Abstract
In this review, we present evidence for the involvement of imidazoline binding sites (IBS) in modulating responses to stress, through central control of monoaminergic and hypothalamo–pituitary–adrenal (HPA) axis activity. Pharmacological and physiological evidence is presented for differential effects of different IBS subtypes on serotoninergic and catecholaminergic pathways involved in control of basal and stress-stimulated HPA axis activity. IBS ligands can modulate behavioural and neuroendocrine responses in animal models of stress, depression and anxiety, and a body of evidence exists for alterations in central IBS expression in psychiatric patients, which can be normalised partially or fully by treatment with antidepressants. Dysfunction in monoaminergic systems and the HPA axis under basal and stress-induced activation has been extensively reported in psychiatric illnesses. On the basis of the literature, we suggest a potential therapeutic role for selective IBS ligands in the treatment of depression and anxiety disorders.
Introduction
Aversive or stressful experiences provoke an array of behavioural and physiological changes, which facilitate an organism's ability to cope. In recent years, thinking has moved away from the stress response as a general response and towards the consensus that adaptive responses to a particular stressor are specific to the nature, duration and intensity of the stressor (Pacak Citation2000; Sapolsky et al. Citation2000; Pacak and Palkovits Citation2001; McEwen Citation2007). The initiation of an appropriately graded response to a particular stressor is of vital importance as inapt responses may lead to chronic stress complications with consequent immunosuppression, and predisposition to infections and illness (Chrousos and Gold Citation1992; Sapolsky et al. Citation2000). Inappropriate responses to stress may also affect personality development and behaviour (Heim et al. Citation2004; Gollan et al. Citation2005; Popma et al. Citation2007) and increase susceptibility to both the onset and severity of mental illness, particularly anxiety disorders, depression (Finlay-Jones and Brown Citation1981; Brown et al. Citation1995; Kendler and Karkowski-Shuman Citation1997; Kendler et al. Citation1999) and schizophrenia (Lahniers and White Citation1976; Gruen and Baron Citation1984; Day et al. Citation1987).
The two major physiological systems mediating the stress response are the sympathetic nervous system and the hypothalamo–pituitary–adrenal (HPA) axis. While there is some evidence for altered sympathetic nervous system activity in psychiatric disorders, little if any strong evidence exists to support a causal role of sympathetic nervous system dysfunction in stress-related psychiatric disorders. In contrast, there is now a significant body of evidence implicating dysfunction of the HPA axis in the aetiology of various psychiatric and stress-related disorders (Zacharko and Anisman Citation1991; Connor et al. Citation1997; Dubrovsky Citation2000).
Depression and the HPA axis
Many studies have examined the association between depressive illness and HPA axis abnormalities (Thomson and Craighead Citation2007). There is evidence that the negative feedback loop regulated by glucocorticoid hormones is deficient in depressed patients and a feed-forward hyperactivation of the HPA axis is considered to occur (Holsboer Citation2000; Parker et al. Citation2003). Specifically, decreased corticosteroid receptor function, elevated plasma cortisol levels, an enhanced adrenal response to adrenocorticotropic hormone (ACTH), a blunted pituitary ACTH response to corticotrophin-releasing factor (CRF) as well as adrenal and pituitary enlargement are commonly observed in patients with depression (Krishnan et al. Citation1991; Rubin et al. Citation1996; Modell et al. Citation1997; Scott and Dinan Citation1998; Arborelius et al. Citation1999; Holsboer Citation2000; Parker et al. Citation2003). Additionally, it has recently been shown that increased ACTH secretion may occur in depressed patients regardless of cortisolemic status (Bornstein et al. Citation2008). Importantly, hyperactivation of the HPA axis is normalized by long-term antidepressant treatment (Greden et al. Citation1983; De Bellis et al. Citation1993; Michelson et al. Citation1997; Pariante et al. Citation2004). Interestingly, the failure of CRF hypersecretion to normalize subsequent to treatment is predictive of early relapse of depressive symptoms (Greden et al. Citation1983; Banki et al. Citation1992; Ribeiro et al. Citation1993; Zobel et al. Citation2001). Lower levels of CRF receptor binding sites and expression are also reported in the cerebral cortex of depressed suicide victims (Nemeroff et al. Citation1988; Bissette et al. Citation2003; Merali Citation2004). This may be due to a down-regulation of receptor signalling in response to excessive CRF release. Finally, there is a high incidence of depressive symptomatology in patients with Cushing's disease (Gibbons and McHugh Citation1962; Carpenter and Bunney Citation1971) and exacerbation of specific depressive disorders such as seasonal bipolar depression (De Bellis et al. Citation1993; Ghadirian et al. Citation2005). Together, these data suggest that HPA axis dysfunction is a state, rather than a trait marker of depression, which may be causally involved in the pathogenesis of this mood disorder. Further support for this hypothesis comes from work demonstrating the antidepressant properties of glucocorticoid synthesis inhibitors such as ketoconazole and metyrapone (Ghadirian et al. Citation1995; Wolkowitz et al. Citation1999). Ketoconazole alleviates depressive symptoms in hypercortisolemic but not in non-hypercortisolemic patients (Wolkowitz et al. Citation1999).
Anxiety disorders and the HPA axis
Anxiety disorders too are linked with CRF-mediated dysregulation of the HPA axis. Evidence for the association between anxiety and HPA axis abnormalities is most established in post-traumatic stress disorder (PTSD) and panic disorders. Elevated CRF levels in plasma (de Kloet et al. Citation2008) and cerebrospinal fluid have been reported in PTSD patients (Bremner et al. Citation1997; Baker et al. Citation1999; Sautter et al. Citation2003). Furthermore, PTSD patients also exhibit hypersuppression of cortisol in dexamethasone-suppression tests (de Kloet et al. Citation2007) and hypersuppression of the ACTH response to cortisol (Yehuda et al. Citation2006). Clinically, the use of pre-operative hydrocortisone treatment is protective against the development of PTSD (Schelling et al. Citation2004). This association is also supported by preclinical work that demonstrates the reduction in the number of rats that display exaggerated anxiety-like behaviours post-exposure to an olfactory stress stimulus when pretreated with corticosterone (Cohen et al. Citation2006). Furthermore, glucocorticoids have also been shown to enhance extinction of fear responding in the rat and in human phobia patients (Soravia et al. Citation2006; Yang et al. Citation2006). In the case of generalised anxiety disorder, the involvement of the HPA axis is less clear. A number of studies report no differences in CRF levels in cerebrospinal fluid of patients versus controls (Banki et al. Citation1992; Jolkkonen et al. Citation1993; Fossey et al. Citation1996). Another interesting piece of evidence implicating CRF in anxiety disorders is the association of a single nucleotide polymorphism in the CRF gene with behavioural inhibition (Smoller et al. Citation2005); a heritable temperamental phenotype in children evidenced by fearful, avoidant or shy behaviour in novel situations. Behavioural inhibition is a risk factor for the development of panic disorder and social phobia (Isolan et al. Citation2005).
Based on the evidence implicating dysfunction of the HPA axis in the aetiology of various psychiatric and stress-related disorders, CRF antagonists have been identified as potential psychopharmaceuticals (Arborelius et al. Citation1999; Grigoriadis Citation2005; Van Den Eede et al. Citation2005). Although some of the psychotropic effects of such compounds may not be attributed to actions on the HPA axis (Ising and Holsboer Citation2007), these compounds reduce depressive symptoms (Zobel et al. Citation2000). Improved understanding of the regulation of HPA axis function during basal and stressful conditions may hold the key to the development of new pharmacological approaches to the treatment of stress-related psychiatric disease.
Modulation of the HPA axis
A plethora of neurotransmitters and neuropeptides have been shown to modulate HPA axis activity and a comprehensive review is outside the scope of this paper. Instead, we will briefly review regulation of the HPA axis by central serotonin and noradrenaline since their regulation by imidazoline binding sites is well-documented and will be discussed in detail later.
Serotoninergic modulation
Serotoninergic (5-HT) neurons of the dorsal and medial raphe nucleus project to the PVN as well as the prefrontal cortex and other limbic and hypothalamic regions. The direct synaptic connections between 5-HT nerve terminals and the CRF-containing neurons of the PVN (Liposits et al. Citation1987) permit both the inhibition and facilitation of HPA axis activity during basal and stressful conditions depending on the specific stressor (Saphier et al. Citation1995), the receptor subtype activated (Welch et al. Citation1993; Saphier and Welch Citation1994; Saphier et al. Citation1995) and route of administration (Welch and Saphier Citation1994; Kageyama et al. Citation1998; Samad et al. Citation2006). Furthermore, chronic variation in circulating corticosterone levels alters the regulation of 5-HT neurones, increasing α1-adrenoceptor-mediated excitation and reducing 5-HT-mediated auto-inhibition at lower corticosterone levels (Judge et al. Citation2004). This alteration has a major impact on control of 5-HT neuronal activity and hence HPA axis activity.
Noradrenergic modulation
Noradrenaline (NA) also plays a key role in regulating the HPA axis. Phasic, fear-associated stimuli produce robust increases in action potential firing in locus coeruleus (LC) NA neurons and in turn, increase NA release in the brain (Valentino and Curtis Citation1991). CRF fibres and receptors are found in the central arousal sympathetic systems including the LC and nucleus tractus solitarii (A2 NA neurons) in the brainstem (Swanson et al. Citation1983). Electrical stimulation of the A1 or A2 region evokes excitatory responses in parvocellular neurons in the PVN, whilst A6 (LC) stimulation evokes a more inhibitory response (Saphier and Feldman Citation1989). Furthermore, electrical stimulation of the ventral noradrenergic ascending bundle, a fibre system primarily comprising catecholaminergic fibres arising from brainstem regions, increases hypophysial-portal plasma immunoreactive CRF levels (Plotsky Citation1987). This suggests that the noradrenergic system plays an important stimulatory role in the modulation of HPA axis activity, and is further corroborated by work demonstrating a dose-dependent facilitation of CRF release by intracerebroventricular (i.c.v.) administration of NA (Plotsky Citation1987). This communication between NA and CRF fibres is bi-directional and it is considered that the CRF-containing neurons of the PVN and NA neurons of the brainstem comprise an autoregulatory negative feedback loop for the HPA axis (Silverman et al. Citation1989; Calogero et al. Citation1990).
A novel group of binding sites that modulate monoamine release have been identified and characterised (Eglen et al. Citation1998). They have been classified as the imidazoline binding sites (IBS) (Ernsberger et al. Citation1995). The effects of IBS on cardiovascular function have been well-investigated since their discovery in 1984. However, evidence would also suggest a role in stress responsivity and psychiatric disease (Halaris and Piletz Citation2003). The remainder of this review will deal with IBS, their importance in regulation of the stress response and their clinical relevance to psychiatric disorders.
Imidazoline binding sites
The existence of IBS was first proposed when clonidine and other compounds containing an imidazoline moiety were found to lower blood pressure by acting at non-adrenoceptor sites in the brainstem (Bousquet et al. Citation1984). These binding sites demonstrate a high affinity for imidazoline-class α2-adrenergic ligands but low affinity for known biogenic amines. Studies by several research groups have indicated their presence in peripheral tissues and in both the central and peripheral nervous systems of various species (Campbell and Potter Citation1994; Ernsberger and Haxhiu Citation1997; Eglen et al. Citation1998; MacInnes and Handley Citation2005). Using radioligands such as [3H]clonidine, [3H]idazoxan, [3H]harmane, [3H]2-BFI and [3H]BU224, three binding site subtypes have been identified: I1, I2 and I3, which can be distinguished on the basis of pharmacology (Parini et al. Citation1996; Mourtada et al. Citation1997; Reis and Piletz Citation1997; Eglen et al. Citation1998), subcellular location (Tesson et al. Citation1995; Ernsberger and Haxhiu Citation1997; Zhang and Abdel-Rahman Citation2006) and regional distribution (Ernsberger and Haxhiu Citation1997; Lione et al. Citation1998; Robinson et al. Citation2002; MacInnes and Handley Citation2005; Anderson et al. Citation2006).
