Abstract
This study investigated the urinary cortisol stress response to one known stressor (anaesthesia) and three unusual events hypothesized to result in increases in cortisol (confinement to one half of an enclosure for several days due to a hurricane, an enrichment exercise, and a change in group composition) in young chimpanzees (Pan troglodytes). Although a cortisol stress response to a variety of laboratory experiences has been documented in captive animals, it is unclear whether other types of atypical events are stressful, including those that are not necessarily negative. Cortisol was measured in 519 urine samples collected from 20 awake, unrestrained chimpanzees; individuals were compared against their own baseline values. A significant increase in urinary cortisol concentration was found as a result of the stress of anaesthesia, but no significant change in urinary cortisol resulted from the three other potential stressors. A lack of a urinary cortisol response to these events may indicate that the events were not actually stressful for the chimpanzees, but may have resulted from the limited temporal resolution of measuring cortisol excretion as an indicator of integrated secretion, or from changes in rates of agonistic behaviors.
Introduction
One of the ways in which the welfare of captive animals can be improved is by a reduction in the levels of stress they experience (Mostl and Palme Citation2002; Honess and Marin Citation2006; Boissy et al. Citation2007). Cortisol is a metabolic hormone that makes more energy available in times of stress (Sapolsky Citation1994; Sapolsky et al. Citation2000). However, despite a broad literature on the various physiological and behavioral manifestations of stress, exactly what kinds of events are likely to cause an increase in circulating cortisol level is still unclear. In rodent studies, unpredictability and loss of control over environmental variables are classic stressors (Weiss Citation1970; Bassett et al. Citation1973), and these findings have been extended to a variety of mammals including primates (Hanson et al. Citation1976; Sapolsky Citation1983, Citation2002, Citation2005). In social animals, agonistic interactions and dominance status can be potent stressors, sometimes resulting in increased cortisol levels in both acute and chronic contexts (Creel Citation2001; Goymann and Wingfield Citation2004). Captive animal studies provide a means to tease apart the physiological and psychosocial responses to stress.
Many studies have documented a variety of stress responses in laboratory animals as a result of routine laboratory procedures such as handling, blood collection, and enclosure transfer (Balcombe et al. Citation2004). Less is known, however, about the potential stress response resulting from events that are specifically not part of a laboratory routine, and therefore not experienced by the animals on a regular basis. In addition, the literature on cortisol responses to events that are positive, rather than negative, is sparse (Pollard Citation1995).
The chimpanzees at the New Iberia Research Center experience few changes in routine from day to day aside from the small changes in type of food or in the personnel rota that occur on a regular basis. However, occasionally certain events occur that are different enough from the routine that they can be considered unusual. This study evaluates the cortisol secretory response to these unusual events in an effort to elucidate what makes an event “stressful” (based on the cortisol response) for a captive chimpanzee. One such event, anaesthesia, acts as a positive control because it is known that it results in elevated cortisol release in this population (Anestis et al. Citation2006). It was hypothesized that restriction to the indoor portion of the chimpanzees' enclosure for an extended period of time would result in increased levels of urinary cortisol, as crowding can increase stress via increased rates of aggression (Southwick Citation1967; Nieuwenhuijsen and de Waal Citation1982) and social unpredictability (Sapolsky Citation2005). Some authors have suggested that increases in cortisol secretion may occur as a result of events that are not necessarily negative (Pollard Citation1995); this study evaluated whether addition of an enrichment tool to the enclosure resulted in increased levels of cortisol in this population. Finally, the chimpanzees experienced a change in enclosure and group composition by the addition of new members to a social group. This major change in social arrangement was hypothesized to cause significantly increased levels of urinary cortisol because of the social stress involved in reassessing dominance relationships with new group members (Sapolsky Citation2005).
Materials and methods
Study site and groups
The chimpanzees live at the University of Louisiana's New Iberia Research Center (NIRC) in New Iberia, LA, USA and the data for this study were collected during two research periods each approximately two months long (June–August 2000 and September–October 2002). The chimpanzees live in indoor–outdoor enclosures with continuous access to toys and water. They receive regular feeding of fruit or vegetables in the morning and monkey chow in the afternoon. The subjects of this study lived in three juvenile and adolescent male/female peer groups (ages four to ten years) and group size ranged between five and nine individuals. Group composition remained stable over the course of an observation period (except for the very end of the research period in 2002, described below), but did change between periods. These chimpanzees were not participating in any other research study during this time, and none had ever participated in a study that could affect neuroendocrine function.
