Abstract
Stress can precipitate major depression and other disorders linked to hippocampal shrinkage. It is hypothesized but not established that treatment of these disorders reverses and prevents the hippocampal changes. Dendritic retraction of individual neurons might in concert with other pathophysiological events contribute to the shrinkage phenomenon. Animal studies have shown that various stress paradigms can induce dendritic retraction in the CA3 pyramidal neurons of the hippocampus. Since electroconvulsive treatment is the most effective treatment in humans with major depression, we investigated whether repeated electroconvulsive stimulations (ECSs) could influence such changes in stressed rats. Furthermore, we investigated whether ECSs per se could influence neuronal branching and total length of the CA3 hippocampal neuronal dendritic tree in normal rats. Rats were stressed using the 21-day 6 h daily restraint stress paradigm. The study shows that stress caused remodelling of the pyramidal neurons by significantly reducing the number of dendritic branch points and total length of the apical dendritic tree. Concomitant administration of ECSs prevented these effects. ECSs had no effect on pyramidal neuron dendrites in normal rats.
Introduction
Sustained stress can have numerous adverse effects on the brain. The hippocampus seems particularly vulnerable to stress and animal models of chronic stress show that stress alters hippocampal neuronal morphology (Watanabe et al. Citation1992a). Stressful conditions can induce retraction of hippocampal dendrites and decrease neurogenesis (Pham et al. Citation2003). The stress-induced shortening of the dendritic tree is seen in the apical tree of the hippocampal CA3 pyramidal neurons and not in the neurons of CA1 and CA2 or the dentate gyrus (Watanabe et al. Citation1992a; Magarinos et al. Citation1996a,Citationb). Following different stress paradigms, phylogenetically distant species like the rat and tree shrews have shown the same dendritic changes with reduction in the number of branch points and total length of the hippocampal CA3 dendritic tree (Watanabe et al. Citation1992a; Magarinos et al. Citation1996a,Citationb). Accordingly, it seems reasonable to presume that these changes might represent a species-independent neurobiological fingerprint of stress.
Stressful life events as well as chronic stress have been shown to be temporally and possibly causatively related to the development of depressive disorder in humans (Post Citation1992; Brown et al. Citation1994; Kessler Citation1997; Kendler et al. Citation2000). This relationship and the presence of hippocampal dendritic retraction in animal models of chronic stress make it tempting to draw parallels to humans with depressive disorder and hippocampal shrinkage (Sheline et al. Citation1996; Bremner et al. Citation2000; MacQueen et al. Citation2003; Videbech and Ravnkilde Citation2004). However, animal models only simulate aspects of complex syndromes such as major depression. The 21-day restraint stress paradigm represents a model in rodents with the potential to cast light on the structural basis of the shrinkage of the human hippocampus in depression. Besides remodelling of neurons, other factors might contribute to these changes in hippocampal volume, that is death of neurons, glial cell loss, decreased angiogenesis and neurogenesis (Sapolsky Citation1996; Lucassen et al. Citation2006; Czéh and Lucassen Citation2007).
Preclinical studies have shown that treatment with antidepressants (e.g., moclobemide and fluoxetine) can reverse stress-induced decreases in hippocampal neurogenesis (Dranovsky and Hen Citation2006). In the 21-day restraint stress paradigm in rats, stress-induced decreases in hippocampal dendritic length and branching can be prevented by the atypical antidepressant tianeptine, by lithium, phenytoin and the benzodiazepine agonist adinazolam (Watanabe et al. Citation1992b,Citationc; Magarinos et al. Citation1999; Wood et al. Citation2004). The adrenal steroid synthesis blocker cyanoketone also blocks these stress-induced changes (Magarinos and McEwen Citation1995). Many of these drugs are used in the treatment or augmentation of treatment in depressive patients. However, not all these substances are antidepressants and antidepressants like the selective 5-HT reuptake inhibitors fluoxetine and fluvoxamine do not counteract the stress-induced changes in hippocampal morphology (Magarinos et al. Citation1999).
