis in CSC mice already after 2 days of DSS treatment suggest that exposure to CSC itself rather than DSS induced colonic inflammation. Indeed, we recently demonstrated an increased secretion of IFN-γ, TNF-α, and IL-10 by mesenteric lymph node cells and an increased histological score after prolonged exposure to CSC (Reber et al. Citation2007). Moreover, even 8 days after termination of CSC exposure, CSC mice of the present study not treated with DSS showed an increased cytokine secretion.
In general, an increased inflammatory state in colonic tissue results in peripherally generated inflammatory mediators and cytokines, and is supposed to rapidly activate the HPA axis. Activation of the HPA axis can occur at various levels, including hypothalamic CRH neurons, pituitary corticotrophs, and the adrenal cortex (Sapolsky et al. Citation1987; Gue et al. Citation1997). Secreted glucocorticoids are important inhibitors of inflammatory processes, due to their ability to block the production and action of several lymphokines, such as IL-2 and IFN-γ (Jones and Romano Citation1984; Besedovsky et al. Citation1986; Suzuki et al. Citation1986; Gue et al. Citation1997). Indeed, in control mice the increased cytokine secretion by mesenteric lymph node cells on day 8 of DSS treatment was paralleled by high plasma corticosterone concentrations. In contrast, the increased inflammatory state in CSC mice after 2 days of DSS treatment was not paralleled by increased levels of plasma corticosterone. These findings suggest that HPA axis reactivity is blunted as a result of CSC exposure during the first days of DSS-induced colitis. Indeed, we recently showed that 19 days of chronic psycho-social stress, induced by either CSC or social defeat/overcrowding (SD/OC) exposure, resulted in an insufficiency of the adrenals to adequately respond to ACTH as shown in vivo as well as in vitro (Reber et al. Citation2006; Reber et al. Citation2007).
However, on day 8 of DSS treatment (i.e. 8 days after termination of CSC exposure) we determined high levels of plasma corticosterone in CSC mice, suggesting that the adrenals of CSC mice have recovered from CSC-induced insufficiency. This increase in immunosuppressive plasma corticosterone concentrations in CSC mice was paralleled by a down-regulated secretion of the pro-inflammatory Th1 cytokines TNF-α, IFN-γ, and IL-6, resulting in cytokine secretion not different from that of the DSS-treated non-stressed control mice. As the high level of secretion of the anti-inflammatory Th2-cytokine IL-10 still remained on day 8 of DSS treatment, an imbalance of the Th2/Th1 cytokine profile is likely to result in glucocorticoid-induced bias towards an anti-inflammatory Th2 milieu (Visser et al. Citation1998; Mysliwiec et al. Citation1999; Citation2001). Thus, these findings suggest a delayed, but efficiently working down-regulation of the inflammation by the secretion of anti-inflammatory glucocorticoids on day 8 of DSS treatment.
Importantly, after 8 days of DSS treatment, CSC mice showed a more pronounced body weight loss, a stronger inflammatory shortening of the colon, and an increased histological damage score of the colon compared with controls. Therefore, the above described subtle regulatory processes, i.e. corticosterone-induced down-regulation of cytokine secretion on day 8 of DSS treatment, could not be confirmed by macroscopic parameters at this time point (colon length and damage score), suggesting that glucocorticoid-induced amelioration of these macroscopic signs of inflammation may take longer to establish (Collins et al. Citation1996). In accordance, after 8 days of DSS treatment mice prior exposed to the SD/OC paradigm showed comparable changes in the macroscopic parameters to those in DSS-treated CSC mice (Reber et al. Citation2006). Interestingly, the cytokine secretion from mesenteric lymph node cells was still increased on day 8 of DSS treatment compared to non-stressed control mice although the adrenals had also recovered from SD/OC-induced insufficiency at day 8 of DSS treatment (Reber et al. Citation2006). This finding suggests that the immunosuppressive effect of corticosterone on inflammatory processes might be attenuated in SD/OC mice, probably promoting the development of systemic inflammation and resulting in the decreased survival rate found on day 4 after termination of DSS treatment in SD/OC mice (Reber et al. Citation2006).