Potential stressors
Urinary cortisol concentrations were measured during and/or after the following events:
Veterinary examinations. The chimpanzees undergo physical examination twice a year. An entire group is restricted to the indoor portion of their enclosure and sedated with Telazol (Fort Dodge, Fort Dodge Iowa, USA) via a dart gun. The sedation process usually takes 15–20 min; once all animals are sedated, they are individually examined by a veterinarian and blood is drawn via femoral venepuncture. The anaesthesia procedure is known to be stressful in a variety of primate species (Sapolsky Citation1982; Hergovitch et al. 2001; Bentson et al. Citation2003; Springer and Baker Citation2007), and to result in elevations in circulating levels of cortisol in this population (Anestis et al. Citation2006). Urine samples were collected from 5 to 26 hours after the anaesthesia procedure, and represent the first and/or several successive voids after anaesthesia for approximately one day following the event.
Forty-eight hours forced restriction to indoor enclosure. A major hurricane in October of 2002 (Lili) forced the chimpanzees indoors for two days, representing a considerable extension of the typical 2-h restriction during cleaning. The hurricane did not result in changes in food or water availability, and the lights remained on during the day as the buildings are equipped with generators. The urine samples included in this stressor category were collected during this period of restriction.
“Surprise box” enrichment. Behavioral enrichment staff provides “surprise boxes” to groups of chimpanzees as an enrichment exercise approximately once per month. These large cardboard boxes contain a variety of food items and toys that are not normally part of the chimpanzees' environment, including peanuts, fruit, paper, and hay. The items in the boxes and the boxes themselves remain in the enclosure until the enclosure is cleaned the following day. On October 23rd, 2002, two of the study groups were provided with surprise boxes, and urine was collected opportunistically during a three and a half hour period as the chimpanzees played with the boxes. The first sample was collected 50 min after the surprise boxes were introduced.
Group composition change. Chimpanzees at the New Iberia Research Center are sometimes moved from one social group to another due to behavioral (e.g. continued and escalating aggression by an animal against another) or practical (e.g. space) considerations. Animals are always moved together with at least one other familiar individual, and newly formed groups are monitored carefully by the primate care staff. On October 24, 2002 one of the study groups was moved to a larger enclosure, and on the following day two new members joined the group (N = 11 post-move). Urine samples were collected opportunistically during this transition and over the course of the following day.
Urine collection and analysis
Measuring hormone levels in urine has several advantages over traditional blood sample analysis, including the lack of stress experienced by the animals as a result of the collection procedure and the fact that urine sample measurements represent the average level of hormone since the last void (Whitten et al. Citation1998). Positive reinforcement was used to train the chimpanzees to void into a paper cup, described in more detail elsewhere (Anestis Citation2005). Because this paper focuses on unusual events in the captive chimpanzee experience, collection was necessarily opportunistic. However, all hypothesized “stressed” samples are compared to a baseline cortisol level obtained from samples collected when the animals were not experiencing any obvious stressors (see ), and animals are only compared against themselves, not against a group average level. This is important as baseline urinary cortisol exhibits individual variation in this population (Anestis Citation2005).
Table I. Year one (2000) and year two (2002) mean baseline and unusual event levels of cortisol for each individual chimpanzee. Raw cortisol/ creatinine values (pmol/mg) are presented in column one, but in order to account for the diurnal pattern of cortisol secretion, residual cortisol levels (obtained by using the regression of cortisol on time of collection) were used in the analyses. Sample sizes are listed in parentheses. Blank cells indicate that no samples were collected from the individual for that event; an asterisk next to a name indicates the animal was sampled in both years. The dagger symbol (†) indicates a condition that was significantly different from baseline.
Urine samples collected for this study are summarized in . The data presented here are from seven animals in the 2000 research period (five males and two females) and 16 animals in the 2002 period (13 males and 3 females). The analyses are largely biased toward male reactions to stressful events. In addition, the possibility that there is a selection bias cannot be refuted in that those individuals who were not trained in the urine collection technique were also (for example) particularly reactive to stressful events. However, samples were collected from all trained chimpanzees that experienced the potential stressors included in this study, and therefore there is no putative stressor collection bias within the subset of trained individuals.
After collection, urine was immediately transferred to labeled tubes and refrigerated within 3 h. Samples were frozen at − 20° C no more than 12 h after collection. Before freezing, samples greater than 0.5 ml in volume were tested on 10 UA Chemstrips (Boehringer–Mannheim, Indianapolis, IN, USA) to determine urine pH, specific gravity, and to check for abnormalities such as the presence of blood. Samples were shipped on dry ice at the end of the collection period via next day air and arrived frozen at the Reproductive Ecology Laboratory at Yale University.