Electroconvulsive treatment (ECT) is one of the most effective antidepressant treatments (Janicak et al. Citation1985; Silverstone and Silverstone Citation2004). Although convulsive therapy has been used in psychiatry for more than 70 years and electroconvulsive therapy for almost 70 years its mechanisms of action are not fully elucidated (Meduna Citation1935; Fink Citation2001). Many studies have investigated the effect of ECT on brain morphology either to demonstrate possible ECT-induced brain damage or to elucidate its mechanism of action. Overall, it appears that ECT does not produce atrophic or necrotic brain changes (Devanand et al. Citation1994). Rather it appears that it increases hippocampal neurogenesis (Madsen et al. Citation2000) and angiogenesis (Hellsten et al. Citation2005).
The aim of the present study was to investigate whether electroconvulsive stimulation (ECS) influence the dendritic length and arborization of hippocampal CA3 neurons in normal as well as in stressed rats.
Materials and methods
Animals and experimental groups
Male Sprague–Dawley rats (weight 260–270 g, Charles River, Germany) were group-housed in plexiglas cages (two rats per cage) with ad libitum access to food and drinking water except during stress sessions. They were kept on a 12 h:12 h light–darkness cycle (lights on at 7 a.m.) with a constant room temperature at 21°C. Rats were weighed on arrival at the laboratory and every week thereafter. They were randomly assigned to the following four groups: (1) six rats were subjected to 6 h restraint stress daily for 21 days (stress), (2) six rats were subjected to 6 h restraint stress daily for 21 days and given ECS treatment three times weekly (stress + ECS), (3) six rats were given ECS treatment three times weekly for 3 weeks (ECS) and (4) finally, six rats were left undisturbed in their cages except for brief daily handling (Cont). In order to avoid the influence of possible ultrasonic sounds from stressed animals on non-stressed rats, stressed and non-stressed rats were kept in separate rooms. After group assignment rats were left undisturbed in their cages (except for daily handling) for 1 week to acclimatize.
Restraint stress
The stress paradigm consisted of 6 h daily restraint stress for 21 consecutive days (Watanabe et al. Citation1992a). This time frame was chosen as dendritic changes in the CA3 region are evident following 21 days and not, for example, 14 days of restraint stress. Each day, rats voluntarily entered wire mesh restrainers that subsequently were closed with wire clips. The movements of the rats were restricted but not prevented totally. The wire mesh was designed to allow natural breathing and to avoid problems of overheating. During the restraint stress session the rats were returned to their cages together with their littermate. The stress paradigm took place from 10 a.m. to 4 p.m.
Electroconvulsive stimulation
ECS (12.5 mC as constant current 50 mA, 50 Hz, 0.5 s, unidirectional square wave pulses, 10 ms pulse width (Woldbye et al. Citation1996)) was administered to unanaesthetised rats with a pair of forceps electrodes on the ears. All stimulations resulted in a tonic–clonic seizure of 20–30 s duration. The dosage was chosen in accordance with studies that have shown biochemical changes and increased neurogenesis in the hippocampus of normal rats after ECS (Kragh et al. Citation1995; Madsen et al. Citation2000). The stimulations started on the same day as the first restraint session, and were given three times weekly (Monday, Wednesday, Friday). In this way, we tried to emulate the clinical schedule and thus gave ECS over a long period. The rats were fully awake when entering the restrainer 1 h later.
Golgi impregnation
Eighteen hours after the last stress session (day 21), rats were deeply anaesthetised with 3% isoflourane and trancardially perfused with 150 ml 4% paraformaldehyde in a 0.1 M phosphate buffer (pH 7.4). Brains were quickly removed and stored overnight at 4°C in the perfusate. The adrenal glands were removed and weighed.
