Abstract
Stressful stimuli cause region-specific increases in c-fos expression within the rat brain. Early maternal separation (EMS) is a model of early life adversity that results in long lasting changes to stress and anxiety responses. This study examined the regional distribution of c-fos mRNA after exposure to the elevated plus-maze (EPM) and how EMS altered this pattern. On each of post-natal days 5–21 pups were separated from the dam for 6 h—control rats remained undisturbed. At 70 days old, male offspring were either exposed to the EPM or left undisturbed in the home cage. After exposure to the EPM, c-fos mRNA expression was significantly increased in specific brain areas, including cingulate cortex, medial amygdala and hippocampus. EMS rats displayed greater anxiety behaviour on the EPM vs. controls. Although EMS caused no overall effect on basal c-fos mRNA, a significant interaction between treatment group and exposure to the EPM occurred in the dentate gyrus and piriform cortex, with lower EPM-induced mRNA levels in EMS rats. The region-specific increase in c-fos mRNA reflects activation of neural circuits associated with EPM-induced anxiety. The effect of EMS on this activation in the two regions suggests these areas may contribute to the differential response to the anxiogenic stress of the EPM.
Introduction
The response of an animal to stress and anxiety is complex and involves specific brain circuits and endocrine responses. Although the physiological responses to different types of stress may be similar for different stimuli, evidence from the expression of the c-fos gene or its protein product, Fos, shows that different brain circuits regulate the response or the adaptation to different classes of stressors (Pacak et al. Citation1998; Briski and Gillen Citation2001; Dayas et al. Citation2001; Ingram Citation2005). This differential pattern of activation is most prominent when stressors are classified into either physical stressors, such as pain and infection (which require an immediate response with little dependence on higher order processing), or psychological stressors, such as restraint and exposure to novel environment (which have a dependence on processing by brain regions regulating memory and emotion; Emmert and Herman Citation1999).
There is growing evidence to suggest that the pathophysiology of anxiety disorders is associated with disruption of multiple brain structures and neurotransmitter systems, possibly involving over-activation or increased sensitivity of stress response circuits. Animal studies measuring c-fos mRNA or Fos expression have investigated the pattern of neuronal activation resulting from exposure to various anxiogenic stimuli (Schreiber et al. Citation1991; Sharp et al. Citation1991; Pezzone et al. Citation1992; Smith et al. Citation1992; Senba et al. Citation1993), but little is known about how this pattern of activation is affected by differences in the anxiety susceptibility of the animal. However, one study has shown that animals bred for differential anxiety levels display a differential pattern of Fos expression in the cingulate cortex and hypothalamic areas after an anxiogenic stimulus (Salome et al. Citation2004).
As well as inherent differences in the basal level of anxiety behaviour, there is also evidence for neonatal programming of anxiety circuits. Differential c-fos expression after a stressful stimulus has been seen in rats born to high licking and grooming (LG) mothers compared to those born to low LG mothers (Menard et al. Citation2004)—high LG behaviour is believed to reduce stress in the offspring. Furthermore, rats exposed to handling during the postnatal period have been shown to display a lower number of Fos-positive cells in stress related brain areas (such as the paraventricular nucleus of the hypothalamus, the central amygdala and the hippocampus) after restraint and ether stress compared to unhandled controls (Abraham and Kovacs Citation2000)—post-natal handling reduces the stress reactivity of the hypothalamic-pituitary-adrenal (HPA) axis in adulthood (Liu et al. Citation1997). These studies suggest that the pattern and relative levels of expression of immediate-early genes can correlate with differential anxiety behaviour.
In this study we examined c-fos mRNA expression in order to identify the pattern of neuronal activation in the brains of rats exposed to the anxiogenic stimulus of placement on the elevated plus maze (EPM). As well as acting as a measure of anxiety, the EPM acts as an anxiogenic stimulus in itself, as indicated by the increase in plasma corticosterone levels, freezing behaviour and production of faecal boli that occur after voluntary or forced entry into the open arms of the maze (Pellow et al. Citation1985). The effect of underlying anxiety susceptibility on the pattern of expression of c-fos mRNA seen after exposure to the EPM was determined by examining the effect of maze exposure in animals that had undergone early maternal separation (EMS). This EMS paradigm, in which neonatal animals are periodically separated from the dam for 6 h per day over 10 occasions between post-natal days 5–21, has been previously used to determine the functional and biochemical effects of early life adversity (Matthews et al. Citation1996). Previous studies have revealed a number of persistent effects of EMS, including changes in anxiety behaviour and in the response to stress (Plotsky and Meaney Citation1993; Wigger and Neumann Citation1999).