The Yale University Animal Care and Use Committee approved all procedures used in this study, and all collection protocols fell under the New Iberia Research Center's guidelines.
Cortisol radioimmunoassay
A competitive binding cortisol radioimmunoassay protocol was used, as developed by Knott (Citation1997) based on a human salivary assay (Ellison Citation1988). The assay uses a (1, 2, 6, and 7) tritium tracer, cortisol-specific antibody, and a charcoal separation step. Briefly, urine samples were assayed after dilution at a 1:10 ratio with HPLC water. Two standard curves were run in each assay using cortisol standards at 800, 400, 200, 100, 50, 20, 10, and 5 ng/ml in a buffer solution (Steraloids Q 3880, Newport, RI, USA), and each standard was run in triplicate. Tracer (Amersham Biosciences, Piscataway, NJ, USA), standard, and unknowns within samples were allowed to compete for binding to the antibody (# 07-121016, MP Biomedicals, Irvine, CA, USA; formerly ICN Biomedicals, Costa Mesa, CA, USA) overnight at 4°C. The following day dextran-coated charcoal was added to the sample and standard curve tubes, vortexed, and incubated at room temperature for 10 min, allowing the unbound tracer to bind to the charcoal. Samples were spun in a refrigerated centrifuge for 20 min to pellet the charcoal and unbound tracer. The supernatant was mixed with scintillation fluid, and counted on a Wallac liquid scintillation counter (model 1409 DSA).
The antibody used in this assay displays 11.4% cross-reactivity with 21-desoxycorticosterone, 8.9% with 11-desoxycortisol, and 1.6% with corticosterone. Other cross-reactivities are less than 1%. Curves were calculated using AssayZap software for the Macintosh (BioSoft, Inc., Cambridge, UK). Assay sensitivity was 5 ng/ml; samples that fell below this value at 1:10 dilution were rerun at full strength, and any that fell below at full strength were eliminated (N = 1). Samples whose duplicates were more than 10% different were run again.
Samples were run sequentially in the order in which they were collected; hypothesized “stress” samples were not run separately from baseline samples. These data are part of a larger study of chimpanzee urinary cortisol spanning four years, in which a total of 59 assays were run. For these 59 assays, intra-assay variation averaged 7.2% and inter-assay variation (estimated for the 59 assays from urine pools with varying levels of cortisol) averaged 21.3% (low), 17.6% (medium), and 9.1% (high).
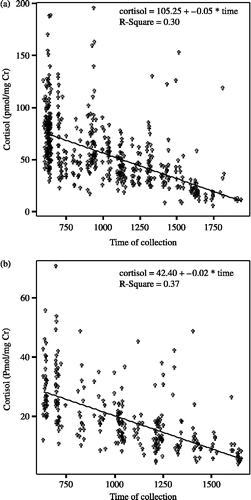
Chimpanzee cortisol secretion exhibits a distinct diurnal rhythm that can affect samples collected as little as half an hour apart (Anestis and Bribiescas Citation2004). Each raw cortisol value (pmol cortisol/mg creatinine) was therefore converted to an unstandardized residual value obtained from the regression of cortisol on time of collection (). A different cortisol on time of collection regression was run for the two observation periods (2000 and 2002) in case there were any between-year differences, especially since the 2000 research period occurred in the summer whereas the 2002 period occurred in the fall.
Creatinine
A colorimetric assay was used for creatinine based on a protocol developed by Knott (Citation1997), using the Jaffe reaction (Taussky Citation1954). Four creatinine standards were used, at concentrations of 0.5, 1.0, 3.0, and 10.0 mg/dL (Sigma–Aldrich, St. Louis, MO, USA). Samples were assayed at the same dilution used to determine cortisol (1:10 in HPLC water).
Behavioral data
Behavioral data included in this study are based on all-occurrences sampling of social behavior, as described in detail elsewhere (Anestis Citation2005). All-occurrences recording was deemed appropriate based on a manageable rate of social interactions due to relatively small group sizes and because only social behaviors were being recorded ad libitum (e.g., resting, feeding, were not recorded). The group was visually scanned continuously in a predetermined order to reduce bias towards more active individuals or those located closer to the observer (the author). Dominance hierarchies were constructed using matrices of pant-grunts and supplants (Bygott Citation1979), and for this study, low-ranking chimpanzees are those in the bottom half of the hierarchy.