Using a vibratome, the dorsal hippocampi (corresponding to − 2.80 to − 3.80 mm caudal to bregma, (Paxinos and Watson Citation1986) were cut into 100 μm coronal slices directly into a solution of 3% potassium dichromate dissolved in distilled water. The sections were then treated according to a slightly modified version of the single-section Golgi impregnation technique (Gabbot and Somogyi Citation1984; Magarinos et al. Citation1996a,Citationb). Briefly, the sections were kept freely floating in potassium dichromate at room temperature for 24 h before further processing. The following day the sections were briefly rinsed in distilled water and mounted on plain slides. Another slide was mounted on top of the brain section using glue at the four corners creating a “sandwich” structure. The slide assemblies were transferred to a solution of 1.5% silver nitrate and stored in the dark for 48 h. The sandwich construction allowed the solution to reach the brain sections that were kept in place by the top slide. Following this process, the slides were quickly dismantled. The sections were rinsed in distilled water and transferred through a graded series of ethanol. Finally, the sections were again mounted on slides and cover-slipped with Eukitt.
Data analysis
All slides were coded prior to analysis. The sections were then carefully examined for Golgi-impregnated pyramidal neurons in the CA3c region of the hippocampus. In order to be selected for quantification a neuron had to have the following characteristics as described by Magarinos et al. (Citation1996a,Citationb): (1) location in the CA3c region of the dorsal hippocampus, (2) a dark and consistent impregnation throughout the whole neuron and (3) distinguishable from neighbouring neurons, that is overlapping of dendrites from different neurons must be resolvable. The CA3 subregion of the hippocampus contains neurons that vary in branching pattern (Fitch et al. Citation1989). Accordingly six to eight representative pyramidal neurons with a single apical shaft were selected from each brain. Each neuron was traced at 500 × magnification and drawn using the camera lucida technique (Leica projection device). This technique made it possible to follow neuronal processes throughout their whole length and to distinguish the dendrites of neighbouring neurons from each other ().
Figure 1 Example of a Golgi-impregnated CA3c neuron with adjoining camera lucida drawing. Scale bar = 50 μm. (Please see colour online)

After drawing, each pyramidal neuron was examined for a number of branch points and total length of the dendritic tree. The number of branch points was determined by simple counting. The length of the dendritic tree (L) was measured using a grid and counting intersections (I) (Diemer and Siemkowicz Citation1981). The total length was measured using the formula L = h × I × π/2 where h is the “grid constant” (the distance between the lines in the grid).
Averages of the dendritic measurements were calculated for each rat, and then a group mean was calculated from these animal means.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test when indicated. A significance level of p < 0.05 was chosen.
Ethics
All animal procedures and protocols were approved by the Danish Committee for the Welfare and Use of Laboratory Animals (The Animal Experiments Inspectorate) and conducted in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Results
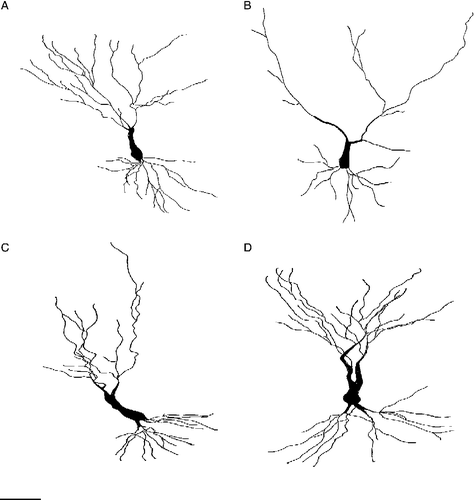
Quantitative analysis of group differences for Golgi-stained CA3 pyramidal cells revealed significant differences in the number of dendritic branch points (F3,20 = 10.23; p < 0.001) and total dendritic length (F3,20 = 17.32; p < 0.001). Post hoc analysis revealed that daily restraint stress resulted in a significant reduction in the number of apical branch points (p < 0.01) and total length of the apical dendritic tree (p < 0.01) of CA3c neurons when compared to control rats ( and ). Concomitant treatment with ECS prevented this stress-induced effect on branch points (p < 0.01) as well as on the total apical dendritic length (p < 0.01). ECS given to normal rats had no effect on the apical dendritic morphology. Stress neither influences the morphology of the basal dendritic tree nor did ECS treatment (). shows representative camera lucida drawings of CA3c neurons for the control, stress, stress + ECS and ECS groups.