Taken together, previous exposure to CSC increased the severity of DSS-induced colitis seen at the macroscopic level after 8 days of DSS treatment. The information about the time course of the development of the DSS-induced colitis provided in the present study further extends knowledge of the effects of chronic psycho-social stress on the pathogenesis of subsequent DSS colitis and its underlying mechanisms.
Our findings of increased cytokine secretion by mesenteric lymph node cells in CSC mice on day 2 and 4 of DSS treatment and in CSC mice not treated with DSS on day 8 after termination of CSC exposure strongly suggest the occurrence of an event during CSC exposure triggering spontaneous inflammation (Reber et al. Citation2007). It is likely that the initial CSC-induced activation of the adrenal glands, as reflected by increased plasma corticosterone levels on day 2 of CSC exposure (Reber et al. Citation2007), plays an important role in the onset of colonic inflammation. In support, the results of the present study show that ADX reduces the severity of DSS-induced colitis in CSC mice. For example, only a minor body weight loss, a diminished inflammatory shortening of the colon, a lower histological score, and a reduced cytokine secretion from mesenteric lymph node cells were found in CSC-exposed ADX mice compared to respective SHAM mice on day 8 of DSS treatment. Increased levels of glucocorticoids have been described to disrupt colonic barrier functions (Meddings and Swain Citation2000) and thus, to trigger the penetration of luminal antigens into the colonic tissue. In support, a decreased pro-inflammatory cytokine secretion by mesenteric lymph node cells and a lower histological score was found in CSC mice of the ADX compared with the SHAM group (Reber et al. Citation2007).
In this context, the acute stress response during the first days of CSC exposure has to be considered in more detail. The initial increase in plasma corticosterone seen until day 2 of CSC exposure (Reber et al. Citation2007) may result in activation of the immune system (Dhabhar et al. Citation1995; Dhabhar and McEwen Citation1997), and furthermore, reduce the epithelial barrier function in the colon (Saunders et al. Citation1994; Meddings and Swain Citation2000; Ferrier et al. Citation2003). Therefore, the coincidence of an enhanced uptake and presentation of luminal antigens (Bailey et al. Citation2006) to an activated intestinal immune system, at least during the initial phase of CSC exposure is likely to be the key factor triggering spontaneous colonic inflammation (Reber et al. Citation2007) and increasing the vulnerability to subsequent DSS treatment and the development of experimentally-induced colitis. These inflammatory processes may be reinforced by the lack of immunosuppressive corticosterone due to CSC-induced adrenal insufficiency after prolonged stressor exposure. Consequently, ADX and lack of the initial activation of the adrenal glands during CSC housing attenuated these inflammatory processes and makes the colonic tissue less vulnerable to subsequent DSS treatment. However, ADX also prevented the anti-inflammatory actions of increased levels of glucocorticoids, as found in SHAM-operated CSC mice on day 8 of subsequent DSS treatment. Consequently, the lack of immunosuppressive glucocorticoid actions at this time point could limit the “beneficial effect” of ADX in the present study. However, further experiments, i.e. ADX in combination with corticosterone replacement or blockade of corticosterone actions by a glucocorticoid receptor antagonist, are needed to clarify whether adrenal glucocorticoids or catecholamines are the main mediators of CSC-induced colonic inflammation. The finding that ADX ameliorates rather than abrogates CSC-induced colonic inflammation indicates that besides hormonal actions also sympathetic nervous mechanisms might be involved in CSC-induced colonic inflammation.
In summary, prior CSC exposure increased the severity of a subsequent DSS-induced colitis which was likely mediated by CSC-induced changes in HPA axis function. This is based on the findings that ADX prior to CSC attenuated the increased severity of a subsequent DSS colitis. Therefore, the recently reported initial increase in plasma corticosterone levels during the first days of CSC exposure (Reber et al. Citation2007) seems to be a key factor for the increased vulnerability of colonic tissue to subsequent DSS treatment. In addition, on days 2 and 4 of DSS treatment, there was no increase in plasma corticosterone in stressed compared with unstressed mice although an elevated cytokine secretion from mesenteric lymph node cells in these animals indicated an increased state of inflammation. The lack of increase in this anti-inflammatory factor may further contribute to the increased severity of DSS-induced colitis after chronic psycho-social stress. The CSC paradigm used in the present study has been proven to be a valuable model for studying further effects of chronic psycho-social stress on distinct immunological parameters.