Rates of behaviors in the following categories were compared: overall (all social interactions); aggressive (all attacks, bites, chases, hits, and threats); agonistic (all aggressive behaviors, as well as pant-grunts, displays, supplants, and stealing of desirable items) (Nishida et al. Citation1999). Baseline rates of behaviors were calculated by dividing raw counts by the number of all-occurrences observation hours. Rates of behaviors during a particular event (the enrichment exercise and the new group condition) were calculated by dividing raw counts during the specified period by the total number of all-occurrences observation hours for that event. Since, the enrichment condition involves only 50 min of behavioral data from approximately 1200 to 1300 h, the data were compared only to rates of behaviors calculated from all observations of that group at the same time of day. Data for the new group condition span a full day, and therefore do not need to be compared to baseline data from a specific diurnal period.
Statistical analyses
Cortisol values used in the analyses presented here are unstandardized residuals from the regression of cortisol on time of day, including all samples analyzed for cortisol for each year (not only the animals included in the stressor dataset presented here). All chimpanzees were included in order to get an as accurate as possible indication of the relationship between decreasing cortisol and time of day (from morning to evening), which should be more accurate if all animals are included. Each of the two years is considered separately to account for any between-year variation in the relationship between cortisol and time of day.
Since individuals are known to differ in baseline cortisol level in primates including humans (Sapolsky Citation1982; Kirschbaum and Hellhammer Citation1994; Stavisky et al. Citation2001), and specifically in this population (Anestis Citation2005), the relationship between the hypothesized stress variables and cortisol was investigated using fixed effects linear regression with robust cluster (Stata, StataCorp LP, College Station, TX, USA) which compares individuals against their own baselines and adjusts standard errors for intra-group (e.g. within-individual) correlations (Dave and Krishnapuram Citation1997). Rates of behaviors in different conditions were compared using Wilcoxon paired tests of individual animals' rates (e.g. baseline vs. enrichment), except in the comparison of aggression and agonism toward low-ranking group members, where the sample size (N = 4) was too small. All tests are considered significant at a p value of 0.05 or less.
Results
The stress of anaesthesia for physical examination resulted in a significant increase in urinary cortisol concentration over baseline values (t = 2.57, p = 0.02; ), while there was no significant change in cortisol resulting from the hurricane (t = − 0.92, p = 0.37), enrichment exercise (t = − 0.89, p = 0.39), and new group condition (t = − 0.66, p = 0.52).
Comparisons of rates of behaviors are presented in Tables and . The overall social interaction rate and rate of agonism (e.g. pant-grunts, stealing of desirable objects) were similar between the enrichment and baseline conditions (overall social interaction: Wilcoxon Z = − 0.18, N = 9, p = 0.86; agonism: Wilcoxon Z = − 0.70, N = 9, p = 0.48), but the rate of aggression was much lower with enrichment than usual (1 event/h in enrichment vs. 8 events/h in the baseline sample; Wilcoxon Z = − 2.03, N = 9, p = 0.04). During the new group condition, the overall rate of aggression and agonism approximately doubled over baseline (aggression: from 7 to 19 events/h, agonism: from 48 to 78 events/h), though a Wilcoxon test was not significant (aggression: Wilcoxon Z = − 1.53, N = 9, p = 0.14; agonism: Wilcoxon Z = − 0.53, N = 9, p = 0.59). In addition, a majority of both agonistic and aggressive behaviors was directed at the two new group members, from whom urine samples were not collected (59% of agonism, 68% of aggression). Despite the overall higher rate of aggressive and agonistic acts during this period, rates of aggression and agonism toward the four low-ranking original group members were actually lower than typical (aggression: from 5 to 3 events/h, agonism: from 9 to 7 events/h; sample size too small for statistical tests).
Table II. Rates of all social behavior, aggression, and agonism in LJ's group (n=9) in the enrichment condition compared to the baseline condition.
Table III. Rates of all social behavior, aggression, and agonism, as well as aggression and agonism directed toward individuals in the bottom-half of the hierarchy, in Mickey's group (N=9 before change, N=11 after change) in the group change and baseline conditions.
Discussion
For all the chimpanzees included in this study, anaesthesia for physical examination resulted in an increase in urinary cortisol, indicating a major disruption of homeostasis and an allostatic load (Korte et al. Citation2005, Citation2007). The physical examination represents a “control” stressor because it is known that pharmacological anaesthesia results in elevated circulating cortisol in these chimpanzees (Anestis et al. Citation2006). The stress is primarily the result of the disorientation that occurs as the drug begins to exert its effects (Puri et al. Citation1981; Sapolsky Citation1982), though the chimpanzees also experience stress as a result of the involuntary nature of the administration of anaesthesia (Bentson et al. Citation2003; Lambeth et al. Citation2006).
