Abstract
The aim of this study was to analyze the effects of chronic mild unpredictable stress (CMS) on the vasoconstrictor response and morphology of the thoracic aorta and serum lipid profiles in rats. Male Sprague–Dawley rats were submitted to CMS, which consisted of the application of different stressors for 7 days per week across 3 weeks. The rats were sacrificed 15 days after CMS expsoure. CMS induced supersensitivity to the vasoconstrictor effect of phenylephrine in endothelium-intact thoracic aortic rings without changes in aortic rings without endothelium, or pre-incubated with nitric oxide (NO) synthesis inhibitor. Rats submitted to CMS showed hypertrophy of the intima and tunica media of thoracic aorta, increased serum levels of triglycerides, total cholesterol, very low-density lipoprotein cholesterol, low-density lipoprotein cholesterol and atherogenic index, without changes in high-density lipoprotein cholesterol levels, when compared with control rats. These data indicate that CMS induces physiological and morphological changes that may contribute to the development of atherosclerosis by mechanisms related to deficiency in NO production and dyslipidemia.
Introduction
During the stress reaction, the sympathetic nervous system and hypothalamic–pituitary–adrenal axis are stimulated and promote adaptive metabolic, cardiovascular and behavioral responses. However, negative effects may occur if glucocorticoid and adrenergic stimulation are maintained without adaptation, in particular under conditions of chronic stress when allostasis can shift a healthy condition toward a pathological state (Broto Citation2003). Consequently, gastrointestinal, psychological and cardiovascular symptoms are observed. With regard to cardiovascular effects, stress plays an important role in the development of atherosclerosis, which is the most important vascular disease and is the main cause of death in western industrialized countries (Hauss et al. Citation1990). Hence the refinement of preventive and therapeutic strategies depends on understanding the mechanisms of the cardiovascular effects of stress.
In this context, animal models of chronic stress are used to study cardiovascular changes induced by stressors. Chronic social stress induces hypertension in laboratory rats (Bernatova and Csizmadiova Citation2006) and reduces the activity of nitric oxide (NO) synthase in the aorta of stressed borderline hypertensive rats (Okruhlicová et al. Citation2008). Chronic stress imposed by restraint and exposure to rat odor induces atherosclerosis in mice (Kumari et al. Citation2003). Chronic restraint and tail shock stress induce accumulation of subendothelial extracellular material in the rat aorta (Gordon et al. Citation1981), as well as hypertrophy of cellular nuclei and increased number of strangulated nuclei in the endothelium of the aorta of rats submitted to chronic stress by muscular activity (Gansburgskii Citation1985). Ricart-Jané et al. (Citation2002) reported that rats submitted to chronic stress by immobilization had increased plasma levels of total cholesterol (TC) and high-density lipoprotein (HDL), without changes in low-density lipoproteins (LDL) in comparison with rats stressed by acute immobilization.
The chronic mild unpredictable stress (CMS) model is characterized by absence of adaptation by rodents to repeated and unpredictable application of stressors. Sprague–Dawley rats submitted to CMS show increased heart rate, decreased heart rate variability and elevated sympathetic cardiac tone in vivo, 4 weeks after the CMS (Grippo et al. Citation2002, Citation2003). Decreased serum levels of TC, HDL, LDL and increased serum levels of triglycerides (TGL) have also been reported in Wistar rats, 6 weeks after CMS (Li et al. Citation2003).
Moreover, CMS is associated with behavioral changes, including reduced sensitivity to reward in rats (anhedonia; Grippo et al. Citation2005b), which is a major symptom of depression (Moreau Citation1997). CMS-induced anhedonia can last for up to 20 days after stressor exposure (Moreau et al. Citation1994). Since stress is involved in the development and maintenance of depression, which is an important risk factor for cardiovascular disease both in physically healthy individuals and in cardiac patients (Grippo et al. Citation2005a), the CMS model can be used in studies about both the behavioral and the cardiovascular effects of stress.
As a complementary tool for in vivo studies, pharmacological approaches are useful to evaluate vascular function. Acute swimming-stress induces subsensitivity to phenylephrine (PE) in the thoracic aorta isolated from male rats, and this effect is related to an increase in the endothelial synthesis of NO (Moura et al. Citation2003). Furthermore, chronic crowding stress induces supersensitivity to noradrenaline in the femoral arteries from rats (Bernatova et al. Citation2007).