I1 binding sites
I1 IBS are characterised by their high affinity for 2-aminoimidazolines such as [3H]clonidine, moderate affinity for imidazolines such as [3H]idazoxan, and a low affinity for guanidines (Dardonville and Rozas Citation2004). I1 IBS have been identified in pre-synaptic neurons in the rostral ventrolateral medulla oblongata (RVLM) region (Ernsberger and Haxhiu Citation1997) and are regionally distributed throughout the striatum, pallidum, dentate gyrus of the hippocampus, amygdala and substantia nigra (King et al. Citation1995) (). Candidate proteins for this binding site have recently been cloned and include the imidazoline receptor antisera-selective (IRAS) protein and its mouse homologue, nischarin (Alahari et al. Citation2000; Piletz et al. Citation2000; Zhang and Abdel-Rahman Citation2006; Sun et al. Citation2007). IRAS/nischarin is a membrane-associated protein with a PX domain that binds to membrane phospholipids (Piletz et al. Citation2000). An acidic region of similarity was found between IRAS-1 and the calcium-binding pore of ryanodine receptors (Piletz et al. Citation2000). IRAS/nisacharin is currently classified as a nexin or a scaffolding protein, which participates in cell signalling (Piletz et al. Citation1996b). Nischarin selectively inhibits Rac-driven signaling cascades that affect migration through p21-activated kinase (Alahari Citation2003). In addition, nischarin can also regulate other elements of Rac1 signalling pathways (Reddig et al. Citation2005). Studies investigating I1 IBS signalling mechanisms have observed the activation of the phosphatidylcholine selective phospholipase C (PC-PLC) pathway (Ernsberger et al. Citation1993; Renouard and Widdowson Citation1993; Separovic and Kester Citation1996; Separovic et al. Citation1997; Takada et al. Citation1997; Zhang et al. Citation2001; Zhang and Abdel-Rahman Citation2005) and increases in extracellular signal-regulated kinases and JNK by selective I1 ligands in PC12 cells (Edwards et al. Citation2001; Zhang et al. Citation2001; Sano et al. Citation2002; Sun and Chang Citation2007) as well as in vivo in the RVLM in the rat (Zhang and Abdel-Rahman Citation2005). The activation of PC-PLC uniquely distinguishes I1 IBS from neuronal α2-adrenoceptors (Separovic et al. Citation1997).
Figure 1 (A) Schematic depicting expression of imidazoline binding sites in the rat brain as determined by receptor autoradiography using [3H]BU224 (I2 selective ligand), [3H]2BFI (I2 selective ligand) and [3H] rilmenidine (I1 selective ligand). The regions highlighted have been shown to express a moderate to high density of imidazoline binding sites (King et al. Citation1995; Lione et al. Citation1998; Robinson et al. Citation2002; Tyacke et al. 2002). The figure also summarises the effects of imidazoline binding site activation on neuronal firing and terminal release of monoamines in these regions. I1 (red) and I2 (blue) binding sites are colocalised (purple) in some brain regions. (B) Schematic depicting [3H]harmane binding (green) in discrete rat brain regions and the effects of harmane administration on neuronal firing and terminal release of monoamines in these regions. Harmane is a putative endogenous IBS ligand. Abbreviations used: 5-HT, serotonin; AP, area postrema; Arc, arcuate nucleus; CG, central grey; Cx, Cortex; DR, dorsal raphe; DM, dorsomedial hypothalamic nucleus; Fr, frontal cortex; ICV, intracerebroventricular; IP, interpeduncular nucleus; LC, locus coeruleus; LM, lateral mammillary nucleus; LS, lateral septal nucleus; MHb, medial habenular nucleus; NA, noradrenaline; Occ, occipital cortex; Pi, pineal gland; PVN, paraventricular nucleus (hypothalamus); Sol, nucleus of the solitary tract; SuG, superficial gray layer of the superior colliculus; VMH, ventromedial hypothalamic nucleus and 12, hypoglossal nucleus.
![Figure 1 (A) Schematic depicting expression of imidazoline binding sites in the rat brain as determined by receptor autoradiography using [3H]BU224 (I2 selective ligand), [3H]2BFI (I2 selective ligand) and [3H] rilmenidine (I1 selective ligand). The regions highlighted have been shown to express a moderate to high density of imidazoline binding sites (King et al. Citation1995; Lione et al. Citation1998; Robinson et al. Citation2002; Tyacke et al. 2002). The figure also summarises the effects of imidazoline binding site activation on neuronal firing and terminal release of monoamines in these regions. I1 (red) and I2 (blue) binding sites are colocalised (purple) in some brain regions. (B) Schematic depicting [3H]harmane binding (green) in discrete rat brain regions and the effects of harmane administration on neuronal firing and terminal release of monoamines in these regions. Harmane is a putative endogenous IBS ligand. Abbreviations used: 5-HT, serotonin; AP, area postrema; Arc, arcuate nucleus; CG, central grey; Cx, Cortex; DR, dorsal raphe; DM, dorsomedial hypothalamic nucleus; Fr, frontal cortex; ICV, intracerebroventricular; IP, interpeduncular nucleus; LC, locus coeruleus; LM, lateral mammillary nucleus; LS, lateral septal nucleus; MHb, medial habenular nucleus; NA, noradrenaline; Occ, occipital cortex; Pi, pineal gland; PVN, paraventricular nucleus (hypothalamus); Sol, nucleus of the solitary tract; SuG, superficial gray layer of the superior colliculus; VMH, ventromedial hypothalamic nucleus and 12, hypoglossal nucleus.](/cms/asset/45c04eef-1173-4fa7-ac25-f90e551d577f/ists_a_330457_f0001_b.gif)
I1 IBS is best known for its role as a regulator of blood pressure (Bousquet and Feldman Citation1984; Chalmers and Pilowsky Citation1991; Ernsberger et al. Citation1995). High densities of I1 IBS are localised in the RVLM, a region involved in cardiovascular regulation. Evidence supporting a role for I1 IBS in sympatho-inhibition comes from work demonstrating that drugs devoid of affinity for α2-adrenoceptors, but with affinity for I1 IBS, reduce blood pressure when injected into the RVLM (Bousquet et al. Citation1984; Laubie et al. Citation1985; Tibirica et al. Citation1989). Moreover, I1 IBS antagonists block the cardiovascular effects of I1 IBS agonists more effectively than pure α2-adrenoceptor antagonists (Wang et al. Citation2007). I1 ligands lower sympathetic tone by acting primarily on cardiovascular regulatory centres in the medulla. However, peripheral presynaptic inhibition of transmitter release from post-ganglionic sympathetic neurons contributes to the overall sympatho-inhibition. The effects of imidazoline antihypertensives have been reviewed in detail elsewhere (Szabo Citation2002; Bousquet et al. Citation2003).
Alterations in I1 IBS expression are evident in patients with depression, which may suggest a role for these receptors in mediating some of the pathophysiological changes that occur in depression and other psychiatric disorders. We will re-visit this issue in greater detail later in the review.
I2 binding sites
These binding sites are heterogenous in nature and have been further subdivided into I2A and I2B according to their sensitivity to amiloride (Olmos et al. Citation1999). I2 IBS are preferentially bound by imidazolines (Nutt et al. Citation1995; Hudson et al. Citation1997; Dardonville and Rozas Citation2004) and show lower affinity for 2-aminoimidazolines (Eglen et al. Citation1998). I2 IBS are widely distributed in the central nervous system (CNS) and peripheral tissues. [3H]Idazoxan, and more recently [3H]2-BFI and [3H]BU224, have proven to be important tools for elucidating the distribution of I2 IBS (). All three ligands possess high affinity for the I2 IBS and the latter two possess a high degree of selectivity for I2 IBS over α2-adrenoceptors and I1 IBS (Dardonville and Rozas Citation2004). Using receptor autoradiography, the highest densities of [3H]2-BFI binding (>90 fmol mg− 1 tissue) were observed in the arcuate nucleus, dorsal surface of the third ventricle, mammillary peduncle, interpeduncular nucleus and area postrema (Lione et al. Citation1998). Significant levels of binding are also found in the pineal gland, surface of the fourth ventricle, ependyma, dorsal raphe and areas such as the hippocampus, frontal cortex and caudate-putamen. Similar regional binding was observed using [3H]BU224 and [3H]idazoxan (Robinson et al. Citation2002). I2 IBS are found largely on the outer membrane of mitochondria and possess a high degree of sequence homology with monoamine oxidases (MAO) that reside there (Tesson et al. Citation1995). This, along with evidence indicating that photolabelled I2 IBS are immuno-precipitated with monoclonal MAO-A and MAO-B antibodies (Raddatz et al. Citation1995) and a study showing that MAO enzymes cannot be separated from I2 IBS during purification processes (Tesson et al. Citation1995), suggests a strong relationship between the I2 IBS and MAO.
[3H]Harmane and its analogues are particularly useful in elucidating the role of IBS on MAO as it acts as a specific and reversible inhibitor of the enzyme (). Harmane is a potent inhibitor of MAO-A (Ki = 55.5 nM) but not MAO-B (Kim et al. Citation1997; Herraiz Citation2005). Harmine, 2-methylharminium, 2,9-dimethylharminium and harmaline are also effective inhibitors of the purified MAO-A, with low Ki values of 5, 69, 15 and 48 nM, respectively (Kim et al. Citation1997). However, residual [3H]harmane binding has been observed in both brain and kidney tissue from MAO-A knockout mice (Anderson et al. Citation2006). This would suggest that although harmane is a potent inhibitor of MAO-A, it may also bind to a non-MAO site. There have also been other reports of a non-MAO associated I2 IBS population. Immunoblotting experiments using a specific polyclonal antibody for IBS have found two peptides in human and rat brain with apparent molecular masses different from those reported for MAO-A (61 kDa) and MAO-B (55 kDa) (Escriba et al. Citation1994). Furthermore, treatment of rats with the irreversible I2 IBS ligand BU99006 results in a decrease in [3H]2-BFI binding to brain membranes without any effect on MAO activity or on the binding of selective MAO-A or MAO-B radioligands (Paterson et al. Citation2006). This would suggest that the site, which binds BU99006 is not MAO-associated and further supports the existence of two distinct populations of I2 sites, one which is associated with MAO and one which is not.
MAO inhibitors (MAOIs) are sometimes prescribed to treat chronic depression in patients that do not respond to selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs). Alterations in MAO activity have been associated with mood disorders (Mayer et al. Citation1976; Nash et al. Citation2005; Yu et al. Citation2005; Christiansen et al. Citation2007; Giraldi et al. Citation2007) and schizophrenia (Li and He Citation2007). Interestingly, chronic MAOI treatment decreases expression of I2 IBS in the brain (Olmos et al. Citation1993). These findings implicate I2 IBS and MAO enzyme activity with the pathogenesis of major depression and possibly other psychiatric disorders and we will present the evidence supporting this hypothesis later in the review.
I3 binding sites
The more recently identified I3 insulinotropic sites are found in the pancreatic β-cells (Morgan et al. Citation1999). These sites are closely related to the adenosine 5′-triphosphate (ATP)-sensitive potassium channel, Kir6.2. Imidazolines can promote intracellular calcium mobilisation (Squires et al. Citation2004) and directly initiate a mechanism of enhanced glucose-dependent insulin secretion (Mest et al. Citation2001; Cooper et al. Citation2003; Bleck et al. Citation2004). A detailed review of I3 imidazoline binding sites is outside the scope of the current article but see Morgan and Chan (Citation2001).