Three events hypothesized to result in cortisol increases—restriction to a small part of the enclosure during a hurricane, addition of new materials into the enclosure, and a change in group composition—did not result in any significant changes in urinary cortisol level. The hurricane experience was hypothesized to have been stressful for the chimpanzees because of the stress caused by crowding. In classic rodent studies, crowding results in increases in aggressive behavior (Calhoun Citation1962), but de Waal (Citation1989) has argued that primates have more flexible responses to changes in the environment, and that aggression is not necessarily the best strategy for a primate concerned with preserving relationships with group-mates. Consistent with this hypothesis, the literature on primate behavioral responses to crowding and confinement is mixed, sometimes resulting in increases in aggression (Macaca mulatta: Southwick Citation1967; Pan troglodytes: Nieuwenhuijsen and de Waal Citation1982), but sometimes not (Cercopithecus aethiops: McGuire et al. Citation1983; Macaca mulatta: de Waal Citation1989; Macaca fascicularis: Aureli et al. Citation1995; Pan troglodytes: Aureli and de Waal Citation1997). Aureli and de Waal (Citation1997) and Videan and Fritz (Citation2007) report decreases in agonistic behaviors in chimpanzees restricted to limited space for long periods, and similarly in this study rates of social interaction during the hurricane were low (pers. obs.), which may be related to a conflict avoidance strategy (Videan and Fritz Citation2007) and may also explain the lack of cortisol increase.
In the surprise box condition, rates of aggressive behavior were eight times lower than typical for that group during that time of day, perhaps because the boxes contain a large number of small items that cannot be monopolized and because the chimpanzees were more interested in engaging with the novel objects than with each other. Similarly, de Waal (Citation1989) reported that long-tailed macaques at the Basel Zoo were provided with small, scattered food items on the enclosure floor during forced restriction as an effective way to reduce escalated fighting (from Angst Citation1980). The negative result reported here does not lend support to the hypothesis that arousal from a positive event results in increases in cortisol secretion (Pollard Citation1995), though the duration of the arousal may play a role, discussed more below.
The finding that the enclosure and group composition change did not result in increased urinary cortisol is surprising. Enclosure changes have been demonstrated to be stressful for mammals such as cats and hyenas (Carlstead et al. Citation1993; Goymann et al. Citation1999), though a study of pigtailed macaques found no such effect (Crockett et al. Citation2000). It is possible that previous experience with a potential stressor (e.g. an enclosure change) mitigates its effect (Crockett et al. Citation2000). Additionally, urine samples were only collected from those individuals in the original group, and not the two who joined them (because those two were not trained in the urine collection technique), and only for two days. Perhaps the original group, because they greatly outnumbered the new members and dominance relationships were not yet determined, felt relatively safe and therefore in control of the social situation (Sapolsky Citation2005), at least initially. Although rates of agonistic and aggressive behaviors were much higher than typical during this period, low-ranking animals actually experienced a buffering effect, receiving less aggression as 68% was directed at the new members. Finally, if aggression is stressful mostly because of the potential damage to important long-term relationships (de Waal Citation1989; Watts Citation2006), it is possible that agonistic and aggressive interactions with the new members—with whom the sampled chimpanzees had no such relationship—was simply not stressful enough to result in detectable increases in urinary cortisol.
These results also highlight the somewhat subtle distinction between the occasional occurrence of unpredictable events and subjection to a continuously unpredictable environment. The occurrence of unpredictable events may not, in itself, be stressful for animals if they typically live in a predictable environment over which they exert some control (Boissy et al. Citation2007). Each unusual event is evaluated and processed by the individual and may or may not result in a change in urinary cortisol levels, depending on whether it disrupts homeostasis (e.g. the anaesthesia procedure does, the enrichment exercise does not). This is determined by a variety of factors including how the event is perceived and processed by the individual and the duration of exposure (e.g., whether or not the individual perceives the event as involving a loss of control; Hanson et al. Citation1976; Coplan et al. Citation1996; Sapolsky Citation2005; Boissy et al. Citation2007).