Materials and methods
Early maternal separation (EMS)
Hooded Lister rats were bred in house from a stock of 12 females and 4 males. Rats were housed under controlled environmental conditions with lights on at 7am/off at 7pm, temperature 22°C, food and water available ad libitum. Breeding females (12 weeks of age) were housed in groups of three prior to each being placed in a cage with a breeding male for 2 days and then housed singly to give birth. Breeding females were assigned as either control or EMS dams.
Between post-natal days 1 and 3 the numbers of pups per litter were counted and, if necessary, reduced to 10 by removal of female pups. EMS was carried out on 10 occasions between post-natal days 5 and 21 (separation occurring on all weekdays), in accordance with an established protocol which has previously been shown to result in long-lasting effects on anxiety behaviour and brain biochemistry in the Hooded Lister rat (Matthews et al. Citation1996, Citation2001). All pups from the litter were removed from the dam and housed in a small polythene cage lined with tissue and nesting material. This cage was placed inside a temperature (34°C) and humidity (45–50%) controlled incubator and water was made available. Pups were separated for 5–6 h per day (during the light phase, beginning at 9 am) before being returned to the dam and home cage. Control litters remained undisturbed apart from normal cage cleaning. Litters were weaned at post-natal day 23 and males housed in litter groups of four. Male offspring were used in preference to females in this current study in order to avoid any potential changes in behaviour and anxiety during the different phases of the oestrous cycle. Rats from several litters were used in this study [six control litters, seven EMS litters (one EMS female contributing two litters)]. All procedures were undertaken in accordance with the UK animals (Scientific Procedures) Act 1986 and were approved by the local ethical committee. There were no effects of EMS on growth rate or body weight of the pups, however EMS offspring showed a decreased body weight at adulthood (70 days of age, mean ± SEM: control 363 ± 23 g EMS 310 ± 24 g; p < 0.001 t-test).
Behavioural testing
Three days before testing, the adult rats (70 days of age) were re-housed into litter group pairs and transferred to a holding room adjacent to the testing room in order to acclimatise. On the day of testing, one rat from each pair was exposed to the EPM. The EPM comprised a cross-shaped platform raised 60 cm above the ground with two opposing arms enclosed by 30 cm high walls (‘closed’ arms), while the other two arms had no walls (‘open’ arms). The rat was placed on the centre of the EPM, facing an open arm and left alone in the room for 10 min. The session was recorded via an overhead video camera and monitored from an adjacent room. Any rat that fell off the maze was immediately retrieved and excluded from the final analysis. The second rat from each pair remained undisturbed in the home cage. At the end of the EPM session the rat was removed from the maze and returned to its cage and cage mate. Both rats were left undisturbed for a further 20 min to allow adequate time for c-fos gene expression to increase. After this time both rats were rapidly killed by decapitation without anaesthetic and brains collected and frozen on dry ice.
Analysis of EPM sessions
EPM sessions were analysed using Etholog software (Ottoni Citation2000). The number and duration of open arm and closed arm entries were recorded, as was time spent in the central hub of the maze. Entry into an arm was regarded as when the head and forepaws crossed over the dividing line. Exploratory behaviour was also examined by counting the number of head dips, rearing events, and stretch attend postures. A ‘head dip’ was defined as the head of the rat dipping below the level of the platform edge—this can only happen in the open arm or central hub and is, therefore, expressed as a proportion of time spent in these regions. ‘Rearing events’ were classed as the rat standing on its hind paws, either unsupported in the central hub or supported by the wall in the closed arm. As rearing rarely happens in an open arm the values are expressed as a proportion of time spent in the closed arms and central hub. A ‘stretch attend posture’ is defined as a sustained forward movement of the head without moving of the forepaws, usually occurring at the entry to the arm or at the end of the open arm. The number of occurrences of grooming behaviour and the number of faecal pellets left on the maze at the end of each session were also recorded.