Figure 2 Number of apical dendritic branch points in hippocampal CA3c neurons. N = 6 rats per group. Values represent means ± SEM. One-way ANOVA followed by Bonferroni post hoc test showed no difference between control, stress + ECS and ECS groups. Asterisk (*) denotes significant difference from control, stress + ECS and ECS rats (p < 0.01).
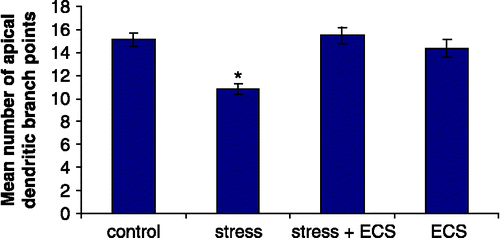
Figure 3 Mean length of apical dendrites in hippocampal CA3c neurons. N = 6 rats per group. Values represent means ± SEM. One-way ANOVA followed by Bonferroni post hoc test showed no difference between control, stress + ECS and ECS groups. Asterisk (*) denotes significant difference from control, stress + ECS and ECS rats (p < 0.01).
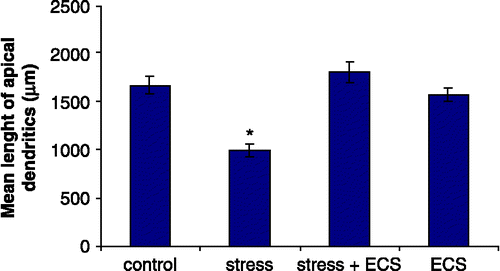
Table I. Stress and ECS effects on CA3c basal dendritic branch points and total length of dendritic tree.
Figure 4 Camera lucida drawings of representative CA3c neurons from (A) control rats, (B) stressed rats, (C) ECS rats and (D) stress + ECS rats. Scale bar = 50 μm.
There was no difference in body weight among rat groups at the start of the study (). However, at the end of the study stressed rats weighed significantly less than control rats (p < 0.01). Likewise, the stress + ECS group weighed less than the control rats at day 21 (p < 0.01). The weight of normal rats treated with ECS did not differ from control rats.
Table II. Stress and ECS effects on body, and adrenal weights.
Measurement of the relative adrenal weights showed that stressed rats had significantly larger adrenal glands than control rats (p < 0.01). A similar, but smaller, difference was found for the stress +ECS group compared with controls (p < 0.05).
Discussion
The data are consistent with previous studies that demonstrate stress-induced remodelling of the apical dendritic tree in the CA3c neurons of the hippocampus. After cessation of the stress sessions all rats seemed healthy, and though the rats were not subjected to any behavioural test, they behaved as usual when observed and handled. However, cognitive and behavioural changes in rats subjected to chronic stress have been found in a variety of tests like the Morris water maze (Sousa et al. Citation2000), the radial arm maze (Kitraki et al. Citation2004) and the Y-maze (Luine et al. Citation1994).
In addition, we demonstrated that ECS is able to prevent the shrinkage phenomena: ECS normalized the stress-induced shortening of the dendritic tree as well as the stress-induced decrease in the number of dendritic branch points. The effects of stress and treatment on CA3c neuron dendritic morphology appear to be selective as no changes in the length or arborization were found in the basal dendritic tree of the pyramidal neurons. Whether the stress-induced changes are indicative of neurons in the early stage of degeneration and neuronal death would follow if the stressor was intensified (Uno et al. Citation1989) is not known (Lucassen et al. Citation2006).