This study measured cortisol secretion non-invasively in chimpanzees by measuring urinary cortisol (Whitten et al. Citation1998), eliminating the confounding variable of sample collection stress (Balcombe et al. Citation2004). However, if increases in cortisol secretion resulting from putatively stressful events are transient, they may be undetectable in samples that are averages of circulating levels over several hours (Zumoff et al. Citation1974; Bahr et al. Citation2000). The chimpanzees may have experienced an increase in cortisol secretion as a result of arousal from the surprise boxes; however, if this effect was small and transient, it may not have left a detectable trace. There is also some evidence that stress-related increases in cortisol may be followed by lower levels of this hormone once the stressor is removed or overcome; for example, salivary cortisol decreases after mobbing calls in common marmosets, suggesting a calming effect of the calls (Cross and Rogers Citation2006). If chimpanzees similarly cope with stressors by participating in calming behaviors that lower cortisol levels (de Waal and van Roosmalen Citation1979; Aureli et al. Citation1989; de Waal and Aureli Citation1996), then initially high levels may be averaged out by subsequent lower than average levels. Collecting post-putative stressor samples from chimpanzees that have been trained for alert blood collection could help address this question.
Changes in cortisol secretion can result from any type of stress, not just social stress. For example, Muller and Wrangham (Citation2004) have described energetically driven differences in cortisol between high and low-ranking chimpanzees: high-ranking animals in their study population exhibited higher cortisol levels, which the authors ascribed to increased levels of physical exertion via aggression. While the events recorded here are hypothesized to increase cortisol secretion for psychosocial reasons, the surprise box condition also resulted in a slight decrease in activity level as measured by the rate of overall social interactions (34–29 events/h), which may have the opposite effect on levels of this hormone. The hurricane event also had this effect (pers. obs.). These two effects may be confounded in this sample, but this hypothesis requires further testing, including measures of overall energy expenditure.
The chimpanzees in this study were probably not hyporesponsive to stress as a result of chronic exposure to stressors because they did not exhibit classic behavioral or physiological signs of chronic stress (Sapolsky Citation1994). Additionally, cortisol levels vary among individuals in this population and are variable over time within the same individual (Anestis Citation2005), and anaesthesia effectively raised urinary cortisol over baseline levels. Nevertheless, a variety of endocrine effects of a chronically stressed state similar to post-traumatic stress disorder in humans has been demonstrated in animal models, especially rodents (Coplan et al. Citation1996; Stam Citation2007), and should be considered in any study of captive animals where expected cortisol responses are not found.
In both captive and wild environments, chimpanzees experience breaks in routine; in this study, such changes in routine did not result in a urinary cortisol stress response. Understanding the etiology of the stress response of captive chimpanzees offers a comparative perspective from which to consider chimpanzees in the wild, where the assessment of stress—especially the stress response to acute events—can be difficult for methodological reasons (Buchanan and Goldsmith Citation2004; Millspaugh and Washburn Citation2004; Cockrem Citation2005). Practically, reducing the anxiety of captive animals is a basic way to increase their overall welfare and health (Mostl and Palme Citation2002; Honess and Marin Citation2006; Boissy et al. Citation2007), and understanding the animals' physiological responses to both routine and atypical events can improve how they are cared for and managed.
Acknowledgments
I am particularly grateful to Richard Bribiescas, David Watts, and Mary Smith at Yale University for their guidance throughout the course of this project, which also included my dissertation research. I would also like to thank the veterinarians and primate care staff at the New Iberia Research Center (especially Dana Hasselschwert, Danny Boutte, and Daphne Briggs) for their help throughout this project, and the director of the NIRC, Jeff Rowell, for giving me permission to conduct my research there. The Schwartz Family Foundation generously granted financial support in the form of a post-doctoral fellowship during the write-up process. Three anonymous reviewers and editor John Russell gave suggestions that greatly improved this manuscript. This work was funded by the National Science Foundation under Grant No. 0120175, The L. S. B. Leakey Foundation, and the Yale University Williams Fund.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Anestis SF. Behavioral style, dominance rank, and urinary cortisol in young chimpanzees (Pan troglodytes). Behaviour 2005; 142: 1245–1268