Acknowledgements
The authors are grateful to S. Selch, L. Traulsen (Institute of Zoology), N. Dunger, N. Grundwald, A. Gräber, B. Riepl (Department of Internal Medicine I) for excellent technical assistance. We also thank Dr D. Slattery (Institute of Zoology) for critical reading of the manuscript. This study was supported by the Deutsche Forschungsgemeinschaft (DFG).
References
- Axelsson LG, Landstrom E, Bylund-Fellenius AC. Experimental colitis induced by dextran sulphate sodium in mice: Beneficial effects of sulphasalazine and olsalazine. Aliment Pharmacol Ther 1998; 12: 925–934
- Bailey MT, Engler H, Sheridan JF. Stress induces the translocation of cutaneous and gastrointestinal microflora to secondary lymphoid organs of C57BL/6 mice. Journal of Neuroimmunology 2006; 171: 29–37
- Besedovsky H, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 1986; 233: 652–654
- Cakir B, Bozkurt A, Ercan F, Yegen BC. The anti-inflammatory effect of leptin on experimental colitis: Involvement of endogenous glucocorticoids. Peptides 2004; 25: 95–104
- Collins SM, McHugh K, Jacobson K, Khan I, Riddell R, Murase K, Weingarten HP. Previous inflammation alters the response of the rat colon to stress. Gastroenterology 1996; 111: 1509–1515
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: A potential role for leukocyte trafficking. Brain Behav Immun 1997; 11: 286–306
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Effects of stress on immune cell distribution. Dynamics and hormonal mechanisms. J Immunol 1995; 154: 5511–5527
- Drossman DA. Is the cotton-topped tamarin a model for behavioral research?. Dig Dis Sci 1985; 30: 24S–27S
- Ferrier L, Mazelin L, Cenac N, Desreumaux P, Janin A, Emilie D, Colombel J, Garcia-Villar R, Fioramonti J, Bueno L. Stress-induced disruption of colonic epithelial barrier: Role of interferon-gamma and myosin light chain kinase in mice. Gastroenterology 2003; 125: 795–804
- Gozalo A, Montoya E. Mortality causes of the moustached tamarin (Saguinus mystax) in captivity. J Med Primatol 1992; 21: 35–38
- Gue M, Bonbonne C, Fioramonti J, More J, Del Rio-Lacheze C, Comera C, Bueno L. Stress-induced enhancement of colitis in rats: CRF and arginine vasopressin are not involved. Am J Physiol 1997; 272: G84–G91
- Gulpinar MA, Ozbeyli D, Arbak S, Yegen BC. Anti-inflammatory effect of acute stress on experimental colitis is mediated by cholecystokinin-B receptors. Life Sciences 2004; 75: 77–91
- Jones SB, Romano FD. Plasma catecholamines in the conscious rat during endotoxicosis. Circ Shock 1984; 14: 189–201
- Kojouharoff G, Hans W, Obermeier F, Maennel DN, Andus T, Scholmerich J, Gross V, Falk W. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol 1997; 107: 353–358
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987; 92: 180–185
- Meddings JB, Swain MG. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology 2000; 119: 1019–1028
- Mendeloff AJ, Monk M, Siegel CI, Lilienfeld A. Illness experience and life stresses in patients with irritable colon and with ulcerative colitis. N Engl J Med 1970; 282: 14–17
- Milde AM, Murison R. A study of the effects of restraint stress on colitis induced by dextran sulphate sodium in singly housed rats. Integr Physiol Behav Sci 2002; 37: 140–150
- Million M, Tache Y, Anton P. Susceptibility of Lewis and Fischer rats to stress-induced worsening of TNB-colitis: Protective role of brain CRF. Am J Physiol Gastrointest Liver Physiol 1999; 276: G1027–G1036
- Mitchell CM, Drossman DA. Survey of the AGA membership relating to patients with functional gastrointestinal disorders. Gastroenterology 1987; 92: 1282–1284