Although it has been reported that chronic stress may induce morphological (Gordon et al. Citation1981) and functional (Bernatova et al. Citation2007) changes in vascular tissue, to our knowledge there are no data about the simultaneous evaluation of the effect of stress on the vasoconstrictor response and the morphology of the aorta in the same experimental protocol. This type of evaluation may help to clarify the mechanisms and temporal relations among stress, lipids and atherosclerosis.
Therefore, the aim of the present study was to evaluate the effects of the CMS protocol on the sensitivity of the thoracic aorta to PE, to the morphology of the thoracic aorta and to blood levels of lipids in rats, after 15 days of recovery from the CMS protocol (Moreau Citation1997).
Material and methods
Animals and experimental design
Male Sprague–Dawley rats (60 days old; 300–350 g), obtained from the Multidisciplinary Center for Biological Investigation of the State University of Campinas, were used. The rats were housed one per cage at 22 ± 2°C with lights on from 6 a.m. to 6 p.m. The rats received commercial rodent chow (Labina-Purina®) and filtered drinking water ad libitum. All procedures were approved by the Institutional Committee on Animal Research Ethics (CEEA–UNICAMP/Protocol Number 900-1) and carried out in accordance with the norms of the Brazilian College of Animal Experimentation.
After an acclimatization period of 1 week in the animal facility, the rats were randomized into two groups: Control and CMS; and were then studied for 7 weeks (n = 10/group). In the CMS group, the stressors were applied during the 3rd, 4th and 5th weeks. During the 1st, 2nd, 6th and 7th week, the rats were submitted only to the procedures related to the normal animal care routine.
Stress protocol
The CMS protocol consisted of the application of different stressors for 7 days per week across three consecutive weeks (3rd, 4th and 5th weeks) of an experimental protocol lasting 7 weeks. The CMS protocol, modified from the methodology described by Moreau (Citation1997), is presented in . Control rats were submitted only to the procedures related to their normal care.
Table I. Chronic, mild, unpredictable stress procedure.
Blood collection and phenylephrine concentration-effect curves in aorta rings
Fifteen days after the end of the CMS protocol, the rats were killed by decapitation, without previous anaesthesia in order to avoid anesthetic-induced high corticosterone plasma levels (Vahl et al. Citation2005). Rats were removed from their home cages and decapitated within 8–10 s. Blood was collected from the trunk in heparin-coated tubes and also in tubes without anticoagulant. The blood collected in heparin-coated tubes was centrifuged (700g for 20 min at 2°C), and the plasma was frozen and stored at − 20°C until corticosterone radioimmunoassay The blood collected in glass tubes without heparin remained at ambient temperature for 2 h and was then centrifuged to separate the serum, which was later used to determine the serum concentrations of lipids.
Immediately after blood collection, the thoracic aorta was removed and dissected free of fatty and connective tissue. Two matched rings 3–5 mm long, taken from the same rat were obtained from the middle portion of each aorta and used for functional assays. One ring was manipulated carefully to avoid damaging the endothelium, and the intimal surface of the second was scraped gently with a scalpel blade to remove the endothelial layer (Moura and Marcondes Citation2001). The rings were suspended in a 20 ml organ bath containing Krebs–Henseleit solution with the following composition (mM): NaCl 115, KCl 4.6, CaCl2·2H2O 2.5, KH2PO4 1.2, MgSO4·7H2O 2.5, NaHCO3 25, glucose 11, ascorbic acid 0.1 and EDTA 0.04 (Moura and Marcondes Citation2001). The Krebs–Henseleit solution was kept at 37.0 ± 0.2°C and aerated continuously with a mixture of 95% O2 and 5% CO2 (pH 7.2–7.4).
After 60 min for stabilization, the intactness of the endothelium was assessed by determining the vasodilating response to 10− 6 M acetylcholine (ACh) in rings pre-contracted with PE (10− 7 M). Only aortic rings that presented 100% relaxation by ACh were considered as rings with intact endothelium. In the rings without endothelium, effectiveness of mechanical removal of the endothelium was confirmed by complete absence of response to ACh. Endothelium intact-aortic rings that presented lower than 100% relaxation in response to ACh, and endothelium denuded rings that presented some response to ACh were discarded. Aortic rings were rinsed three times with Krebs–Henseleit buffer and allowed to re-equilibrate for 45–60 min.