- Anestis SF, Bribiescas RG. Rapid changes in chimpanzee (Pan troglodytes) urinary cortisol excretion. Horm Behav 2004; 45: 209–214
- Anestis SF, Bribiescas RG, Hasselschwert DL. Age, rank, and personality effects on the cortisol sedation stress response in young chimpanzees. Physiol Behav 2006; 89: 287–294
- Angst W. Aggression bei affen und menschen. Springer, Berlin 1980
- Aureli F, van Schaik CP, van Hooff JARAM. Functional aspects of reconciliation among captive long-tailed macaques (Macaca fascicularis). Am J Primatol 1989; 19: 39–51
- Aureli F, Eck CJVPV, Veenema HC. Long-tailed macaques avoid conflicts during short-term crowding. Aggr Behav 1995; 21: 113–122
- Aureli F, de Waal FBM. Inhibition of social behavior in chimpanzees under high-density conditions. Am J Primatol 1997; 41: 213–228
- Bahr NI, Palme R, Mohle U, Hodges JK, Heistermann M. Comparative aspects of the metabolism and excretion of cortisol in three individual nonhuman primates. Gen Comp Endocrinol 2000; 117: 427–438
- Balcombe J, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Topics Lab Anim Sci 2004; 43: 42–51
- Bassett JR, Cairncross KD, King MG. Parameters of novelty, shock predictability and response contingency in corticosterone release in the rat. Physiol Behav 1973; 10: 901–907
- Bentson KL, Capitanio JP, Mendoza SP. Cortisol responses to immobilization with Telazol or ketamine in baboons (Papio cynocephalus/anubis) and rhesus macaques (Macaca mulatta). J Med Primatol 2003; 32: 148–160
- Boissy A, Manteuffel G, Jensen MB, Moe RO, Spruijt B, Keeling LJ, Winckler C, Forkman B, Dimitrov I, Langbein J, Bakken M, Veissier I, Aubert A. Assessment of positive emotions in animals to improve their welfare. Physiol Behav (Special Issue: Stress and Welfare in Farm Animals) 2007; 92: 375–397
- Buchanan KL, Goldsmith AR. Noninvasive endocrine data for behavioural studies: The importance of validation. Anim Behav 2004; 67: 183–185
- Bygott D. Agonistic behaviour and dominance in wild chimpanzees. DA Hamburg, ER McCown. Benjamin/Cummings, The Great Apes. Menlo Park, CA 1979; 405–427, editors
- Calhoun JB. Population density and social pathology. Scientific Am 1962; 206: 139–148
- Carlstead K, Brown J, Seidensticker J. Behavioural and adrenocortical responses to environmental changes in leopard cats Felis bengalensis. Zool Biol 1993; 12: 321–331
- Cockrem JF. Conservation and behavioral neuroendocrinology. Horm Behav 2005; 48: 492–501
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: Implications for the pathophysiology of mood and anxiety disorders. Proc Nat Acad Sci USA 1996; 93: 1619–1623
- Creel S. Social dominance and stress hormones. Trends Ecol Evol 2001; 16: 491–497
- Crockett CM, Shimoji M, Bowden DM. Behavior, appetite, and urinary cortisol responses by adult female pigtailed macaques to cage size, cage level, room change, and ketamine sedation. Am J Primatol 2000; 52: 63–80
- Cross N, Rogers LJ. Mobbing vocalizations as a coping response in the common marmoset. Horm Behav 2006; 49: 237–245
- Dave RN, Krishnapuram R. Robust clustering methods: a unified view. IEEE Trans Fuzzy Syst 1997; 5: 270–293
- Ellison PT. Human salivary steroids: Methodological considerations and applications in physical anthropology. Yearbook Phys Anthropol 1988; 31: 115–142
- Goymann W, Mostl E, Van't Hof T, East ML, Hofer H. Noninvasive fecal monitoring of glucocorticoids in spotted hyenas, Crocuta crocuta. Gen Comp Endocrinol 1999; 114: 340–348
- Goymann W, Wingfield JC. Allostatic load, social status and stress hormones: The costs of social status matter. Anim Behav 2004; 67: 591–602
- Hanson JD, Larson ME, Snowdon CT. The effects of control over high intensity noise on plasma cortisol levels in rhesus monkeys. Behav Biol 1976; 16: 333–340
- Hergovich N, Singer E, Agneter E, Eichler HG, Graselli U, Simhandl C, Jilma B. Comparison of the effects of ketamine and memantine on prolactin and cortisol release in men: A randomized, double-blind, placebo-controlled trial. Neuropsychopharmacology 2001; 24: 590–593
- Honess PE, Marin CM. Enrichment and aggression in primates. Neurosci Biobehav Rev (Special Issue: Relationship between the Brain and Aggression) 2006; 30: 413–436