- Mysliwiec J, Kretowski A, Szelachowska M, Mikita A, Kinalska I. Serum pro- and anti-inflammatory cytokines in patients with Graves' disease with ophthalmopathy during treatment with glucocorticoids. Rocz Akad Med Bialymst 1999; 44: 160–169
- Mysliwiec J, Kretowski A, Topolska J, Siewko K, Jakubczyk M, Szelachowska M, Mikita A, Kinalska I. Serum Th1 and Th2 profile cytokine level changes in patients with Graves' ophthalmopathy treated with corticosteroids. Horm Metab Res 2001; 33: 739–743
- North CS, Alpers DH, Helzer JE, Spitznagel EL, Clouse RE. Do life events or depression exacerbate inflammatory bowel disease? A prospective study. Ann Intern Med 1991; 114: 381–386
- Obermeier F, Kojouharoff G, Hans W, Scholmerich J, Gross V, Falk W. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol 1999; 116: 238–245
- Obermeier F, Dunger N, Deml L, Herfarth H, Scholmerich J, Falk W. CpG motifs of bacterial DNA exacerbate colitis of dextran sulfate sodium-treated mice. Eur J Immunol 2002; 32: 2084–2092
- Obermeier F, Dunger N, Strauch UG, Grunwald N, Herfarth H, Scholmerich J, Falk W. Contrasting activity of cytosin-guanosin dinucleotide oligonucleotides in mice with experimental colitis. Clin Exp Immunol 2003; 134: 217–224
- Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990; 98: 694–702
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 1985; 14: 149–167
- Pfeiffer CJ, Qiu B, Lam SK. Reduction of colonic mucus by repeated short-term stress enhances experimental colitis in rats. J Physiol Paris 2001; 95: 81–87
- Qiu BS, Vallance BA, Blennerhassett PA, Collins SM. The role of CD4+ lymphocytes in the susceptibility of mice to stress-induced reactivation of experimental colitis. Nat Med 1999; 5: 1178–1182
- Reber SO, Obermeier F, Straub RH, Falk W, Neumann ID. Chronic intermittent psychosocial stress (social defeat/overcrowding) in mice increases the severity of an acute DSS-induced colitis and impairs regeneration. Endocrinology 2006; 147: 4968–4976
- Reber SO, Birkeneder L, Veenema AH, Obermeier F, Falk W, Straub RH, Neumann ID. Adrenal insufficiency and colonic inflammation after a novel chronic psycho-social stress paradigm in mice: Implications and mechanisms. Endocrinology 2007; 148: 670–682
- Riley SA, Mani V, Goodman MJ, Lucas S. Why do patients with ulcerative colitis relapse?. Gut 1990; 31: 179–183
- Robertson DA, Ray J, Diamond I, Edwards JG. Personality profile and affective state of patients with inflammatory bowel disease. Gut 1989; 30: 623–626
- Salem SN, Shubair KS. Non-specific ulcerative colitis in Bedouin Arabs. Lancet 1967; 1: 473–475
- Sapolsky RM, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukine-1 stimulates the secretion of hypothalamic corticotropin releasing factor. Science Wash DC 1987; 238: 522–524
- Saunders PR, Kosecka U, McKay DM, Perdue MH. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am J Physiol Gastrointest Liver Physiol 1994; 267: G794–G799
- Stefanski V, Knopf G, Schulz S. Long-term colony housing in Long Evans rats: Immunological, hormonal, and behavioral consequences. J Neuroimmunol 2001; 114: 122–130
- Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of Murine Colitis by Lactococcus lactis Secreting Interleukin-10. Science 2000; 289: 1352–1355
- Suzuki S, Oh C, Nakano K. Pituitary-dependent and -independent secretion of CS caused by bacterial endotoxin in rats. Am J Physiol Endocrinol Metab 1986; 250: E470–E474
- Visser J, van Boxel-Dezaire A, Methorst D, Brunt T, de Kloet ER, Nagelkerken L. Differential regulation of interleukin-10 (IL-10) and IL-12 by glucocorticoids in vitro. Blood 1998; 91: 4255–4264