ECS had no effect on hippocampal dendrites in normal rats. One might have expected more branch points and/or longer dendrites when compared with controls as ECS increases neurogenesis and axonal, mossy fiber, sprouting in normal rats (Vaidya et al. Citation1999; Madsen et al. Citation2000). However, the lack of remodelling effects of ECS on normal rats adds to other studies that have examined and failed to find any brain damage in animals or humans undergoing ECS/ECT (Scott et al. Citation1991; Devanand et al. Citation1994; Vaidya et al. Citation1999; Ende et al. Citation2000).
ECS did not block the peripheral stress effect examined here, i.e., it did not prevent stress-induced loss in body weight or stress-induced increase in relative adrenal gland weight. The same phenomenon has been observed with other treatments (Watanabe et al. Citation1992b), thus the atypical tricyclic antidepressant tianeptine was able to prevent stress-induced changes in the hippocampus but was not able to abolish the peripheral effects of stress. Chronic ECS per se has been shown to increase relative adrenal gland weight in normal rats (Young et al. Citation1990), but this was not seen in this study. May be this discrepancy is due to differences in the pattern of administration of ECS as we tried to emulate the clinical schedule and gave ECS over a longer period: that is, three weekly ECS sessions over 3 weeks when compared with one daily ECS session on 8 consecutive days as in Young et al. (Citation1990). These authors also found that repeated ECS led to increased corticosterone levels. Thus, a reasonable explanation for the divergent results seems to be that the rats in our study adapted to the less frequent ECS treatment, which is more similar to the clinical situation.
Histopathological studies examining the hippocampus of steroid-treated and -depressed patients have not confirmed massive cell loss or suppression of neurogenesis (Czéh and Lucassen Citation2007). However, it seems plausible that morphological changes taking place in the hippocampus in animal models of stress could form the cellular substrate of the hippocampal shrinkage observed in humans with major depression. At the microscopic level, dendritic retraction in existing neurons, decreased neurogenesis, remodelling of neurons, death of neurons (Sapolsky Citation1996), glial cell loss (Wennstrom et al. Citation2006) and decreased angiogenesis are likely pathophysiological candidates for hippocampal shrinkage and plasticity. Preclinical studies have already shown that ECS is capable of increasing hippocampal neurogenesis (Madsen et al. Citation2000), inducing hippocampal angiogenesis (Hellsten et al. Citation2005) and counteracting corticosterone-induced inhibition of gliogenesis (Wennstrom et al. Citation2006). However, here we show that ECS also acts by protecting CA3c neurons from the effects of stress on the dendritic tree.
Previous studies have shown that ECS is able to induce sprouting of the granule cell mossy fiber pathway of the hippocampus (Vaidya et al. Citation1999). The functional significance of this phenomenon is not known. However, the sprouting process might be linked to the ability of ECS to induce expression of the principal neurotrophic factor brain-derived neurotrophic factor (BDNF) as ECS-induced sprouting is significantly reduced in BDNF heterozygotic knockout ( ± ) mice (Vaidya et al. Citation1999). ECS has also been shown to induce BDNF expression in the CA3 subfield (Nibuya et al. Citation1995). Thus, it seems plausible that the prevention of stress-induced remodelling of CA3 neurons by ECS in this study might be linked to the induction of BDNF expression.
The clinical effect of ECT is linked to the increase in seizure threshold observed through the course of ECT (Sackeim et al. Citation1983). The molecular mechanism of this anti-excitatory effect is not fully understood. Seizures prior to neuronal injury as in ischemia have been shown to protect hippocampal CA3c and CA1 neurons against neuronal damage (Sasahira et al. Citation1995; Towfighi et al. Citation1999). Thus, seizures (excitatory by nature) can confer tolerance to subsequent hippocampal damage. Analogous to this, ECS might confer protection against the damaging effects of stress. The anti-excitatory effect may be related to an increased levels of inhibitory neurotransmitters such as γ-aminobutyric acid (GABA) and neuropeptide Y (NPY), since preclinical studies have shown that ECS is able to increase GABA and NPY levels in a variety of brain regions including the hippocampus (Ferraro et al. Citation1990; Bolwig et al. Citation1999; Mikkelsen and Woldbye Citation2006). Also, cortical GABA concentrations appear to increase in depressed patients following ECT (Sanacora et al. Citation2003). In the present model, the stress-induced changes appear to be elicited by an excitatory if not an excitotoxic mechanism, since this is dependent on the excitatory fibers from the entorhinal cortex (Sunanda et al. Citation1997) and can be prevented by glutamate inhibitors (Watanabe et al. Citation1992c; Magarinos and McEwen Citation1995). Furthermore, the GABA agonist adinazolam prevents the stress-induced morphological changes in this model (Magarinos et al. Citation1999). Thus, ECS-activated GABA and NPY neurotransmission might counteract the stress-induced increase in excitatory neurotransmission and may be an important mechanism in the modulation of neuronal plasticity.