Endogenous imidazoline binding site ligands
Clonidine displacing substance (CDS), from the first crude isolation of an endogenous ligand for the imidazoline binding sites (Atlas Citation1994), was associated with a number of biological functions including arterial pressure regulation (Bousquet and Feldman Citation1986), smooth muscle contraction (Felsen et al. Citation1987) and catecholamine release from adrenal chromaffin cells (Regunathan et al. Citation1991). The components of CDS were later identified as tryptamine, agmatine and β-carbolines: harmane and harmalan (Parker et al. Citation2004). However, tryptamine's retention time, relatively short-half life (Durden et al. Citation1988) and tendency to convert into other active bioactive components suggested that it was not the bioactive component of classical CDS but rather a modulator of both α2-adrenoceptors and IBS (Parker et al. Citation2004). Although agmatine is widely distributed throughout the body, binds to α2-adrenoceptors and IBS subtypes (Dardonville and Rozas Citation2004) and possesses many qualities of a neurotransmitter (Reis and Regunathan Citation2000), agmatine does not have the high potency or affinity at IBS nor diverse tissue distribution ascribed to the bioactive constituent of CDS (Parker et al. Citation2000). Harmane and harmalan are the proposed bioactive constituents of CDS and the endogenous ligands for IBS (Parker et al. Citation2004). Both harmane and harmalan have many of the functional characteristics associated with CDS and exhibit a high affinity for both the I1 IBS and I2 IBS. Harmane significantly reduces blood pressure by activating I1 IBS (Musgrave and Badoer Citation2000), modulates monoamine oxidases via the I2 IBS (Albores et al. Citation1990; Rommelspacher and May Citation1994; Kim et al. Citation1997; Holt et al. Citation2004) and increases insulin secretion in a glucose-dependent manner by activation of the I3 IBS in pancreatic β-cells (Cooper et al. Citation2003). Although the physiological role of endogenous harmane has yet to be elucidated, harmane and its β-carboline analogues are implicated in the modulation of body temperature (hyperthermia) (Adell et al. Citation1996), the forebrain reward pathway (Ergene and Schoener Citation1993) and fear-related behaviours (Kalin et al. Citation1992; Talalaenko et al. Citation2006). The latter topic is discussed later
Imidazoline-4-acetic acid ribotide (IAA-RP) has also been extracted from CDS (Prell et al. Citation2004). IAA is a GABAA receptor agonist that also exhibits a low efficacy, partial agonist effect at GABAC receptors (Tunnicliff Citation1998). Pulse-chase studies showed IAA could be conjugated with phosphoribosyl-pyrophosphate (while hydrolyzing ATP) to produce an IAA-ribotide (Fernandes et al. Citation1960; Crowley Citation1964). IAA-RP is considered to bind to I1 IBS in adrenal tissue and promote the release of arachidonic acid from PC12 cells (Prell et al. Citation2004). However, as a potential I1 IBS agonist, one would expect IAA-RP administration into the RVLM to lower blood pressure, but in contrast, IAA-RP produces a rapid, transient increase in mean arterial pressure (Prell et al. Citation2004). Similar to CDS and other I3 IBS agonists (Chan et al. 1997; Eglen et al. Citation1998), IAA-RP increases insulin secretion from islets (Prell et al. Citation2004). Potentiation of glucose-induced insulin release is the best-characterised I3 IBS-mediated response (Chan et al. 1997; Eglen et al. Citation1998; Morgan et al. Citation1999). I3 IBS agonists also interact with K+ATP channels of the pancreatic islets and these channels are found in high density in the RVLM (Golanov and Reis Citation1999) and promote cell excitability (Chan et al. 1997). These IAA-RP effects are characteristic of I3R agonists. Interestingly, IAA-RP was more potent than the prototypical I3 agonist /I1 antagonist efaroxan, with EC50 = 30–50 nM (Prell et al. Citation2004) versus 100 μM (Chan and Morgan Citation1990). Moreover, efaroxan induces hypertension when administered directly into the RVLM (Ernsberger Citation1998). Thus, it is speculated that IAA-RP may act at an I3 IBS-like receptor located in the brainstem. Evidence also indicates that IAA-RP participates in trans-synaptic signalling in the brain. It is distributed throughout forebrain and limbic system regions including the amygdala, hypothalamus and hippocampus as well as in midbrain and brainstem nuclei (Friedrich et al. Citation2007), exhibits depolarisation-induced Ca2 + -dependent release from P2 synaptosomal elements and possesses a relatively high affinity for membrane-bound IBS (Prell et al. Citation2004). Furthermore, IAA-RP is rapidly metabolised by phosphatases and ecto-5′-nucleotidases (Thomas and Prell Citation1995). This would strongly suggest that not only is IAA-RP a proposed endogenous ligand for the I3 IBS it may indeed act as a neurotransmitter.
The regulation of monoamine neurons by imidazoline binding sites and their ligands
The modulation of noradrenergic neuron activity in the LC by IBS has been investigated by blocking the action of α2-adrenoceptors with N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) (; ). Systemic administration of low doses of clonidine characteristically inhibits neuronal activity (Szabo Citation2002). However, in EEDQ-treated rats, central administration of clonidine directly into the RVLM increases cell firing rate in the LC by 88 ± 24% versus basal firing (Ruiz-Ortega Citation1997). Similarly, the characteristic inhibitory effect of low doses of clonidine on LC noradrenergic neurons was abolished and a paradoxical, dose-dependent increase in firing rate was observed at higher doses (Pineda et al. Citation1993). In the same study, neuronal activation was also stimulated by cirazoline and rilmenidine; both I1 IBS agonists. These results would suggest that IBS ligands can modulate the activity of LC noradrenergic neurones through the activation of I1 IBS. However, the direct effect of these ligands on catecholaminergic neurons in the LC is questionable. In a similar study, central administration of clonidine into the LC did not increase the firing rate of neurons in the EEDQ-pretreated anaesthetized rat (Ruiz-Ortega Citation1997). Physiological recordings during this experiment showed there was no effect on blood pressure after clonidine administration in EEDQ-pretreated rats. Moreover, no functional IBS have been observed in midpontine slices prepared from the rat brain following application of clonidine, rilmenidine and moxonidine (Szabo and Urban Citation1995). Thus, the effects of I1 IBS ligands may be mediated by an indirect mechanism involving IBS located on RVLM neurons projecting to the LC.
Table I. Modulation of brain monoamine activity by IBS ligandsa in rodents in vivo.
Another study investigating the mechanisms underpinning the modulation of noradrenergic neuronal activity in the LC by clonidine has implicated an excitatory amino acid pathway, modulated by an inhibitory serotoninergic mechanism. The stimulatory effect of clonidine on LC noradrenergic neurones in the rat is completely antagonized by pretreatment with kynurenic acid, an excitatory amino acid receptor antagonist (Ruiz-Ortega and Ugedo Citation1997). In this study, clonidine-induced increases in LC noradrenergic neuronal activity were potentiated by both resperine and p-chloro-phenylalanine pretreatment (irreversible inhibitor of tryptophan hydroxylase, a rate-limiting enzyme in the biosynthesis of serotonin) but not with α-methyl-p-tyrosine (reversible inhibitor of tyrosine hydroxylase, a rate-limiting enzyme in the biosynthesis of catecholamines).
An important role for I2 IBS in the modulation of brain monoamine neurons has also been demonstrated. Both central and systemic administration of the selective I2 ligand, 2-BFI, increases the firing rate of LC cells in the anaesthetised rat (Ugedo et al. Citation1998). This effect, however, is antagonised by chronic pretreatment with clorgyline, an irreversible MAOI. In the same study, rat midpontine brain slices containing the LC were bathed in 2-BFI, BU224 and idazoxan. Each agonist reversibly stimulated the LC cells, although when two agonists were administered simultaneously, cell firing did not increase in an additive fashion. When 2-BFI was applied to the bath, it reversed the inhibitory effects of the ATP-sensitive K+-channel opener diazoxide. Furthermore, glibenclamide, an ATP-sensitive K+-channel blocker, partially blocked the effects of 2-BFI. This study indicates that the stimulation of LC cells by selective I2 IBS ligands is not mediated by I1 or I2 IBS, despite the high density of I2 IBS present in the LC (King et al. Citation1995; MacKinnon et al. Citation1995) but possibly via an IBS subtype yet to be identified. These data suggest that this IBS subtype is located extracellularly and modulates ATP-sensitive K+-channels.
IBS ligands also modulate the activity of dorsal raphe neurons in the rat (; ). Harmane inhibits the firing of serotonergic dorsal raphe neurons (Ugedo et al. Citation1999; Touiki et al. Citation2005). It has been suggested that harmane achieves this by acting directly on specific neurotransmitter receptors (Muller et al. Citation1981), although it possesses very low affinity for 5-HT2C receptors and no affinity for 5-HT1A receptors, dopamine D2, or benzodiazepine receptors (Glennon et al. Citation2000). The mechanism by which harmane inhibits serotonergic neurons remains to be elucidated. Harmane and norharmane have also been implicated in the modulation of mesolimbic dopaminergic neuron activity (Ergene and Schoener Citation1993). Here again, the precise pharmacological mechanisms have yet to be clarified.
Presynaptic IBS modulate the extracellular levels of monoamines by allosteric inhibition of MAO activity (Holt et al. Citation2004). Although IBS ligands were once considered to act only on peripheral noradrenergic cells (Gothert et al. Citation1995; Molderings et al. Citation1997), microdialysis studies show that systemic administration of selective IBS ligands increases extracellular levels of noradrenaline in the cerebral cortex (Meana et al. Citation1997), the frontal cortex and hippocampus (Nutt et al. Citation1995, 1997; Hudson et al. Citation1999; Abu Ghazaleh et al. Citation2007) and the hypothalamic PVN (Finn et al. Citation2002), Similarly, I2 IBS ligands increase extracellular levels of serotonin in the hippocampus and LC in the rat (Adell et al. Citation1996; Ugedo et al. Citation1999). I2 IBS ligands also increase extracellular levels of dopamine in the striatum (Hudson et al. Citation1999) and frontal cortex (Abu Ghazaleh et al. Citation2007) of the rat.
Regulation of stress responses by imidazoline binding sites
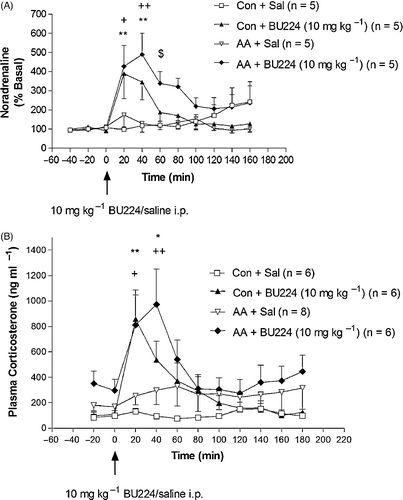
HPA axis. As discussed above, neurobiological systems mediating the stress response are subject to regulation by IBS. Moreover, there is direct evidence to suggest that IBS play a role in mediating and modulating neurochemical, neuroendocrine and behavioural responses to stress. The localisation of I2 IBS in stress responsive brain regions (Lione et al. Citation1998) and their modulation of monoamine levels via inhibition of MAO are of particular relevance to the stress response as monoamines play an essential role in HPA axis regulation. Consistent with a role for central NA in IBS-mediated modulation of HPA axis function, we have demonstrated that systemic administration of the I2 selective ligand BU224 significantly increases extracellular levels of NA in the rat PVN (Finn et al. Citation2002). This elevation, as measured by microdialysis in awake, behaving rats, was observed at 20 and 40 min post-injection (). NA levels were also significantly increased by BU224 in rats with adjuvant-induced arthritis 20, 40 and 60 min post-injection. The BU224-induced increase in extracellular levels of NA in this animal model of chronic inflammatory stress was significantly greater than that observed in non-stressed rats. In the same study, BU224 increased plasma corticosterone levels in both control and adjuvant-induced arthritis rats, an increase that was positively correlated with the increase in extracellular NA. These data suggest that (a) I2 IBS ligands stimulate HPA axis activity by increasing release of NA in the PVN and (b) the noradrenergic response to an I2 IBS ligand is enhanced in the PVN of rats with adjuvant-induced arthritis. This indicates that I2 IBS is an important pharmacological target as its activation facilitates NA release under basal and chronic stress conditions.
Figure 2 Effect of systemic (i.p., imraperitoneal) administration of the selective I2 IBS ligand, BU224, on (A) extracellular levels of NA in the hypothalamic PVN region and (B) plasma corticosterone concentrations in control rats and rats with adjuvant-induced arthritis (AA; a chronic stressor). Values are expressed as means ± s.e.m. **p < 0.01, *p < 0.05 comparing post-injection levels with basal levels in the Con + BU224 group; +p < 0.05, ++p < 0.01 comparing post-injection levels with basal levels in the AA + BU224 group; $p < 0.05 comparing AA + BU224 with Con + BU224 group at 60 min. Con, Control; Sal, Saline vehicle. Reproduced with permission from Finn et al. (Citation2002).