Cumulative concentration-effect curves (CEC) for PE were obtained by stepwise increases in the agonist concentration (0.5 log units). A maximum response was obtained when a 0.5 log unit increase in agonist concentration did not produce an additional response. Changes in thoracic aorta sensitivity to PE were evaluated by determining the concentration that produced 50% of the maximum response and were expressed as the mean negative logarithm (pD2; Miller et al. Citation1948).
To evaluate the role of NO in the modulation of thoracic aorta sensitivity to PE, rings with intact endothelium, obtained from different rats were incubated for 40 min with the NO synthesis inhibitor, NG-l-arginine-methyl-ester (l-NAME) (10− 5 M) before CECs were obtained. Isolation of thoracic rings, evaluation of endothelium integrity and concentration-effect measurements were performed as described above.
The stock solution of PE (Sigma Chemical®, St Louis, MO, USA) was dissolved in 2% ascorbic acid and stored at − 10°C for 1 week. The dilutions for the CECs were prepared immediately before use and later discarded. For the Krebs–Henseleit solution, standard salts (Merck®, Darmstadt, Germany) were used and prepared in distilled and deionized water. The quality of the water was assessed weekly by measuring its conductivity and pH.
Analytic methods and atherogenic index
Plasma corticosterone was assayed by radioimmunoassay, using the Coat-A-Count® Rat Corticosterone Kit (Diagnostic Products, Thousand Oaks, CA, USA), with sensitivity of 5.7 ng/ml; the intra- and inter-assay coefficients of variation were 4.3 and 5.8%, respectively. Serum TGL were determined by using a commercially available kit CELM®, with sensitivity of 0.7 μmol/L. Very-low-density lipoprotein (VLDL) was calculated from TGL by Friedewald's formula: TGL/5. Serum TC and HDL were determined by using a commercially available kit Laborlab®, with sensitivity of 0.14 and 0.5 μmol/l, respectively. Serum LDL was determined by Friedewald's formula: LDL = TC − HDL − (TGL × 0.2) (Friedewald et al. Citation1972). The Atherogenic index (AI) was determined by using the formula: AI = TC − HDL/TC in accordance with Kamgang et al. (Citation2005).
Tissue processing and morphometric study
The inferior third of the thoracic aorta from 5 mm above the diaphragm was immediately excised and placed in Karnovsky's fixative (Karnovsky Citation1965) for 6 h at 4°C. Next, the aorta fragments were washed in 0.1 M phosphate buffer, pH 7.3 and postfixed in 1% osmium tetroxide in 0.1 M phosphate buffer, pH 7.3, for 2 h at room temperature and dehydrated with 50, 70, 90 and 100% acetone. The fragments were embedded in Araldite-502 resin (Luft Citation1961) and polymerized for 48 h at 60°C.
Semi-thin (1 μm) transverse sections of the aorta were cut on a SORVALL® Porter-Blum MT2-B ultramicrotome with a glass knife, and collected at 10 μm intervals. The sections were stained with 0.5% toluidine blue in 1% sodium borate for 50 s followed by 1% basic fuchsin for 30 s. We obtained five sections of each aorta in five rats per group, totalling 25 sections in each studied group for analysis by light microscopy.
The measurements of internal diameter (ID), intima thickness (IT) and tunica media thickness (TMT) and total intima-media-thickness (IMT) of the thoracic aorta were made via a photomicroscope (Carl Zeiss, Oberkochen, West Germany) connected to a millimeter eyepiece (Ernest Leitz, Wetzlar, Germany, 12.5 × ), and using a millimeter ruler (Carl Zeiss 5 + 100/100 mm) for calibration. From each section, three measurements of the smaller diameter and three of the larger diameter were made to determine the mean ID. For these measurements a 1 × objective lens and 1.6 × optovar were used; the results are given in mm. In addition, 24 measurements were made from each section to calculate the IT and 24 measurements to calculate TMT. For the IT measurements a 100 × objective lens and 1.6 × optovar were used, and the intima limits were considered to be between the luminal surface and the internal elastic lamina. For the tunica media measurements a 10 × objective lens and 2.0 × optovar were used, and the tunica media limits were considered to be between the internal and external elastic lamina. To calculate the IMT, 24 measurements were made from each section, using a 25 × objective lens and 1.6 × optovar, considering the IMT limits to be between the luminal surface and external elastic lamina. The results of IT, TMT and IMT are shown in micrometers.