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 1994; 19: 313–333
- Knott CD. Field collection and preservation of urine in orangutans and chimpanzees. Trop Biodivers 1997; 4: 95–102
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev 2005; 29: 3–38
- Korte SM, Olivier B, Koolhaas JM. A new animal welfare concept based on allostasis. Physiol Behav (Special Issue: Stress and Welfare in Farm Animals) 2007; 92: 422–428
- Lambeth SP, Hau J, Perlman JE, Martino M, Schapiro SJ. Positive reinforcement training affects hematologic and serum chemistry values in captive chimpanzees (Pan troglodytes). Am J Primatol 2006; 68: 245–256
- McGuire M, Raleigh M, Johnson C. Social dominance in adult male vervet monkeys: General considerations. Soc Sci Inform 1983; 22: 89–123
- Millspaugh JJ, Washburn BE. Use of fecal glucocorticoid metabolite measures in conservation biology research: Considerations for application and interpretation. Gen Comp Endocrinol 2004; 138: 189–199
- Mostl E, Palme R. Hormones as indicators of stress. Domest Anim Endocrinol (Fourth International Conference on Farm Animal Endocrinology) 2002; 23: 67–74
- Muller MN, Wrangham R. Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii). Behav Ecol Sociobiol 2004; 55: 332–340
- Nieuwenhuijsen K, de Waal FBM. Effects of spatial crowding on social behavior in a chimpanzee colony. Zool Biol 1982; 1: 5–28
- Nishida T, Kano T, Goodall J, McGrew WC, Nakamura M. Ethogram and ethnography of Mahale chimpanzees. Anthropol Sci 1999; 107: 141–188
- Pollard TM. Use of cortisol as a stress marker: Practical and theoretical problems. Am J Hum Biol 1995; 7: 265–274
- Puri CP, Puri V, Kumar CA. Serum levels of testosterone, cortisol, prolactin and bioactive luteinizing hormone in adult male rhesus monkeys following cage-restraint or anaesthetizing with ketamine hydrochloride. Acta Endocrinol 1981; 97: 118–124
- Sapolsky RM. The endocrine stress–response and social status in the wild baboon. Horm Behav 1982; 16: 279–292
- Sapolsky RM. Endocrine aspects of social instability in the olive baboon (Papio anubis). Am J Primatol 1983; 5: 365–379
- Sapolsky RM. Why zebras don't get ulcers: A guide to stress, stress-related diseases, and coping. W.H. Freeman and Company, New York 1994
- Sapolsky RM. Endocrinology of the stress response. Behavioral endocrinology, JB Becker, SM Breedlove, D Crews, M McCarthy. MIT Press, Cambridge, MA 2002; 409–450
- Sapolsky RM. The influence of social hierarchy on primate health. Science 2005; 308: 648–652
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses?. Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 2000; 21: 55–89
- Southwick C. An experimental study of intra-group agonistic behavior in rhesus monkeys (Macaca mulatta). Behaviour 1967; 28: 182–209
- Springer DA, Baker KC. Effect of ketamine anesthesia on daily food intake in Macaca mulatta and Cercopithecus aethiops. Am J Primatol 2007; 69: 1080–1092
- Stam R. PTSD and stress sensitisation: A tale of brain and body. Part 2: Animal models. Neurosci Biobehav Rev 2007; 31: 558–584
- Stavisky RC, Adams MR, Watson SL, Kaplan JR. Dominance, cortisol, and behavior in small groups of female Cynomolgus monkeys (Macaca fascicularis). Horm Behav 2001; 39: 232–238
- Taussky HH. A microcolormetric determination of creatinine in urine by the Jaffe reaction. J Biol Chem 1954; 208: 853–861
- Videan EN, Fritz J. Effects of short- and long-term changes in spatial density on the social behavior of captive chimpanzees (Pan troglodytes). Appl Anim Behav Sci 2007; 102: 95–105
- de Waal FBM. The myth of a simple relation between space and aggression in captive primates. Zool Biol 1989; 8: 141–148
- de Waal FBM, van Roosmalen A. Reconciliation and consolation among chimpanzees. Behav Ecol Sociobiol 1979; 5: 55–66
- de Waal FBM, Aureli F. Consolation, reconciliation, and possible cognitive differences between macaque and chimpanzee. Reaching into thought: the minds of the great apes, AE Russon, KA Bard, ST Parker. Cambridge University Press, Cambridge 1996; 80–110
- Watts DP. Conflict resolution in chimpanzees and the valuable-relationships hypothesis. Int J Primatol 2006; 27: 1337–1364
- Weiss JM. Somatic effects of predictable and unpredictable shock. Psychosom Med 1970; 32: 397–408
- Whitten PL, Brockman DK, Stavisky RC. Recent advances in noninvasive techniques to monitor hormone–behavior interactions. Yearbook Phys Anthropol 1998; 41: 1–23
- Zumoff B, Fukushima DK, Hellman L. Intercomparison of four methods for measuring cortisol production. J Clin Endocrinol Metabol 1974; 38: 169–175