Selective I2 IBS ligands have been shown to potentiate stress-induced neuroendocrine changes in other stress paradigms. We have shown previously that the plasma corticosterone response to acute psychological restraint stress in rats is enhanced by systemic administration of BU224 () and idazoxan compared with saline-treated restraint controls (Finn et al. Citation2004). Thus, I2 IBS ligands are capable of increasing corticosterone levels under basal conditions and during exposure to stress (). In addition to the BU224-induced increase in NA in the PVN discussed above, further evidence indicating that I2 IBS-mediated stimulation of HPA axis activity is centrally driven comes from our work demonstrating that plasma ACTH levels are significantly increased by BU224 in both non-stressed rats and rats exposed to acute swim stress (Finn et al. Citation2003). In addition, work with the endogenous IBS ligand harmaline has shown that expression of CRF mRNA is increased in the inferior olivary complex of cats 8 h following administration of this tremor-inducing β-carboline (Cummings et al. Citation1994) although the relevance of this change to HPA axis activity is unclear. Interestingly, both preclinical and clinical trials show that plasma ACTH and glucocorticoid levels are significantly decreased by clonidine administration (Alexander and Irvine Citation2000; Munoz-Hoyos et al. Citation2000). This finding is consistent with the rationale that I1 IBS activation may reduce monoaminergic activity in some brain regions and exert a consequent inhibitory effect on HPA axis activity. In contrast, acute administration of the I2 IBS ligand idazoxan has been shown to attenuate the normal diurnal fall in plasma cortisol in humans (Glue et al. Citation1992).
Figure 3 Effects of the selective I2 binding site ligand BU224 (10 mg/kg, i.p.) on plasma corticosterone concentrations in control and restraint-stressed rats. Results are expressed as means ± s.e.m. (n = 5–7). **p < 0.01 and ++p < 0.01 compared with saline-treated controls at 30 and 60 min, respectively. ‡p < 0.05 and †p < 0.01 compared with BU224-treated controls (not restrained) at 30 and 60 min, respectively. $$p < 0.05 compared with saline-treated restrained rats at 60 min. Sal + Con, Saline + Control (no restraint); BU + Con: BU224 + Control; Sal + Res, Saline + Restraint; and BU + Res, BU224 + Restraint. Reproduced with permission from Finn et al. (Citation2004).
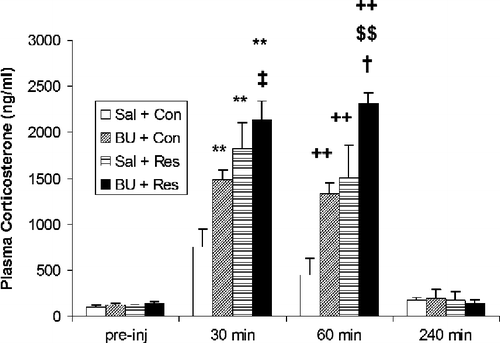
Table II. Modulation of stress hormones by IBS ligands.
Sympathetic nervous system. Sympathetic activation is also an important part of the stress response as it initiates the immediate physiological changes that enable the ‘fight or flight’ behavioural response. The I1 IBS agonist moxonidine does not alter blood pressure in healthy human subjects at rest but decreases plasma noradrenaline levels (Wenzel et al. Citation2004). Physical exercise using bicycle ergometry (50 W increasing to 100, 150 and 200 W every 2 min) significantly increases both blood pressure and plasma NA levels and this stress-induced increase in plasma NA, but not blood pressure, is attenuated with moxonidine pretreatment. However, moxonidine pretreatment significantly reduces the stress-induced increase in both plasma NA levels and blood pressure seen during mental stress testing (Wenzel et al. Citation2004). Another study showed that moxonidine reduces blood pressure during driving simulation without a concomitant impairment of performance (Schmidt Citation1992). These studies suggest that moxonidine decreases total sympathetic tone under basal and during physical exercise as well as mental stress conditions without limiting maximal exercise capacity. Similarly, rilmenidine, an I1 IBS agonist, reduces blood pressure in healthy and hypertensive resting subjects (Fauvel et al. Citation1999; Esler et al. Citation2004) and decreases peak exercise heart rate without modifying peak aerobic power (Teixeira de Castro et al. Citation2006). Teixeira de Castro et al. (Citation2006) have also shown that rilmenidine inhibits the stress-induced blood pressure increases exerted by mental stress testing. However, evidence with respect to the effects of rilmenidine on sympathetic nervous system activity during physical and mental stress is equivocal and there are a number of studies which report that rilmendine does not alter responses in blood pressure and heart rate induced by physical (Fauvel et al. Citation1999; Esler et al. Citation2004) or mental (Esler et al. Citation2004) stress. Moreover, studies in conscious rabbits have demonstrated that rilmenidine fails to inhibit sympathetic activation in response to noise, a psychological stressor (Burke et al. Citation1998; Head and Burke Citation2004). Thus, modulation of sympathetic physiological responses by IBS ligands appears to be dependent on the type of ligand and stressor under investigation and further research in this area is warranted.
Preclinical evidence for a potential therapeutic role of imidazoline binding sites in psychiatric disease
The effects of IBS ligands on behavioural responses to stress have also been investigated using animal models. The forced swim test (FST) is a predictive test of anti-depressant efficacy. In the FST, selective ligands for the I2 IBS (BU224 and 2-BFI) significantly reduce the immobility time of rats compared with saline-treated controls (Nutt et al. Citation1995; Finn et al. Citation2002). These behavioural effects of BU224 are accompanied by modulation of both the HPA axis and brain monoamine tissue levels. This strongly implicates the I2 IBS in mediating antidepressant-like behaviour. However, BU224 has no effect in the mouse FST (O'Neill et al. Citation2001). Agmatine, which has moderate to high affinity for both the I1 and I2 IBS, reduces immobility time in the FST in both rats (1.25, 2.5 and 5 mg/kg s.c.) (Li et al. Citation2003) and mice (20 mg/kg s.c.) (Zomkowski et al. Citation2002; Li et al. Citation2003). The antidepressant-like effects of agmatine (10 mg/kg, i.p.) in the mouse FST can be blocked by pretreatment with efaroxan, idazoxan and antazoline (a ligand with high affinity for the I2 IBS) (Zeidan et al. Citation2007). Furthermore, subeffective dosing with 0.001 mg/kg, (i.p.) agmatine produces a synergistic antidepressant-like effect with clonidine, moxonidine, antazoline and MK-801 (a non-competitive NMDA receptor antagonist). Pretreatment of mice with yohimbine (a non-imidazoline-α2 antagonist) blocks the synergistic antidepressant-like effect of agmatine with clonidine. These data suggest that the anti-immobility effect of agmatine in the FST is dependent on its interaction with I1 and I2 IBS.
Agmatine also reduces immobility in the tail suspension test (TST) (40 and 80 mg/kg p.o.) (Zomkowski et al. Citation2002; Li et al. Citation2003) and elicits acute anxiolytic-like behavioural changes in the rat and mouse in the elevated plus maze (EPM) following i.p. injection (10, 20, 40, 80 or 100 mg/kg, i.p), increasing time spent on the open arms, without accompanying changes in locomotor activity (Lavinsky et al. Citation2003; Gong et al. Citation2006). Moreover, harmane, the endogenous ligand for IBS also increases time spent on the open arms of the EPM and reduces immobility in the rat FST (Aricioglu and Altunbas Citation2003). However, the modulation of behaviours elicited by IBS ligands appears to be stressor specific. In contrast to the effects of IBS ligands in models that examine escape behaviour and learned helplessness, analogues of the endogenous ligand β-carboline potentiated fear-related behaviour and increased plasma levels of both ACTH and cortisol in infant rhesus monkeys when administered immediately after maternal separation (Kalin et al. Citation1992). These differences may be due to the influence of other receptors activated by the particular compounds or possibly by modulatory effects stimulated by the psychological nature of the stressor. Differences in neuroendocrine and behavioural responses to physical versus psychological stressors have been previously reported (Marti and Armario Citation1998)
Clinical evidence for involvement of imidazoline binding sites in pathogenesis of psychiatric disease
Alterations in binding site density
Additional evidence to suggest that IBS play a direct role in the pathology of stress-related disorders and psychiatric disease comes from work demonstrating alterations in binding site densities in psychiatric patients. For example the density of I2 IBS expression on platelet membranes is significantly reduced in platelets of depressed patients (Piletz et al. Citation1994). Furthermore, I2 IBS expression is altered in brain tissue. I2 IBS are reduced by 40% in the frontal cortex of suicide victims (Sastre et al. Citation1995). Since, the association between MAO and I2 IBS is well-established, studies investigating the correlation between MAO-B and suicidal behaviour are of particular relevance to the role of I2-IBS in psychiatric disorders. Many of these studies, however, have reported negative findings, until a recent study showed that suicide victims express >30% increase in binding sites for lazabemide, a MAO-B inhibitor, in the frontal cortex versus matched controls (Ballesteros et al. Citation2008). This study controlled for the influence of confounding variables such as age at death. Alterations in I1 IBS densities have also been reported. Radioligand binding density (Bmax) of I1 IBS on platelet plasma membranes is elevated in depressed patients compared with healthy control subjects (Piletz et al. Citation1994, 1996a).
Evidence from immuno-blotting studies
Western blotting studies have correlated 33 and 85 kDa bands found using imidazoline-binding protein antiserum with platelet I1 IBS as detected by [125I]p-iodoclonidine (Zhu et al. Citation2003). Other bands have been identified and correlated with the I1 IBS (Garcia-Sevilla et al. Citation1999). Interestingly, immunoreactivity of these bands (35 and 45 kDa) is upregulated in the platelets and brain membranes of depressed patients compared to matched controls (Garcia-Sevilla et al. Citation1999). The 45 kDa band is also upregulated in the brains of suicide victims compared to matched controls (Garcia-Sevilla et al. Citation1996). In contrast, the immunoreactivity of both the 35 and 45 kDa bands is decreased in hippocampal homogenates from depressed patients relative to matched controls (Piletz et al. Citation2000). This brain region is renowned for morphological changes such as decreased neurogenesis and cellular remodelling, following stress exposure (Joca et al. Citation2007). Surprisingly, no elevation of platelet I1 IBS expression is evident in patients with generalised anxiety disorder (Piletz et al. Citation1996a). Further correlations have also been made between the immunoreactivity of the 45 kDa band and that of Gαq/11, Gαi2 and Gβ proteins (Garcia-Sevilla et al. Citation1996). Such correlations suggest that the 45 kDa protein may couple to a phosphoinositide pathway in platelets. This pathway has been postulated to function abnormally in mood disorders (Manji Citation1992; Hudson et al. Citation1993; Jope and Williams Citation1994). More specifically, a 30% deficit in G protein-mediated [3H]PI hydrolysis (phosphoinositide signalling) has been reported in the prefrontal cortex of suicide victims with depression compared with matched controls (Pacheco et al. Citation1996). These data highlight the functional implications of the abnormally high expression of I1 IBS (45 kDa protein bands) and its importance in the pathogenesis of major depression and suicide.
Western blotting has also identified an 85 kDa band and a correlation with I1 IBS (Ivanov et al. Citation1998). The authors deduced that lower molecular weight proteins identified using the same antiserum may be possible breakdown fragments of the 85 kDa band by omitting the mixture of protease inhibitors used to prepare the membranes. This 85 kDa band is up-regulated in response to administration of the IBS ligands moxonidine, idazoxan and agmatine (Ivanov et al. Citation1998). Moreover, IRBP-immunoreactivity of the 85 kDa band is increased in the presence of NA and idazoxan whilst yohimbine has no effect. This would suggest this protein is imidazoline specific and up-regulated only with an increase in NA levels and/or administration of IBS ligands.