Statistical analysis
Statistical differences were determined by the Student's-t test with GraphPad Prism® 4 software (San Diego, CA, USA) License G3-A 14920-830. Differences were considered significant at p < 0.05. The results are presented as means ± SEM.
Results
Plasma concentration of corticosterone was significantly increased in the CMS group (21.29 ± 0.63 ng/ml) compared with the control group (6.18 ± 0.48 ng/ml) (p < 0.05; five rats per group), 15 days after the end of the CMS protocol.
shows the CEC for PE obtained in isolated thoracic aortas. As there was no difference in the dry-weight of the aorta rings or in the maximum response to PE between the control and CMS groups (p>0.05; ), the CEC were expressed as percentage of maximum response. In endothelium-intact aortic rings, CMS increased the pD2 value of PE when compared with the control, with a 3.8-fold shift to the left in the CEC (p < 0.05; ), with no effect in endothelium-denuded aortic rings (p>0.05; ), or in the presence of l-NAME (inhibitor of NO synthesis) (p>0.05; ).
Figure 1 Concentration-effect curves for phenylephrine (PE) obtained in endothelium-intact (A), endothelium-denuded (B) and in the presence of l-NAME (10− 5 M) in endothelium-intact (C) thoracic aorta rings isolated from rats submitted to chronic mild unpredictable stress (CMS), 15 days after the stress protocol, and controls (n = 5/group). *p < 0.05 (Student's-t test) vs. control group. pD2 = Negative logarithm of the molar concentration of agonist producing 50% of the maximum response. The values are expressed as means ± SEM.
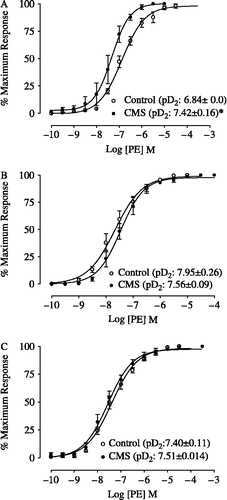
Table II. Dry-weight (DW) of aorta rings and Maximum Response (MR) to phenylephrine in Endothelium-Intact, Endothelium-Denuded Thoracic Aortic Rings and in the presence of l-NAME (10−5 M), isolated from Control and CMS groups.
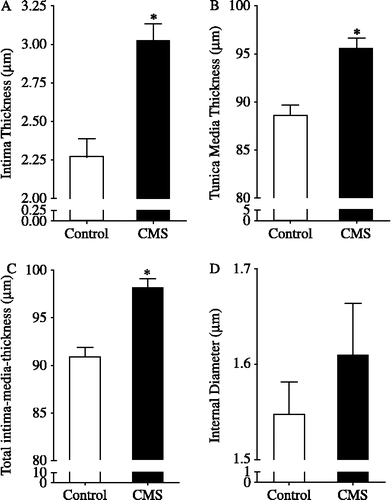
Thoracic aortas isolated from rats submitted to CMS presented a significant increase in the IT (p < 0.05; ) and TMT (p < 0.05; ) compared with control. Total IMT in the CMS group was significantly increased compared with the control group (p < 0.05; ), without change in the aorta ID (p > 0.05; ).
Figure 2 Intima (A), tunica media (B), total intima-media (C) thickness and internal diameter (D) of thoracic aortas isolated from rats submitted to chronic mild stress (CMS), 15 days after the stress protocol, and controls (n = 5/group). *p < 0.05 vs. control group. The values are expressed as means ± SEM.
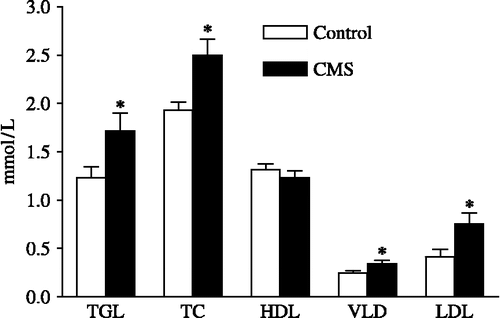
Serum concentrations of TGL, TC, VLDL and LDL were significantly greater in the CMS group (p < 0.05, ) with no statistical difference in the serum levels of HDL between the groups 15 days after the end of the CMS protocol (p>0.05). AI was significantly greater in the CMS rats (1.99 ± 0.20) than in the control rats (1.25 ± 0.09; p < 0.05).