Also, alterations in serotonergic transmission presumably contribute to the dendritic remodelling seen in the restraint stress paradigm (Magarinos et al. Citation1999). Under normal circumstances the apical dendritic tree receives serotonergic afferents with a high concentration of serotonin transporters (5-HTT) (Mongeau et al. Citation1997). In a model of chronic social stress, which showed similar hippocampal morphological changes of the pyramidal CA3 neurons as in this study, downregulation of the 5-HTT was found (McKittrick et al. Citation2000). Increased levels of 5-HT in the hippocampus might enhance N-methyl-d-aspartate (NMDA) receptor excitatory currents via 5-HT2A receptor-mediated increased phosphorylation of NMDA subunits as suggested by Magarinos et al. (Citation1999). In this way, the excitatory input to the CA3 neurons may be enforced, leading to the observed dendritic changes as discussed above. ECS has been shown to increase 5-HTT in the frontal cortex in normal rats (Hayakawa et al. Citation1995; Shen et al. Citation2003) and might have the same effect in the hippocampal dendritic tree. If this holds true, chronic ECS may counteract the stress-induced decrease in 5-HTTs and thereby protect the CA3 neurons from stress-induced morphological changes. We are presently investigating this possibility.
In humans with stress-related disorders like major depression and post-traumatic stress disorder (PTSD), it is hypothesized but not established that treatment reverses and prevents hippocampal shrinkage. Studies of PTSD patients showed that paroxetine (Vermetten et al. Citation2003) and phenytoin (Bremner et al. Citation2005) are able to increase the hippocampal volume. In another study on patients with major depression, fluoxetine did not increase the hippocampal volume (Vythilingam et al. Citation2004). In the present study, we have demonstrated that repetitive ECS is able to abolish stress-induced morphological changes in the hippocampal CA3 subfield. ECS prevented stress-induced shortening of the dendritic tree and stress-induced reduction in dendritic branch points. We suggest that the molecular mechanisms underlying the effect of ECT on depression are the same mechanisms, which in the present experimental ECT paradigm prevent the stress-induced changes in the hippocampal CA3 subfield.
Acknowledgements
Special thanks to Professor Bruce McEwen and Dr Ana Maria Magarinos (The Rockefeller University, NY, USA) for their help with the Golgi impregnation method. The study was supported by The Danish Medical Research Council, The Ivan Nielsen Foundation and The Wørzner Foundation.