IBS density and antidepressants
Importantly, all of the IBS alterations (Zhu et al. Citation1997a) observed in depression are essentially reversible following antidepressant treatment (Piletz et al. Citation1991; Garcia-Sevilla et al. Citation1996; Piletz et al. Citation1996b; Zhu et al. Citation1997b; Zhu et al. Citation1999; Halaris and Piletz Citation2001; Halaris et al. Citation2002). Indeed, patients with bipolar depression that were treated with lithium show normal IRBP-immunodensities (Garcia-Sevilla et al. Citation1996; Garcia-Sevilla et al. Citation1999). The down regulation of bands associated with I1 IBS was found only in depressed patients and not in healthy control subjects that received the antidepressant treatment (Piletz et al. Citation2008). This would suggest that the down-regulation/normalization of platelet I1 IBS is limited to treated depressed patients and is likely to be related to antidepressant-induced mood improvement. However, a platelet 33 kDa band is up-regulated during desipramine treatment of healthy subjects (Piletz et al. Citation2008). This conflicts directly with findings in antidepressant-treated patients (Zhu et al. Citation1999). This discrepancy supports observations by Ivanov et al. (Citation1998), which identified the 33 kDa band as a possible proteolytic breakdown product. Further investigation is required to elucidate the molecular nature of IBS and the intracellular mechanisms involved here.
Concluding remarks
IBS-mediated modulation of central monoamine neurons, HPA axis activity and stress-related behaviours strongly suggests a role for these novel targets in the pathophysiology and treatment of psychiatric disorders. Precisely how I1 and I2 IBS modulate the stress response is still relatively unclear, but with the availability of more selective synthetic ligands this task is becoming more achievable. Further work is also required to elucidate the signalling mechanism and functional role of the I2 IBS. This is essential to fully understand how this binding site is implicated in stress and might be manipulated for therapeutic effect. The physiological functions of the recently isolated endogenous ligands of IBS have also yet to be fully determined. The hope is that these findings will help us to develop more selective drugs with improved tolerability for patients with depression, anxiety and other stress-related psychiatric disorders. These binding sites offer a new and innovative target for the treatment of a myriad of stress-related disorders and psychiatric disease.
Acknowledgement
This work was supported by a grant from Science Foundation Ireland.
Declaration of interest The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abu Ghazaleh H, Lalies MD, Husbands SM, Nutt DJ, Hudson AL. The effect of 1-(4,5-dihydro-1H-imidazol-2-yl) isoquinoline on monoamine release and turnover in the rat frontal cortex. Neurosci Lett 2007; 422: 109–113
- Adell A, Biggs TA, Myers RD. Action of harman (1-methyl-beta-carboline) on the brain: Body temperature and in vivo efflux of 5-HT from hippocampus of the rat. Neuropharmacology 1996; 35: 1101–1107
- Alahari SK, Lee JW, Juliano RL. Nischarin, a novel protein that interacts with the integrin alpha 5 subunit and inhibits cell migration. J Cell Biol 2000; 151: 1141–1154
- Alahari SK. Nischarin inhibits Rac induced migration and invasion of epithelial cells by affecting signaling cascades involving PAK. Exp Cell Res 2003; 288: 415–424
- Albores R, Neafsey EJ, Drucker G, Fields JZ, Collins MA. Mitochondrial respiratory inhibition by N-methylated beta-carboline derivatives structurally resembling N-methyl-4-phenylpyridine. Proc Natl Acad Sci USA 1990; 87: 9368–9372
- Alexander SL, Irvine CH. The effect of the alpha-2-adrenergic agonist, clonidine, on secretion patterns and rates of adrenocorticotropic hormone and its secretagogues in the horse. J Neuroendocrinol 2000; 12: 874–880
- Anderson NJ, Seif I, Nutt DJ, Hudson AL, Robinson ES. Autoradiographical distribution of imidazoline binding sites in monoamine oxidase A deficient mice. J Neurochem 2006; 96: 1551–1559
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 1999; 160: 1–12
- Aricioglu F, Altunbas H. Harmane induces anxiolysis and antidepressant-like effects in rats. Ann N Y Acad Sci 2003; 1009: 196–201
- Atlas D. Identifying clonidine-displacing substance. Science 1994; 266: 462–464
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD, Jr. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry 1999; 156: 585–588
- Ballesteros J, Maeztu AI, Callado LF, Meana JJ, Gutierrez M. Specific binding of [(3)H]Ro 19-6327 (lazabemide) to monoamine oxidase B is increased in frontal cortex of suicide victims after controlling for age at death. Eur Neuropsychopharmacol 2008; 18: 55–61
- Banki CM, Karmacsi L, Bissette G, Nemeroff CB. CSF corticotropin-releasing hormone and somatostatin in major depression: Response to antidepressant treatment and relapse. Eur Neuropsychopharmacol 1992; 2: 107–113
- Bissette G, Klimek V, Pan J, Stockmeier C, Ordway G. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology 2003; 28: 1328–1335
- Bleck C, Wienbergen A, Rustenbeck I. Glucose dependence of imidazoline-induced insulin secretion: Different characteristics of two ATP-Sensitive K+ channel-blocking compounds. Diabetes 2004; 53(3)S135–S139
- Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab 2008, 19:175–180
- Bousquet P, Feldman J, Schwartz J. Central cardiovascular effects of alpha adrenergic drugs: Differences between catecholamines and imidazolines. J Pharmacol Exp Ther 1984; 230: 232–236
- Bousquet P, Feldman J, Atlas D. An endogenous, non-catecholamine clonidine antagonist increases mean arterial blood pressure. Eur J Pharmacol 1986; 124: 167–170
- Bousquet P, Greney H, Bruban V, Schann S, Ehrhardt JD, Monassier L, Feldman J. I(1) imidazoline receptors involved in cardiovascular regulation: Where are we and where are we going?. Ann NY Acad Sci 2003; 1009: 228–233
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry 1997; 154: 624–629
- Brown CM, MacKinnon AC, Redfern WS, Williams A, Linton C, Stewart M, Clague RU, Clark R, Spedding M. RS-45041-190: a selective, high-affinity ligand for I2 imidazoline receptors. Br J Pharmacol 1995; 116: 1737–1744
- Burke SL, Malpas SC, Head GA. Effect of rilmenidine on the cadiovascular responses to stress in the conscious rabbit. J Auton Nerv Syst 1998; 72: 177–186
- Calogero AE, Bagdy G, Szemeredi K, Tartaglia ME, Gold PW, Chrousos GP. Mechanisms of serotonin receptor agonist-induced activation of the hypothalamic–pituitary–adrenal axis in the rat. Endocrinology 1990; 126: 1888–1894
- Campbell WR, Potter DE. Potential role of imidazoline (I1) receptors in modulating aqueous humor dynamics. J Ocul Pharmacol 1994; 10: 393–402
- Carpenter WT, Jr, Bunney WE, Jr. Behavioral effects of cortisol in man. Semin Psychiatry 1971; 3: 421–434
- Chalmers J, Pilowsky P. Brainstem and bulbospinal neurotransmitter systems in the control of blood pressure. J Hypertens 1991; 9: 675–694
- Chan SL, Morgan NG. Stimulation of insulin secretion by efaroxan may involve interaction with potassium channels. Eur J Pharmacol 1990; 176: 97–101
- Christiansen L, Tan Q, Iachina M, Bathum L, Kruse TA, McGue M, Christensen K. Candidate gene polymorphisms in the serotonergic pathway: Influence on depression symptomatology in an elderly population. Biol Psychiatry 2007; 61: 223–230
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Jama 1992; 267: 1244–1252
- Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, Kozlovsky N, Kaplan Z. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry 2006; 59: 1208–1218
- Connor TJ, Kelly JP, Leonard BE. Forced swim test-induced neurochemical endocrine, and immune changes in the rat. Pharmacol Biochem Behav 1997; 58: 961–967
- Cooper EJ, Hudson AL, Parker CA, Morgan NG. Effects of the beta-carbolines, harmane and pinoline, on insulin secretion from isolated human islets of Langerhans. Eur J Pharmacol 2003; 482: 189–196
- Crowley GM. The enzymatic synthesis of 5′-phosphoribosylimidazoleacetic acid. J Biol Chem 1964; 239: 2593–2601
- Cummings S, Hinds D, Young WS, 3rd. Corticotropin-releasing factor mRNA increases in the inferior olivary complex during harmaline-induced tremor. Brain Res 1994; 660: 199–208
- Dardonville C, Rozas I. Imidazoline binding sites and their ligands: an overview of the different chemical structures. Med Res Rev 2004; 24: 639–661
- Day R, Nielsen JA, Korten A, Ernberg G, Dube KC, Gebhart J, Jablensky A, Leon C, Marsella A, Olatawura M, et al. Stressful life events preceding the acute onset of schizophrenia: A cross-national study from the World Health Organization. Cult Med Psychiatry 1987; 11: 123–205
- De Bellis MD, Gold PW, Geracioti TD, Jr, Listwak SJ, Kling MA. Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry 1993; 150: 656–657
- Diaz A, Mayet S, Dickenson AH. BU-224 produces spinal antinociception as an agonist at imidazoline I2 receptors. Eur J Pharmacol 1997; 333: 9–15
- Dubrovsky B. The specificity of stress responses to different nocuous stimuli: Neurosteroids and depression. Brain Res Bull 2000; 51: 443–455
- Durden DA, Nguyen TV, Boulton AA. Kinetics of intraventricularly injected trace amines and their deuterated isotopomers. Neurochem Res 1988; 13: 943–950
- Edwards L, Fishman D, Horowitz P, Bourbon N, Kester M, Ernsberger P. The I1-imidazoline receptor in PC12 pheochromocytoma cells activates protein kinases C, extracellular signal-regulated kinase (ERK) and c-jun N-terminal kinase (JNK). J Neurochem 2001; 79: 931–940
- Eglen RM, Hudson AL, Kendall DA, Nutt DJ, Morgan NG, Wilson VG, Dillon MP. Seeing through a glass darkly’: casting light on imidazoline ‘I’ sites. Trends Pharmacol Sci 1998; 19: 381–390
- Ergene E, Schoener EP. Effects of harmane (1-methyl-beta-carboline) on neurons in the nucleus accumbens of the rat. Pharmacol Biochem Behav 1993; 44: 951–957
- Ernsberger P. Arachidonic acid release from PC12 pheochromocytoma cells is regulated by I1-imidazoline receptors. J Auton Nerv Syst 1998; 72: 147–154
- Ernsberger P, Haxhiu MA. The I1-imidazoline-binding site is a functional receptor mediating vasodepression via the ventral medulla. Am J Physiol 1997; 273: R1572–R1579
- Ernsberger P, Damon TH, Graff LM, Schafer SG, Christen MO. Moxonidine, a centrally acting antihypertensive agent, is a selective ligand for I1-imidazoline sites. J Pharmacol Exp Ther 1993; 264: 172–182
- Ernsberger P, Graves ME, Graff LM, Zakieh N, Nguyen P, Collins LA, Westbrooks KL, Johnson GG. I1-imiddazoline receptors. Definition, characterization, distribution, and transmembrane signaling. Ann NY Acad Sci 1995; 763: 22–42