Figure 3 Effects of CMS on serum concentrations of triglycerides (TGL), total cholesterol (TC), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL) and low-density lipoprotein (LDL) in rats submitted to chronic mild stress (CMS), 15 days after the stress protocol, and controls (n = 9–12/group). *p < 0.05 vs. control group. The values are expressed as means ± SEM.
Discussion
The data presented in this study show that chronic stress simultaneously induces functional and morphological changes in the rat thoracic aorta, and suggest that these functional effects may be related to decreased synthesis of NO. To our knowledge this is the first evaluation of this type in the same experimental protocol, thereby avoiding variations related to animal strain, quality and intensity of stressors.
The effectiveness of the stress protocol was confirmed by the significant increase in corticosterone plasma levels in rats submitted to CMS compared with the control group at 15 days after the end of the CMS protocol. This result shows that CMS is a useful protocol to study late effects of chronic stress in rats.
CMS induced supersensitivity to PE in endothelium-intact aorta rings, but not in the endothelium-denuded aorta, indicating that the effect of CMS was endothelium-dependent. Since there was no difference between groups in the CECs obtained in the presence of l-NAME, our data also indicate that stress-induced supersensitivity to PE seems to be related to a decrease in NO production by the endothelium. Furthermore, considering that the pD2 values did not differ between the curves obtained in the presence and in the absence of l-NAME in aortic rings from rats submitted to chronic stress, endothelial NO synthase (eNOS) seems to have been inhibited by some other factor in vivo, not related to the addition of l-NAME in the incubation bath.
The data obtained in the present study are partially in agreement with the effects of chronic crowding stress in Wistar and spontaneously hypertensive rats (SHR) reported by Bernatova et al. (Citation2007). Femoral arteries isolated from SHR and Wistar rats submitted to chronic crowding stress for 8 weeks showed increased noradrenaline-induced vasoconstriction. Moreover, crowding reduced aortic eNOS activity in the thoracic aorta from SHR rats, without changes in the tissue isolated from Wistar rats. However, acute immobilization (Cordellini and Vassilieff Citation1998) and swimming stress (Moura et al. Citation2003) seem to increase eNOS in Wistar rats.
Endothelial NO plays an important role in the regulation of vascular contractile activity. Therefore, a decrease in NO availability may exert an opposite effect to relaxation (Garg and Hassid Citation1989). The results obtained in the present study, considered with the above-mentioned studies, suggest that stress can modulate endothelial NO synthesis and that this effect depends on the stressor characteristics.
According to Grunfeld and Eloy (Citation1987), endogenous glucocorticoids increase vascular reactivity to noradrenaline. Glucocorticoids reduce the expression of guanosine triphosphate cyclohydrolase 1 mRNA, which is necessary for the synthesis of tetrahydrobiopterin cofactor (Mitchell et al. Citation2003, Citation2004), essential for stabilizing eNOS. Thus, greater levels of corticosterone observed in rats submitted to CMS could be related to the supersensitivity of thoracic aorta to PE.
In the present study, in vitro functional changes observed in the thoracic aorta were associated with a significant increase in the total IMT, with significant IT as well as of TMT in the CMS group. The IMT of the carotid artery is a clinically important parameter to quantify, via ultrasound, in patients to diagnose and to follow up all phases of atherosclerosis (Almeida et al. Citation2007). IMT can also be evaluated by histomorphometric analysis in experimental studies, as was done in the present study, according to Razuvaev et al. (Citation2008). Our findings are in agreement with Hadjiisky et al. (Citation1987), who studied alterations related to atherosclerosis in the aorta tunica media of SHR rats and observed an increase in vessel wall thickness and hypertrophy of vascular smooth muscle cells (VSMC).