References
- Bolwig TG, Woldbye DP, Mikkelsen JD. Electroconvulsive therapy as an anticonvulsant: A possible role of neuropeptide Y (NPY). J ECT 1999; 15: 93–101
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry 2000; 157: 115–118
- Bremner JD, Mletzko T, Welter S, Quinn S, Williams C, Brummer M, Siddiq S, Reed L, Heim CM, Nemeroff CB. Effects of phenytoin on memory, cognition and brain structure in posttraumatic stress disorder: A pilot study. J Psychopharmacol 2005; 19(2)159–165
- Brown GW, Harris TO, Hepworth C. Life events and endogenous depression. A puzzle reexamined. Arch Gen Psychiatry 1994; 51: 525–534
- Czéh B, Lucassen PJ. What causes the hippocampal volume decrease in depression?. Eur Arch Psychiatry Clin Neurosci 2007; 257: 250–260
- Devanand DP, Dwork AJ, Hutchinson ER, Bolwig TG, Sackeim HA. Does ECT alter brain structure?. Am J Psychiatry 1994; 151: 957–970
- Diemer NH, Siemkowicz E. Regional neurone damage after cerebral ischaemia in the normo- and hypoglycemic rat. Neuropath Appl Neurobiol 1981; 7: 217–227
- Dranovsky A, Hen R. Hippocampal neurogenesis: Regulation by stress and antidepressants. Biol Psychiatry 2006; 59: 1136–1143
- Ende G, Braus DF, Walter S, Weber-Fahr W, Henn FA. The hippocampus in patients treated with electroconvulsive therapy: A proton magnetic resonance spectroscopic imaging study. Arch Gen Psychiatry 2000; 57: 937–943
- Ferraro TN, Golden GT, Hare TA. Repeated electroconvulsive shock selectively alters gamma-aminobutyric acid levels in the rat brain: Effect of electrode placement. Convuls Ther 1990; 6: 199–208
- Fink M. Convulsive therapy: A review of the first 55 years. J Affect Disord 2001; 63: 1–15
- Fitch JM, Juraska JM, Washington LW. The dendritic morphology of pyramidal neurons in the rat hippocampus CA3 area. I. Cell types. Brain Res 1989; 479: 105–114
- Gabbot PL, Somogyi J. The single section Golgi impregnation procedure: Methodological description. J Neurosci Methods 1984; 11: 221–230
- Hayakawa H, Okamoto Y, Shimizu M, Nishida A, Motohashi N, Yamawaki S. Single or repeated treatment with electroconvulsive shock increases number of serotonin uptake binding sites in the frontal cortex. Neuropsychobiology 1995; 31: 1–5
- Hellsten J, West MJ, Arvidsson A, Ekstrand J, Jansson L, Wennstrom M, Tingstrom A. Electroconvulsive seizures induce angiogenesis in adult rat hippocampus. Biol Psychiatry 2005; 58: 871–878
- Janicak PG, Davis JM, Gibbons RD, Ericksen S, Chang S, Gallagher P. Efficacy of ECT: A meta-analysis. Am J Psychiatry 1985; 142: 297–302
- Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: An evaluation of the “kindling” hypothesis. Am J Psychiatry 2000; 157: 1243–1251
- Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol 1997; 48: 191–214
- Kitraki E, Kremmyda O, Youlatos D, Alexis M, Kittas C. Spatial performance and corticosteroid receptor status in the 21-day restraint stress paradigm. Ann NY Acad Sci 2004; 1018: 323–327
- Kragh J, Jorgensen MB, Diemer NH, Bolwig TG. Long-term decrease in the hippocampal [3H]inositoltriphosphate binding following repeated electroshock in the rat. Biol Psychiatry 1995; 38: 471–474
- Lucassen PJ, Heine VM, Muller MB, van der Beek EM, Wiegant VM, De Kloet ER, Joels M, Fuchs E, Swaab DF, Czeh B. Stress, depression and hippocampal apoptosis. CNS Neurol Disord Drug Targets 2006; 5: 531–546
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated restraint stress causes reversible impairments of spatial memory performance. Brain Res 1994; 639: 167–170
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA 2003; 100: 1387–1392
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry 2000; 47: 1043–1049
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 1995; 69: 89–98
- Magarinos AM, Fuchs E, Flugge G, McEwen BS. Golgi technique used to study stress and glucocorticoid effects on hippocampal neuronal morphology. Methods in Neurosci 1996a; 30: 315–325
- Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci 1996b; 16: 3534–3540
- Magarinos AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol 1999; 371: 113–122
- McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse 2000; 36: 85–94
- Meduna LJ. Die Konvulsionstherapie der Schizophrenie. Psychr Neurol Wochenschr 1935; 37: 317–319
- Mikkelsen JD, Woldbye DP. Accumulated increase in neuropeptide Y and somatostatin gene expression of the rat in response to repeated electroconvulsive stimulation. J Psychiatr Res 2006; 40: 153–159
- Mongeau R, Blier P, de Montigny C. The serotonergic and noradrenergic systems of the hippocampus: Their interactions and the effects of antidepressant treatments. Brain Res Brain Res Rev 1997; 23: 145–195
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 1995; 15: 7539–7547
- The rat brain in stereotaxic coordinates, G Paxinos, C Watson. Academic Press, New York 1986
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci 2003; 17: 879–886
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry 1992; 149: 999–1010
- Sackeim HA, Decina P, Prohovnik I, Malitz S, Resor SR. Anticonvulsant and antidepressant properties of electroconvulsive therapy: A proposed mechanism of action. Biol Psychiatry 1983; 18: 1301–1310
- Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, Ostroff RB, Berman RM, Krystal JH. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry 2003; 160: 577–579
- Sapolsky RM. Why stress is bad for your brain. Science 1996; 273: 749–750
- Sasahira M, Lowry T, Simon RP, Greenberg DA. Epileptic tolerance: Prior seizures protect against seizure-induced neuronal injury. Neurosci Lett 1995; 185: 95–98
- Scott AI, Turnbull LW, Blane A, Douglas RH. Electroconvulsive therapy and brain damage. Lancet 1991; 338: 264
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 1996; 93: 3908–3913
- Shen H, Numachi Y, Yoshida S, Fujiyama K, Toda S, Awata S, Matsuoka H, Sato M. Electroconvulsive shock increases serotonin transporter in the rat frontal cortex. Neurosci Lett 2003; 341: 170–172
- Silverstone PH, Silverstone T. A review of acute treatments for bipolar depression. Int Clin Psychopharmacol 2004; 19: 113–124
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioural improvement. Neurosci 2000; 97: 253–266
- SunandaMeti BL, Raju TR. Entorhinal cortex lesioning protects hippocampal CA3 neurons from stress-induced damage. Brain Res 1997; 770: 302–306
- Towfighi J, Housman C, Mauger D, Vannucci RC. Effect of seizures on cerebral hypoxic–ischemic lesions in immature rats. Brain Res Dev Brain Res 1999; 113: 83–95
- Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci 1989; 9: 1705–1711
- Vaidya VA, Siuciak JA, Du F, Duman RS. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience 1999; 89: 157–166
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress diosorder. Biol Psychiatry 2003; 54: 693–702
- Videbech P, Ravnkilde B. Hippocampal volume and depression: A meta-analysis of MRI studies. Am J Psychiatry 2004; 161: 1957–1966
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, Staib LH, Charney DS, Bremner JD. Hippocampal volume, memory and cortisol status in major depressive disorder: Effects of treatment. Biol Psychiatry 2004; 56: 101–112
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res 1992a; 588: 341–345
- Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharmacol 1992b; 222: 157–162
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus 1992c; 2: 431–435
- Wennstrom M, Hellsten J, Ekstrand J, Lindgren H, Tingstrom A. Corticosterone-induced inhibition of gliogenesis in rat hippocampus is counteracted by electroconvulsive seizures. Biol Psychiatry 2006; 59: 178–186
- Woldbye DP, Greisen MH, Bolwig TG, Larsen PJ, Mikkelsen JD. Prolonged induction of c-fos in neuropeptide Y- and somatostatin-immunoreactive neurons of the rat dentate gyrus after electroconvulsive stimulation. Brain Res 1996; 720: 111–119
- Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: Prevention by lithium treatment. Proc Natl Acad Sci USA 2004; 101: 3973–3978
- Young EA, Spencer RL, McEwen BS. Changes at multiple levels of the hypothalamo-pituitary adrenal axis following repeated electrically induced seizures. Psychoneuroendocrinology 1990; 15: 165–172