- Escriba PV, Sastre M, Wang H, Regunathan S, Reis DJ, Garcia-Sevilla JA. Immunodetection of putative imidazoline receptor proteins in the human and rat brain and other tissues. Neurosci Lett 1994; 178: 81–84
- Esler M, Lux A, Jennings G, Hastings J, Socratous F, Lambert G. Rilmenidine sympatholytic activity preserves mental and orthostatic sympathetic response and epinephrine secretion. Arch Mal Coeur Vaiss 2004; 97: 786–792
- Fauvel JP, Najem R, Ryon B, Ducher M, Laville M. Effects of rilmenidine on stress-induced peak blood pressure and renal function. J Cardiovasc Pharmacol 1999; 34: 41–45
- Felsen D, Ernsberger P, Meeley MP, Reis DJ. Clonidine displacing substance is biologically active on smooth muscle. Eur J Pharmacol 1987; 142: 453–455
- Fernandes JF, Castellani O, Plese M. Biosynthesis of histamine ribotide and imidazoleacetate ribotide. Biochem Biophys Res Commun 1960; 3: 679–684
- Finlay-Jones R, Brown GW. Types of stressful life event and the onset of anxiety and depressive disorders. Psychol Med 1981; 11: 803–815
- Finn DP, Lalies MD, Harbuz MS, Jessop DS, Hudson AL, Nutt DJ. Imidazoline(2) (I(2)) binding site- and alpha(2)-adrenoceptor-mediated modulation of central noradrenergic and HPA axis function in control rats and chronically stressed rats with adjuvant-induced arthritis. Neuropharmacology 2002; 42: 958–965
- Finn DP, Marti O, Harbuz MS, Valles A, Belda X, Marquez C, Jessop DS, Lalies MD, Armario A, Nutt DJ, Hudson AL. Behavioral, neuroendocrine and neurochemical effects of the imidazoline I2 receptor selective ligand BU224 in naive rats and rats exposed to the stress of the forced swim test. Psychopharmacology (Berl) 2003; 167: 195–202
- Finn DP, Hudson AL, Kinoshita H, Coventry TL, Jessop DS, Nutt DJ, Harbuz MS. Imidazoline 2 (I2) receptor- and alpha2-adrenoceptor-mediated modulation of hypothalamic–pituitary–adrenal axis activity in control and acute restraint stressed rats. J Psychopharmacol 2004; 18: 47–53
- Fossey MD, Lydiard RB, Ballenger JC, Laraia MT, Bissette G, Nemeroff CB. Cerebrospinal fluid corticotropin-releasing factor concentrations in patients with anxiety disorders and normal comparison subjects. Biol Psychiatry 1996; 39: 703–707
- Friedrich VL, Jr, Martinelli GP, Prell GD, Holstein GR. Distribution and cellular localization of imidazoleacetic acid-ribotide, an endogenous ligand at imidazol(in)e and adrenergic receptors, in rat brain. J Chem Neuroanat 2007; 33: 53–64
- Garcia-Sevilla JA, Escriba PV, Guimon J. Imidazoline receptors and human brain disorders. Ann NY Acad Sci 1999; 881: 392–409
- Garcia-Sevilla JA, Escriba PV, Sastre M, Walzer C, Busquets X, Jaquet G, Reis DJ, Guimon J. Immunodetection and quantitation of imidazoline receptor proteins in platelets of patients with major depression and in brains of suicide victims. Arch Gen Psychiatry 1996; 53: 803–810
- Ghadirian AM, Marcovitz S, Pearson Murphy BE. A case of seasonal bipolar disorder exacerbated by Cushing's disease. Compr Psychiatry 2005; 46: 155–158
- Ghadirian AM, Engelsmann F, Dhar V, Filipini D, Keller R, Chouinard G, Murphy BE. The psychotropic effects of inhibitors of steroid biosynthesis in depressed patients refractory to treatment. Biol Psychiatry 1995; 37: 369–375
- Gibbons JL, McHugh Pr. Plasma cortisol in depressive illness. J Psychiatr Res 1962; 1: 162–171
- Giraldi T, De Vanna M, Malagoli M, Tuveri G, Sutto K, Schillani G, Grassi L. Mental adaptation to cancer: Depression and blood platelet monoamine oxidase activity in breast cancer patients. Anticancer Res 2007; 27: 1715–1719
- Glennon RA, Dukat M, Grella B, Hong S, Costantino L, Teitler M, Smith C, Egan C, Davis K, Mattson MV. Binding of beta-carbolines and related agents at serotonin (5-HT(2) and 5-HT(1A)), dopamine (D(2)) and benzodiazepine receptors. Drug Alcohol Depend 2000; 60: 121–132
- Glue P, Wilson S, Campling GM, Knightly M, Franklin M, Cowen PJ, Nutt DJ. Alpha-2-adrenoceptor control of cortisol and ACTH in normal volunteers: Preliminary open trial of the effects of acute and chronic idazoxan. Psychoneuroendocrinology 1992; 17: 261–266
- Golanov EV, Reis DJ. A role for KATP+-channels in mediating the elevations of cerebral blood flow and arterial pressure by hypoxic stimulation of oxygen-sensitive neurons of rostral ventrolateral medulla. Brain Res 1999; 827: 210–214
- Gollan JK, Lee R, Coccaro EF. Developmental psychopathology and neurobiology of aggression. Dev Psychopathol 2005; 17: 1151–1171
- Gong ZH, Li YF, Zhao N, Yang HJ, Su RB, Luo ZP, Li J. Anxiolytic effect of agmatine in rats and mice. Eur J Pharmacol 2006; 550: 112–116
- Gothert M, Molderings GJ, Fink K, Schlicker E. Alpha 2-adrenoceptor-independent inhibition by imidazolines and guanidines of noradrenaline release from peripheral, but not central noradrenergic neurons. Ann NY Acad Sci 1995; 763: 405–419
- Greden JF, Gardner R, King D, Grunhaus L, Carroll BJ, Kronfol Z. Dexamethasone suppression tests in antidepressant treatment of melancholia. The process of normalization and test-retest reproducibility. Arch Gen Psychiatry 1983; 40: 493–500
- Grigoriadis DE. The corticotropin-releasing factor receptor: A novel target for the treatment of depression and anxiety-related disorders. Expert Opin Ther Targets 2005; 9: 651–684
- Gruen R, Baron M. Stressful life events and schizophrenia. Relation to illness onset and family history. Neuropsychobiology 1984; 12: 206–208
- Halaris A, Piletz JE. Imidazoline receptors: Possible involvement in the pathophysiology and treatment of depression. Hum Psychopharmacol 2001; 16: 65–69
- Halaris A, Piletz JE. Relevance of imidazoline receptors and agmatine to psychiatry: A decade of progress. Ann NY Acad Sci 2003; 1009: 1–20
- Halaris A, Zhu H, Ali J, Nasrallah A, Lindsay De Vane C, Piletz JE. Down-regulation of platelet imidazoline-1-binding sites after bupropion treatment. Int J Neuropsychopharmacol 2002; 5: 37–46
- Head GA, Burke SL. Sympathetic responses to stress and rilmenidine in 2K1C rabbits: Evidence of enhanced nonvascular effector mechanism. Hypertension 2004; 43: 636–642
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology 2004; 29: 641–648
- Herraiz T, Chaparro C. Human monoamine oxidase is inhibited by tobacco smoke: Beta-carboline alkaloids act as potent and reversible inhibitors. Biochem Biophys Res Commun 2005; 326: 378–386
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 2000; 23: 477–501
- Holt A, Wieland B, Baker GB. Allosteric modulation of semicarbazide-sensitive amine oxidase activities in vitro by imidazoline receptor ligands. Br J Pharmacol 2004; 143: 495–507
- Hudson CJ, Young LT, Li PP, Warsh JJ. CNS signal transduction in the pathophysiology and pharmacotherapy of affective disorders and schizophrenia. Synapse 1993; 13: 278–293
- Hudson AL, Chapleo CB, Lewis JW, Husbands S, Grivas K, Mallard NJ, Nutt DJ. Identification of ligands selective for central I2-imidazoline binding sites. Neurochem Int 1997; 30: 47–53
- Hudson AL, Gough R, Tyacke R, Lione L, Lalies M, Lewis J, Husbands S, Knight P, Murray F, Hutson P, Nutt DJ. Novel selective compounds for the investigation of imidazoline receptors. Ann NY Acad Sci 1999; 881: 81–91
- Ising M, Holsboer F. CRH-sub-1 receptor antagonists for the treatment of depression and anxiety. Exp Clin Psychopharmacol 2007; 15: 519–528
- Isolan LR, Zeni CP, Mezzomo K, Blaya C, Kipper L, Heldt E, Manfro GG. Behavioral inhibition and history of childhood anxiety disorders in Brazilian adult patients with panic disorder and social anxiety disorder. Rev Bras Psiquiatr 2005; 27: 97–100
- Ivanov TR, Zhu H, Regunathan S, Reis DJ, Dontenwill M, Vonthron C, Bousquet P, Piletz JE. Co-detection by two imidazoline receptor protein antisera of a novel 85 kilodalton protein. Biochem Pharmacol 1998; 55: 649–655
- Joca SR, Ferreira FR, Guimaraes FS. Modulation of stress consequences by hippocampal monoaminergic, glutamatergic and nitrergic neurotransmitter systems. Stress 2007; 10: 227–249
- Jolkkonen J, Lepola U, Bissette G, Nemeroff C, Riekkinen P. CSF corticotropin-releasing factor is not affected in panic disorder. Biol Psychiatry 1993; 33: 136–138
- Jope RS, Williams MB. Lithium and brain signal transduction systems. Biochem Pharmacol 1994; 47: 429–441
- Judge SJ, Ingram CD, Gartside SE. Moderate differences in circulating corticosterone alter receptor-mediated regulation of 5-hydroxytryptamine neuronal activity. J Psychopharmacol 2004; 18: 475–483
- Kageyama K, Tozawa F, Horiba N, Watanobe H, Suda T. Serotonin stimulates corticotropin-releasing factor gene expression in the hypothalamic paraventricular nucleus of conscious rats. Neurosci Lett 1998; 243: 17–20
- Kalin NH, Shelton SE, Turner JG. Effects of beta-carboline on fear-related behavioral and neurohormonal responses in infant rhesus monkeys. Biol Psychiatry 1992; 31: 1008–1019
- Kendler KS, Karkowski-Shuman L. Stressful life events and genetic liability to major depression: Genetic control of exposure to the environment?. Psychol Med 1997; 27: 539–547
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 1999; 156: 837–841
- Kim H, Sablin SO, Ramsay RR. Inhibition of monoamine oxidase A by beta-carboline derivatives. Arch Biochem Biophys 1997; 337: 137–142
- King PR, Gundlach AL, Louis WJ. Quantitative autoradiographic localization in rat brain of alpha 2-adrenergic and non-adrenergic I-receptor binding sites labelled by [3H]rilmenidine. Brain Res 1995; 675: 264–278
- de Kloet CS, Vermetten E, Heijnen CJ, Geuze E, Lentjes EG, Westenberg HG. Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology 2007; 32: 215–226
- de Kloet CS, Vermetten E, Geuze E, Lentjes EG, Heijnen CJ, Stalla GK, Westenberg HG. Elevated plasma corticotrophin-releasing hormone levels in veterans with posttraumatic stress disorder. Prog Brain Res 2008; 167: 287–291
- Krishnan KR, Doraiswamy PM, Lurie SN, Figiel GS, Husain MM, Boyko OB, Ellinwood EH, Jr, Nemeroff CB. Pituitary size in depression. J Clin Endocrinol Metab 1991; 72: 256–259
- Lahniers CE, White K. Changes in environmental life events and their relationship to psychiatric hospital admissions. J Nerv Ment Dis 1976; 163: 154–158
- Laubie M, Poignant JC, Scuvee-Moreau J, Dabire H, Dresse A, Schmitt H. Pharmacological properties of (N-dicyclopropylmethyl) amino-2-oxazoline (S 3341), an alpha-2 adrenoceptor agonist. J Pharmacol 1985; 16: 259–278
- Lavinsky D, Arteni NS, Netto CA. Agmatine induces anxiolysis in the elevated plus maze task in adult rats. Behav Brain Res 2003; 141: 19–24
- Li D, He L. Meta-study on association between the monoamine oxidase A gene (MAOA) and schizophrenia. Am J Med Genet B Neuropsychiatr Genet 2007