It has been postulated that one of the functions of the vascular endothelium is to maintain the mitogenic quiescence of VSMC through NO activity (Costa and Assreuy Citation2005). Therefore a lack of NO may induce excessive proliferation of these cells (Garg and Hassid Citation1989). Thus, it seems reasonable to suggest that CMS-induced inhibition of NO production evidenced in the CECs for PE obtained in thoracic aortas, could also be related to the aortic wall hypertrophy induced by CMS observed in our histomorphometric analysis.
In this study, there was a significant increase in serum levels of TGL, TC, LDL and VLDL in rats submitted to the CMS compared with the control. Stress hormones modulate the breakdown of proteins, glycogen, and TGL into molecules that can be rapidly metabolized in order to generate energy (Black Citation2002), supporting the fight or flight strategy in the face of an aggressive agent. However if these metabolic responses are sustained, they can induce an atherogenic lipid profile including high levels of cholesterol, lipoproteins, TGL, and free fatty acids (Stoney et al. Citation1999; Ferreira et al. Citation2006). Electric shock stress increases plasma cholesterol concentrations (Berger et al. Citation1980), and immobilization stress decreases HDL and increases LDL and VLDL blood levels in rats (Bryant et al. Citation1988). According to these findings, the data of the present study strengthen the hypothesis that chronic stress contributes to unfavorable concentrations of lipoproteins that may predispose to atherosclerosis (Brindley et al. Citation1993).
HDL exerts a protective effect against atherogenesis (Schmidt et al. Citation1992), and although no changes in HDL levels were observed in rats submitted to CMS, higher levels of TGL, TC, LDL, and VLDL were associated with a higher AI in comparison with the control group. Therefore it seems that the protective effect of HDL was disturbed. The AI is a parameter for determining the risk of atherosclerosis developing in man (Wakabayashi Citation2004) and this index has also been used in rats (Kamgang et al. Citation2005; Montilla et al. Citation2005) for the same purpose. As stress may produce an atherogenic lipid profile (Black Citation2002), and atherosclerosis is related to vessel stiffening (Taniwaki et al. Citation1999), the change in this index indicates the role of CMS as a risk factor for cardiovascular disease.
It is well established that risk factors for atherosclerosis, such as chronic stress, high LDL and VLDL levels (Badimón and Martínez-Gonzalez, Citation2002), may promote alterations in the endothelial barrier with regard to blood constituents (Margariti et al. Citation2006). For instance, the catecholamines are capable of enhancing endothelial permeability to the traffic of lipoproteins and oxidized lipids (Ross Citation1999), causing functional and structural changes in the arterial endothelium (Kao et al. Citation1995). Moreover, incubation of isolated rabbit aorta with LDL reduces endothelium-dependent relaxation (Jacobs et al. Citation1990), LDL decreases the expression of eNOS (Liao et al. Citation1995), and LDL can oppose the NO vasodilating effect induced by anaerobic training in rats (Cunha et al. Citation2005). Accordingly, it may be suggested that the increase in LDL levels observed in the CMS group in the present study may have contributed to endothelial supersensitivity to PE and decreased NO production in the thoracic aorta isolated from stressed rats.
In summary, the results of the present study indicate that CMS increased the in vitro vasoconstrictor response of the thoracic aorta and induced vascular wall hypertrophy. Since these effects seem to be related to the inhibition of endothelial NO synthesis production, dyslipidemia and increase in AI, we conclude that CMS induced proatherosclerotic effects in rats 15 days after the end of the chronic stress exposure.