- Lione LA, Nutt DJ, Hudson AL. Characterisation and localisation of [3H]2-(2-benzofuranyl)-2-imidazoline binding in rat brain: A selective ligand for imidazoline I2 receptors. Eur J Pharmacol 1998; 353: 123–135
- Liposits Z, Uht RM, Harrison RW, Gibbs FP, Paull WK, Bohn MC. Ultrastructural localization of glucocorticoid receptor (GR) in hypothalamic paraventricular neurons synthesizing corticotropin releasing factor (CRF). Histochemistry 1987; 87: 407–412
- Li YF, Gong ZH, Cao JB, Wang HL, Luo ZP, Li J. Antidepressant-like effect of agmatine and its possible mechanism. Eur J Pharmacol 2003; 469: 81–88
- MacInnes N, Handley SL. Autoradiographic localisation of [3H]2-BFI imidazoline I2 binding sites in mouse brain. Eur J Pharmacol 2005; 516: 139–144
- MacKinnon AC, Redfern WS, Brown CM. [3H]-RS-45041-190: A selective high-affinity radioligand for I2 imidazoline receptors. Br J Pharmacol 1995; 116: 1729–1736
- Manji HK. G proteins: Implications for psychiatry. Am J Psychiatry 1992; 149: 746–760
- Marti O, Armario A. Anterior pituitary response to stress: Time-related changes and adaptation. Int J Dev Neurosci 1998; 16: 241–260
- Mayer KH, Stamler J, Dyer A, Freinkel N, Stamler R, Berkson DM, Farber B. Epidemiologic findings on the relationship of time of day and time since last meal to glucose tolerance. Diabetes 1976; 25: 936–943
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 2007; 87: 873–904
- Meana JJ, Herrera-Marschitz M, Goiny M, Silveira R. Modulation of catecholamine release by alpha 2-adrenoceptors and I1-imidazoline receptors in rat brain. Brain Res 1997; 744: 216–226
- Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Anisman H. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci 2004; 24: 1478–1485
- Mest HJ, Raap A, Schloos J, Treinies I, Paal M, Giese U, Koivisto V. Glucose-induced insulin secretion is potentiated by a new imidazoline compound. Naunyn Schmiedebergs Arch Pharmacol 2001; 364: 47–52
- Michelson D, Galliven E, Hill L, Demitrack M, Chrousos G, Gold P. Chronic imipramine is associated with diminished hypothalamic–pituitary–adrenal axis responsivity in healthy humans. J Clin Endocrinol Metab 1997; 82: 2601–2606
- Modell S, Yassouridis A, Huber J, Holsboer F. Corticosteroid receptor function is decreased in depressed patients. Neuroendocrinology 1997; 65: 216–222
- Molderings GJ, Likungu J, Jakschik J, Gothert M. Presynaptic imidazoline receptors and non-adrenoceptor [3H]-idazoxan binding sites in human cardiovascular tissues. Br J Pharmacol 1997; 122: 43–50
- Morgan NG, Chan SL. Imidazoline binding sites in the endocrine pancreas: Can they fulfil their potential as targets for the development of new insulin secretagogues?. Curr Pharm Des 2001; 7: 1413–1431
- Morgan NG, Chan SL, Mourtada M, Monks LK, Ramsden CA. Imidazolines and pancreatic hormone secretion. Ann NY Acad Sci 1999; 881: 217–228
- Mourtada M, Smith SA, Morgan NG. Insulin secretagogues with an imidazoline structure inhibit arginine-induced secretion from isolated glucagon secretion from isolated rat islets of Langerhans. Biochem Biophys Res Commun 1997; 236: 162–166
- Muller WE, Fehske KJ, Borbe HO, Wollert U, Nanz C, Rommelspacher H. On the neuropharmacology of harmane and other beta-carbolines. Pharmacol Biochem Behav 1981; 14: 693–699
- Munoz-Hoyos A, Fernandez-Garcia JM, Molina-Carballo A, Macias M, Escames G, Ruiz-Cosano C, Acuna-Castroviejo D. Effect of clonidine on plasma ACTH, cortisol and melatonin in children. J Pineal Res 2000; 29: 48–53
- Musgrave IF, Badoer E. Harmane produces hypotension following microinjection into the RVLM: Possible role of I(1)-imidazoline receptors. Br J Pharmacol 2000; 129: 1057–1059
- Nash MW, Sugden K, Huezo-Diaz P, Williamson R, Sterne A, Purcell S, Sham PC, Craig IW. Association analysis of monoamine genes with measures of depression and anxiety in a selected community sample of siblings. Am J Med Genet B Neuropsychiatr Genet 2005; 135: 33–37
- Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry 1988; 45: 577–579
- Nutt DJ, French N, Handley S, Hudson A, Husbands S, Jackson H, Jordan S, Lalies MD, Lewis J, Lione L, et al. Functional studies of specific imidazoline-2 receptor ligands. Ann NY Acad Sci 1995; 763: 125–139
- Nutt DJ, Lalies MD, Lione LA, Hudson AL. Noradrenergic mechanisms in the prefrontal cortex. J Psychopharmacol 1997; 11: 163–168
- O'Neill MF, Osborne DJ, Woodhouse SM, Conway MW. Selective imidazoline I2 ligands do not show antidepressant-like activity in the forced swim test in mice. J Psychopharmacol 2001; 15: 18–22
- Olmos G, Gabilondo AM, Miralles A, Escriba PV, Garcia-Sevilla JA. Chronic treatment with the monoamine oxidase inhibitors clorgyline and pargyline down-regulates non-adrenoceptor [3H]-idazoxan binding sites in the rat brain. Br J Pharmacol 1993; 108: 597–603
- Olmos G, Alemany R, Boronat MA, Garcia-Sevilla JA. Pharmacologic and molecular discrimination of I2-imidazoline receptor subtypes. Ann NY Acad Sci 1999; 881: 144–160
- Pacak K. Stressor-specific activation of the hypothalamic–pituitary–adrenocortical axis. Physiol Res 2000; 49(Suppl 1)S11–S17
- Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: Implications for stress-related disorders. Endocr Rev 2001; 22: 502–548
- Pacheco MA, Stockmeier C, Meltzer HY, Overholser JC, Dilley GE, Jope RS. Alterations in phosphoinositide signaling and G-protein levels in depressed suicide brain. Brain Res 1996; 723: 37–45
- Pariante CM, Papadopoulos AS, Poon L, Cleare AJ, Checkley SA, English J, Kerwin RW, Lightman S. Four days of citalopram increase suppression of cortisol secretion by prednisolone in healthy volunteers. Psychopharmacology (Berl) 2004; 177: 200–206
- Parini A, Moudanos CG, Pizzinat N, Lanier SM. The elusive family of imidazoline binding sites. Trends Pharmacol Sci 1996; 17: 13–16
- Parker CA, Hudson AL, Nutt DJ, Dillon MP, Eglen RM, Crosby J. Isolation of RP-HPLC pure clonidine-displacing substance from NG108-15 cells. Eur J Pharmacol 2000; 387: 27–30
- Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav 2003; 43: 60–66
- Parker CA, Anderson NJ, Robinson ES, Price R, Tyacke RJ, Husbands SM, Dillon MP, Eglen RM, Hudson AL, Nutt DJ, Crump MP, Crosby J. Harmane and harmalan are bioactive components of classical clonidine-displacing substance. Biochemistry 2004; 43: 16385–16392
- Paterson L.M., Tyacke R.J., Robinson E.S., Nutt D.J., Hudson A.L. In vitro and in vivo effect of BU99006 (5-isothiocyanato-2-benzofuranyl-2-imidazoline) on I(2) binding in relation to MAO: Evidence for two distinct I(2) binding sites. Neuropharmacology 2006; 52: 395–404
- Piletz J, Baker R, Halaris A. Platelet imidazoline receptors as state marker of depressive symptomatology. J Psychiatr Res 2008; 42: 41–49
- Piletz JE, Halaris A, Saran A, Marler MR. Desipramine lowers tritiated para-aminoclonidine binding in platelets of depressed patients. Arch Gen Psychiatry 1991; 48: 813–820
- Piletz JE, Halaris A, Ernsberger PR. Psychopharmacology of imidazoline and alpha 2-adrenergic receptors: Implications for depression. Crit Rev Neurobiol 1994; 9: 29–66
- Piletz JE, Halaris A, Nelson J, Qu Y, Bari M. Platelet I1-imidazoline binding sites are elevated in depression but not generalized anxiety disorder. J Psychiatr Res 1996a; 30: 147–168
- Piletz JE, Halaris AE, Chikkala D, Qu Y. Platelet I1-imidazoline binding sites are decreased by two dissimilar antidepressant agents in depressed patients. J Psychiatr Res 1996b; 30: 169–184
- Piletz JE, Ivanov TR, Sharp JD, Ernsberger P, Chang CH, Pickard RT, Gold G, Roth B, Zhu H, Jones JC, Baldwin J, Reis DJ. Imidazoline receptor antisera-selected (IRAS) cDNA: Cloning and characterization. DNA Cell Biol 2000; 19: 319–329
- Pineda J, Ugedo L, Garcia-Sevilla JA. Stimulatory effects of clonidine, cirazoline and rilmenidine on locus coeruleus noradrenergic neurones: Possible involvement of imidazoline-preferring receptors. Naunyn Schmiedebergs Arch Pharmacol 1993; 348: 134–140
- Plotsky PM. Facilitation of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation after activation of catecholaminergic pathways or central norepinephrine injection. Endocrinology 1987; 121: 924–930
- Popma A, Doreleijers TA, Jansen LM, Van Goozen SH, Van Engeland H, Vermeiren R. The diurnal cortisol cycle in delinquent male adolescents and normal controls. Neuropsychopharmacology 2007; 32: 1622–1628
- Prell GD, Martinelli GP, Holstein GR, Matulic-Adamic J, Watanabe KA, Chan SL, Morgan NG, Haxhiu MA, Ernsberger P. Imidazoleacetic acid-ribotide: An endogenous ligand that stimulates imidazol(in)e receptors. Proc Natl Acad Sci USA 2004; 101: 13677–13682
- Raddatz R, Parini A, Lanier SM. Imidazoline/guanidinium binding domains on monoamine oxidases. Relationship to subtypes of imidazoline-binding proteins and tissue-specific interaction of imidazoline ligands with monoamine oxidase B. J Biol Chem 1995; 270: 27961–27968
- Reddig PJ, Xu D, Juliano RL. Regulation of p21-activated kinase-independent Rac1 signal transduction by nischarin. J Biol Chem 2005; 280: 30994–31002
- Regunathan S, Meeley MP, Reis DJ. Clonidine-displacing substance from bovine brain binds to imidazoline receptors and releases catecholamines in adrenal chromaffin cells. Mol Pharmacol 1991; 40: 884–888
- Reis DJ, Regunathan S. Is agmatine a novel neurotransmitter in brain?. Trends Pharmacol Sci 2000; 21: 187–193
- Reis DJ, Piletz JE. The imidazoline receptor in control of blood pressure by clonidine and allied drugs. Am J Physiol 1997; 273: R1569–R1571
- Renouard A, Widdowson PS, Cordi A. [3H]-idazoxan binding to rabbit cerebral cortex recognises multiple imidazoline I2-type receptors: Pharmacological characterization and relationship to monoamine oxidase. Br J Pharmacol 1993; 109: 625–631
- Ribeiro SC, Tandon R, Grunhaus L, Greden JF. The DST as a predictor of outcome in depression: A meta-analysis. Am J Psychiatry 1993; 150: 1618–1629
- Robinson ES, Tyacke RJ, Nutt DJ, Hudson AL. Distribution of [(3)H]BU224, a selective imidazoline I(2) binding site ligand, in rat brain. Eur J Pharmacol 2002; 450: 55–60
- Rommelspacher H, May T, Salewski B. Harman (1-methyl-beta-carboline) is a natural inhibitor of monoamine oxidase type A in rats. Eur J Pharmacol 1994; 252: 51–59
- Rubin RT, Phillips JJ, McCracken JT, Sadow TF. Adrenal gland volume in major depression: Relationship to basal and stimulated pituitary–adrenal cortical axis function. Biol Psychiatry 1996; 40: 89–97
- Ruiz-Durantez E, Ruiz-Ortega JA, Pineda J, Ugedo L. Stimulatory effect of harmane and other beta-carbolines on locus coeruleus neurons in anaesthetized rats. Neurosci Lett 2001; 308: 197–200