Acknowledgements
The authors thank FAPESP for its support (grant 05/060284-6). VJN and RC and MLT were recipient of FAPESP fellowships. RF was recipient of a CNPq fellowship. The authors thank Dr. Oduvaldo Câmara Marques Pereira – São Paulo State University, for corticosterone radioimmunoassay, Margery Galbraith for editing the English of the manuscript and Marcelo Corêa Alves for the statistical analysis.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Almeida CA, Teixeira PFD, Soares DV, Cabral MD, Costa SM, Salles EF, Silva NAO, Morais FFC, Buescu A, Henriques JM, Vaisman M. Espessura íntima-média carotídea como marcador de risco cardiovascular em pacientes com hipotireoidismo subclínico. Arq Bras Endocrinol Metab 2007; 51: 472–477
- Badimón L, Martinez-González J. Endotelio en la protección vascular: nuevos conocimientos. Rev Esp Cardiol 2002; 55: 17–26
- Berger DF, Starzed JJ, Mason EB. The effects of differential psychological stress on plasma cholesterol levels in rats. Psychosom Med 1980; 42: 481–492
- Bernatova I, Csizmadiova Z. Effect of chronic social stress on nitric oxide synthesis and vascular function in rats with family history of hypertension. Life Sci 2006; 78: 1726–1732
- Bernatova I, Csizmadiova Z, Kopincova J, Puzserova A. Vascular function and nitric oxide production in chronic social-stress-exposed rats with various family history of hypertension. J Physiol Pharmacol 2007; 58: 487–501
- Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun 2002; 16: 622–653
- Brindley DN, McCann BS, Niaura R, Stoney CM, Suarez EC. Stress and lipoprotein metabolism: modulators and mechanisms. Metabolism 1993; 42: 3–15
- Broto MAP. Temporal effects of stress by immobilization and sensitivity of the isolated rat pacemaker to isoproterenol: roles of corticosterone, neuronal uptake, and β-adrenergic homogeneity. J Pharmacol Exp Ther 2003; 306: 1152–1158
- Bryant HU, Story JA, Yim GKW. Assessment of endogenous opioid mediation in stress-induced hypercholesterolemia in the rat. Psychosom Med 1988; 50: 576–585
- Cordellini S, Vassilieff VS. Decreased endothelium-dependent vasoconstriction to noradrenaline in acute-stressed rats is potentiated by previous chronic stress: nitric oxide involvement. Gen Pharmacol 1998; 30: 79–83
- Costa RSA, Assreuy J. Multiple potassium channels mediate nitric oxide-induced inhibition of rat vascular smooth muscle cell proliferation. Nitric Oxide 2005; 13: 145–151
- Cunha TS, Moura MJCS, Bernardes CF, Tanno AP, Marcondes FK. Vascular sensitivity to phenylephrine in rats submitted to anaerobic training and nandrolone treatment. Hypertension 2005; 46: 1010–1015
- Ferreira R, Costa R, Tramascia ML, Neves VJ, Marcondes FK. Effects of chronic mild stress on the body weight of rats. Physiol Mini Rev 2006; 2: 204
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502
- Gansburgskii AN. State of the endothelium of the aorta during chronic stress. Arkh Anat Gistol Embriol 1985; 88: 38–44
- Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 1989; 83: 1774–1777
- Gordon D, Guyton JR, Karnovsky MJ. Intimal alterations in rat aorta induced by stressful stimuli. Lab Invest 1981; 45: 14–27
- Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol 2002; 282: R1333–R1341
- Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav 2003; 78: 703–710
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav 2005a; 84: 697–706
- Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, Chen Z, Garcia F, Muma NA, Kar LDV. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psycopharmacology 2005b; 179: 769–780
- Grunfeld JP, Eloy L. Glucocorticoids modulate vascular reactivity in the rat. Hypertension 1987; 10: 608–618
- Hadjiisky P, Peyri N, Gorsgogeat Y. Tunica media changes in the spontaneously hypertensive rat (SHR). Atherosclerosis 1987; 65: 125–137
- Hauss WH, Bauch HJ, Schulte H. Adrenaline and noradrenaline as possible chemical mediators in the pathogenesis of arteriosclerosis. Ann N Y Acad Sci 1990; 598: 91–101
- Jacobs M, Plane F, Bruckdorfer KR. Native and oxidized low-density lipoproteins have different inhititory effects on endothelium-derived relaxing factor in the rabbit aorta. Br J Pharmacol 1990; 100: 21–26