- Ruiz-Ortega JA, Ugedo L. The stimulatory effect of clonidine on locus coeruleus neurons of rats with inactivated alpha 2-adrenoceptors: Involvement of imidazoline receptors located in the nucleus paragigantocellularis. Naunyn Schmiedebergs Arch Pharmacol 1997; 355: 288–294
- Sanchez-Blazquez P, Boronat MA, Olmos G, Garcia-Sevilla JA, Garzon J. Activation of I(2)-imidazoline receptors enhances supraspinal morphine analgesia in mice: a model to detect agonist and antagonist activities at these receptors. Br J Pharmocol 2000; 130: 146–152
- Samad N, Perveen T, Haider S, Haleem MA, Haleem DJ. Inhibition of restraint-induced neuroendocrine and serotonergic responses by buspirone in rats. Pharmacol Rep 2006; 58: 636–642
- Sano H, Liu SC, Lane WS, Piletz JE, Lienhard GE. Insulin receptor substrate 4 associates with the protein IRAS. J Biol Chem 2002; 277: 19439–19447
- Saphier D, Feldman S. Catecholaminergic projections to tuberoinfundibular neurones of the paraventricular nucleus: II. Effects of stimulation of the ventral noradrenergic ascending bundle: Evidence for cotransmission. Brain Res Bull 1989; 23: 397–404
- Saphier D, Welch JE. Central stimulation of adrenocortical secretion by 5-hydroxytryptamine1 A agonists is mediated by sympathomedullary activation. J Pharmacol Exp Ther 1994; 270: 905–917
- Saphier D, Farrar GE, Welch JE. Differential inhibition of stress-induced adrenocortical responses by 5-HT1A agonists and by 5-HT2 and 5-HT3 antagonists. Psychoneuroendocrinology 1995; 20: 239–257
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 2000; 21: 55–89
- Sastre-Coll A, Esteban S, Miralles A, Zanetti R, Garcia-Sevilla JA. The imidazoline receptor ligand 2-(2-benzofuranyl)-2-imidazoline is a dopamine-releasing agent in the rat striatum in vivo. Neurosci Lett 2001; 301. 32
- Sastre M, Escriba PV, Reis DJ, Garcia-Sevilla JA. Decreased number and immunoreactivity of I2-imidazoline receptors in the frontal cortex of suicide victims. Ann NY Acad Sci 1995; 763: 520–522
- Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, Johnson JE, Cerbone A, Malaspina D. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry 2003; 54: 1382–1388
- Schelling G, Kilger E, Roozendaal B, de Quervain DJ, Briegel J, Dagge A, Rothenhausler HB, Krauseneck T, Nollert G, Kapfhammer HP. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: A randomized study. Biol Psychiatry 2004; 55: 627–633
- Schmidt U, Frerick H, Kraft K, Schenk N, Löw-Kröger A. Hypertension: A possible risk in road traffic. J Cardiovasc Pharmacol 1992; 20: S50–S56
- Scott LV, Dinan TG. Vasopressin and the regulation of hypothalamic–pituitary–adrenal axis function: Implications for the pathophysiology of depression. Life Sci 1998; 62: 1985–1998
- Separovic D, Kester M, Ernsberger P. Coupling of I1-imidazoline receptors to diacylglyceride accumulation in PC12 rat pheochromocytoma cells. Mol Pharmacol 1996; 49: 668–675
- Separovic D, Kester M, Haxhiu MA, Ernsberger P. Activation of phosphatidylcholine-selective phospholipase C by I1-imidazoline receptors in PC12 cells and rostral ventrolateral medulla. Brain Res 1997; 749: 335–339
- Silverman AJ, Hou-Yu A, Chen WP. Corticotropin-releasing factor synapses within the paraventricular nucleus of the hypothalamus. Neuroendocrinology 1989; 49: 291–299
- Smoller JW, Yamaki LH, Fagerness JA, Biederman J, Racette S, Laird NM, Kagan J, Snidman N, Faraone SV, Hirshfeld-Becker D, Tsuang MT, Slaugenhaupt SA, Rosenbaum JF, Sklar PB. The corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorder. Biol Psychiatry 2005; 57: 1485–1492
- Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, Roozendaal B, de Quervain DJ. Glucocorticoids reduce phobic fear in humans. Proc Natl Acad Sci USA 2006; 103: 5585–5590
- Squires PE, Hills CE, Rogers GJ, Garland P, Farley SR, Morgan NG. The putative imidazoline receptor agonist, harmane, promotes intracellular calcium mobilisation in pancreatic beta-cells. Eur J Pharmacol 2004; 501: 31–39
- Sun Z, Chang CH, Ernsberger P. Identification of IRAS/Nischarin as an I1-imidazoline receptor in PC12 rat pheochromocytoma cells. J Neurochem 2007; 101: 99–108
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: An immunohistochemical study. Neuroendocrinology 1983; 36: 165–186
- Szabo B, Urban R. Mechanism of sympathoinhibition by imidazolines. Ann NY Acad Sci 1995; 763: 552–565
- Szabo B. Imidazoline antihypertensive drugs: A critical review on their mechanism of action. Pharmacol Ther 2002; 93: 1–35
- Takada K, Hayashi Y, Kamibayashi T, Mammoto T, Yamatodani A, Kitamura S, Yoshiya I. The involvement of pertussis toxin-sensitive G proteins in the post receptor mechanism of central I1-imidazoline receptors. Br J Pharmacol 1997; 120: 1575–1581
- Talalaenko AN, Krivobok GK, Pankrat'ev DV, Goncharenko NV. Neurochemical mechanisms of the dorsal pallidum in the antiaversive effects of anxiolytics in various models of anxiety. Neurosci Behav Physiol 2006; 36: 749–754
- Teixeira de Castro RR, Tibirica E, de Oliveira MA, Moreira PB, Catelli MF, Rocha MN, Nobrega AC. Reduced hemodynamic responses to physical and mental stress under low-dose rilmenidine in healthy subjects. Cardiovasc Drugs Ther 2006; 20: 129–134
- Tesson F, Limon-Boulez I, Urban P, Puype M, Vandekerckhove J, Coupry I, Pompon D, Parini A. Localization of I2-imidazoline binding sites on monoamine oxidases. J Biol Chem 1995; 270: 9856–9861
- Thomas B, Prell GD. Imidazoleacetic acid, a gamma-aminobutyric acid receptor agonist, can be formed in rat brain by oxidation of histamine. J Neurochem 1995; 65: 818–826
- Thomson F, Craighead M. Innovative approaches for the treatment of depression: Targeting the HPA axis. Neurochem Res 2007
- Tibirica E, Mermet C, Feldman J, Gonon F, Bousquet P. Correlation between the inhibitory effect on catecholaminergic ventrolateral medullary neurons and the hypotension evoked by clonidine: A voltammetric approach. J Pharmacol Exp Ther 1989; 250: 642–647
- Touiki K, Rat P, Molimard R, Chait A, de Beaurepaire R. Harmane inhibits serotonergic dorsal raphe neurons in the rat. Psychopharmacology (Berl) 2005; 182: 562–569
- Tunnicliff G. Pharmacology and function of imidazole 4-acetic acid in brain. Gen Pharmacol 1998; 31: 503–509
- Ugedo L, Pineda J, Ruiz-Ortega JA, Martin-Ruiz R. Stimulation of locus coeruleus neurons by non-I1/I2-type imidazoline receptors: An in vivo and in vitro electrophysiological study. Br J Pharmacol 1998; 125: 1685–1694
- Ugedo L, Pineda J, Martin-Ruiz R, Ruiz-Ortega JA, Artigas F. Imidazoline-induced inhibition of firing rate of 5-HT neurons in rat dorsal raphe by modulation of extracellular 5-HT levels. Ann NY Acad Sci 1999; 881: 365–368
- Valentino RJ, Curtis AL. Pharmacology of locus coeruleus spontaneous and sensory-evoked activity. Prog Brain Res 1991; 88: 249–256
- Van Den Eede F, Van Broeckhoven C, Claes SJ. Corticotropin-releasing factor-binding protein, stress and major depression. Ageing Res Rev 2005; 4: 213–239
- Wang LG, Gao L, Wang W, Yuan WJ, Wang WZ. Sympathoexcitation of moxonidine in the caudal ventrolateral medulla is dependent on I1-imidazoline receptors in anesthetized rats. Neurosci Lett 2007; 426: 91–96
- Welch JE, Saphier D. Central and peripheral mechanisms in the stimulation of adrenocortical secretion by the 5-hydroxytryptamine2 agonist, (+)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane. J Pharmacol Exp Ther 1994; 270: 918–928
- Welch JE, Farrar GE, Dunn AJ, Saphier D. Central 5-HT1A receptors inhibit adrenocortical secretion. Neuroendocrinology 1993; 57: 272–281
- Wenzel RR, Mitchell A, Siffert W, Buhrmann S, Philipp T, Schafers RF. The I1-imidazoline agonist moxonidine decreases sympathetic tone under physical and mental stress. Br J Clin Pharmacol 2004; 57: 545–551
- Wolkowitz OM, Reus VI, Chan T, Manfredi F, Raum W, Johnson R, Canick J. Antiglucocorticoid treatment of depression: Double-blind ketoconazole. Biol Psychiatry 1999; 45: 1070–1074
- Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology 2006; 31: 912–924
- Yehuda R, Yang RK, Buchsbaum MS, Golier JA. Alterations in cortisol negative feedback inhibition as examined using the ACTH response to cortisol administration in PTSD. Psychoneuroendocrinology 2006; 31: 447–451
- Yu YW, Tsai SJ, Hong CJ, Chen TJ, Chen MC, Yang CW. Association study of a monoamine oxidase a gene promoter polymorphism with major depressive disorder and antidepressant response. Neuropsychopharmacology 2005; 30: 1719–1723
- Zacharko RM, Anisman H. Stressor-induced anhedonia in the mesocorticolimbic system. Neurosci Biobehav Rev 1991; 15: 391–405
- Zeidan MP, Zomkowski AD, Rosa AO, Rodrigues AL, Gabilan NH. Evidence for imidazoline receptors involvement in the agmatine antidepressant-like effect in the forced swimming test. Eur J Pharmacol 2007; 565: 125–131
- Zhang J, Abdel-Rahman AA. Mitogen-activated protein kinase phosphorylation in the rostral ventrolateral medulla plays a key role in imidazoline (i1)-receptor-mediated hypotension. J Pharmacol Exp Ther 2005; 314: 945–952
- Zhang J, Abdel-Rahman AA. Nischarin as a functional imidazoline (I1) receptor. FEBS Lett 2006; 580: 3070–3074
- Zhang J, El-Mas MM, Abdel-Rahman AA. Imidazoline I(1) receptor-induced activation of phosphatidylcholine-specific phospholipase C elicits mitogen-activated protein kinase phosphorylation in PC12 cells. Eur J Pharmacol 2001; 415: 117–125
- Zhu H, Halaris A, Piletz JE. Chronic imipramine treatment downregulates IR1-imidazoline receptors in rat brainstem. Life Sci 1997a; 61: 1973–1983
- Zhu H, Paul IA, McNamara M, Redmond A, Nowak G, Piletz JE. Chronic imipramine treatment upregulates IR2-imidazoline receptive sites in rat brain. Neurochem Int 1997b; 30: 101–107
- Zhu H, Halaris A, Madakasira S, Pazzaglia P, Goldman N, DeVane CL, Andrew M, Reis D, Piletz JE. Effect of bupropion on immunodensity of putative imidazoline receptors on platelets of depressed patients. J Psychiatr Res 1999; 33: 323–333
- Zhu H, Hayes J, Chen M, Baldwin J, Piletz JE. Relationship between platelet imidazoline receptor-binding peptides and candidate imidazoline-1 receptor, IRAS. Ann NY Acad Sci 2003; 1009: 439–446
- Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, Holsboer F. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R12 in major depression: The first 20 patients treated. J Psychiatr Res 2000; 34: 171–181
- Zobel AW, Nickel T, Sonntag A, Uhr M, Holsboer F, Ising M. Cortisol response in the combined dexamethasone/CRH test as predictor of relapse in patients with remitted depression. a prospective study. J Psychiatr Res 2001; 35: 83–94
- Zomkowski AD, Hammes L, Lin J, Calixto JB, Santos AR, Rodrigues AL. Agmatine produces antidepressant-like effects in two models of depression in mice. Neuroreport 2002; 13: 387–391