- Kamgang R, Mboumi RY, N'dillé GPRM, Yonkeu JN. Cameroon local diet-induced glucose intolerance and dyslipidemia in adult wistar rat. Diabetes Res Clin Pract 2005; 69: 224–230
- Kao CH, Chen JK, Kuo JS, Yang VC. Visualization of the transport pathways of low density lipoproteins across the endothelial cells in the branched regions of rat arteries. Atherosclerosis 1995; 116: 27–41
- Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 1965; 27: 137A
- Kumari M, Grahanme-Clarke C, Shanks N, Marmot M, Lightman S, Vallance P. Chronic stress accelerates atherosclerosis in the apolipoprotein E deficient mouse. Stress 2003; 6: 297–299
- Li J, Kong L, Wang Y, Cheng CHK, Zhang W, Tan W. Behavioral and biochemical studies on chronic mild stress models in rats treated with a Chinese traditional prescription Banxia-houpu decoction. Life Sci 2003; 74: 55–73
- Liao JK, Shin WS, Lee WY, Clark SL. Oxidized low-density lipoprotein decreases the expression of endothelial nitric oxide synthase. J Biol Chem 1995; 270: 319–324
- Luft JH. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol 1961; 9: 409
- Margariti A, Zeng L, Xu Q. Stem cells, vascular smooth muscle cells and atherosclerosis. Histol Histopathol 2006; 21: 979–985
- Miller LC, Becker TJ, Tainter ML. The quantitative evalutation of spasmolytic drugs “in vivo”. J Pharmacol Exp Ther 1948; 92: 260–268
- Mitchell BM, Dorrance AM, Webb RC. GTP cyclohydrolase 1 downregulation contributes to glucocorticoid hypertension in rats. Hypertension 2003; 41: 669–674
- Mitchell BM, Dorrance AM, Mack EA, Webb RC. Glucocorticoids decrease GTP cyclohydrolase and tetrahydrobiopterin-dependent vasorelaxation through glucocorticoid receptors. J Cardiovasc Pharmacol 2004; 43: 8–13
- Montilla P, Barcos M, Muñoz MC, Bujalance I, Muñoz-Castañeda JR, Tunez I. Red wine prevents brain-oxidative stress and nephropathy in streptozotocin-induced diabetic rats. J Biochem Mol Biol 2005; 38: 539–544
- Moreau JL. Validation d'un modèle animal de l'anhédonie, symptôme mejeur de la depresión [Validation of an animal model of anhedonia, a major symptom of depression]. L'Encephale 1997; 23: 280–289
- Moreau JL, Bourson A, Jenck F, Martin JR, Mortas P. Curative effects of the atypical antidepressant mianserin in the chronic mild stress-induced anhedonia model of depression. J Psychiatr Neurosci 1994; 19: 51–56
- Moura MJCS, Marcondes FK. Influence of estradiol and progesterone on the sensitivity of rat thoracic aorta to noradrenaline. Life Sci 2001; 68: 881–888
- Moura MJCS, Quintal MM, Marcondes FK. A natação forçada induz subsensibilidade à fenilefrina em aorta torácica de rato. Revista Brasileira de Ciências Farmacêuticas 2003; 39: 433–439
- Okruhlicová L, Dlugosová K, Mitasíková M, Bernátová I. Ultrastructural characteristics of aortic endothelial cells in borderline hypertensive rats exposed to chronic social stress. Physiol Res 2008; 57(2)S31–S37
- Razuvaev A, Lund K, Roy J, Hedin U, Caidahl K. Noninvasive real-time imaging of intima thickness after rat carotid artery balloon injury using ultrasound biomicroscopy. Atherosclerosis 2008; 199: 310–316
- Ricart-Jané D, Rodríguez-Sureda V, Benavides A, Peinado-Onsurbe J, López-Tejero MD, Llobera M. Immobilization stress alters intermediate metabolism and circulating lipoproteins in the rat. Metabolism 2002; 51: 925–931
- Ross A. Atherosclerosis – an inflammatory disease. N Engl J Med 1999; 340: 115–126
- Schmidt K, Werner ER, Mayer B, Wachter H, Kukovetz WR. Tetrahydrobiopterin-dependent formation of endothelium-derived relaxing factor (nitric oxide) in aortic endothelial cells. Biochem J 1992; 281: 297–300
- Stoney CM, Niaura R, Bausserman L, Matacin M. Lipid reactivity to stress: I Comparison of chronic and acute stress responses in middle-aged airline pilots. Health Psychol 1999; 18: 241–250
- Taniwaki H, Kawagishi T, Emoto M, Shoji T, Kanda H, Maekawa K, Nishizawa Y, Morii H. Correlation between the intima-media thickness of the carotid artery and aortic pulse-wave velocity in patients with type 2 diabetes. Diabetes Care 1999; 22: 1851–1857
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D'Alessio DA, Herman JP. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab 2005; 289: E823–E828
- Wakabayashi I. Effects of age on relationship of alcohol drinking and obesity to atherosclerosis risks. Geriatrics Gerontol Int 2004; 4: S292–S295