In situ hybridisation histochemistry
For each brain area of interest, four 12 μm coronal sections per slide (approximately 72 μm apart) were collected using a cryostat. Sections were thaw mounted onto RNAase-free, gelatin-coated slides and stored at − 80°C. Areas of interest were chosen from previous c-fos expression studies and by relevance to putative anxiety circuitry (Windle et al. Citation2004, Citation2006). Sections were pre-treated by initial fixation for 5 min in 4% paraformaldehyde in phosphate-buffered saline (pH 7.4), two washes in phosphate-buffered saline, acetylation for 10 min with 0.25% acetic anhydride in 0.1 M triethanolamine buffer, dehydration through graded alcohol washes, defatting by a 10 min chloroform wash, and treatment with 100 and 95% alcohol. Slides were then air dried and stored at − 20°C.
In situ hybridisation histochemistry was carried out using a cocktail of three oligonucleotide probes directed against three specific regions of rat c-fos mRNA—probes were complimentary to bases 208–252, 333–372 and 546–590 and were synthesised by MGW Biotech (Ebersberg, Germany). Probes were 3′ end labelled with 35S-adenosine triphosphate using terminal deoxynucleotide transferase (Roche, Manheim, Germany). Probes were purified using a commercial kit (Qiagen Nucleotide Removal Kit) to remove unbound radioactivity and were added to hybridisation mixture (50% formamide, 4 × standard saline citrate, 10% dextran sulphate, 5 μl Denhart's, 200 μg/ml salmon sperm DNA, 100 μg/ml poly A, 25 mM sodium pyrophosphate and 5% dithiothreitol). Hybridisation mixture was added at approximately 2 × 106 cpm per slide and sections were hybridised overnight at 37°C. Excess hybridisation mixture was washed off in 2 × standard sodium citrate, before slides were rinsed in deionised water and air dried (McQuade et al. Citation2004). Sections were exposed to 35S-sensitive film (MR Biomax film, Kodak) in an autoradiographic cassette for 6–8 weeks along with 14C calibration strips (GE Healthcare, Amersham, UK). Time of optimum exposure for each region was judged by including and analysing (after 6 weeks) another set of standards and both positive and negative control sections that were exposed to film in a separate cassette. Films were developed using an automatic Agfa Curix compact plus daylight™ processor.
Quantitative analysis of the films was performed using Scion Image software. Sections were matched for rostrocaudal level before capturing images from each area. Density of signal was calibrated using the radioactive standards included on each film (the background measured in an area of each region without evident hybridisation signal was negligible in all groups) and data are expressed as a percentage change from the value in the control group (no EMS, no EPM).
Statistical analysis
Data are shown as group mean ± SEM. All statistical analysis was carried out using SPSS (version 11 for Windows) or GraphPad Prism (Version 3.0). Behavioural data were analysed using the Independent samples t-test for normally distributed data and the Kruskal Wallis test for non-parametric data. ANOVA with post hoc t-tests, and Bonferroni corrections for pairwise comparisons, was used to analyse the c-fos mRNA expression data. Significance above the 95% level is reported.
Results
Behavioural results
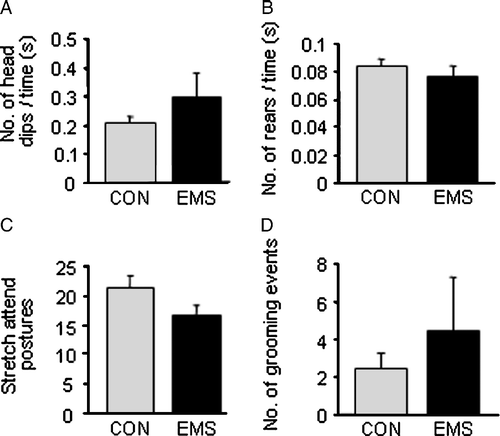
Male Hooded–Lister rats that had undergone EMS showed a decreased proportion of entries into the open arm of the EPM (), indicating an increased level of anxiety. There was no significant difference in overall locomotion, as shown by total number of arm entries (), nor were there significant differences in any of the other parameters examined, such as time in open arms () and latency to first open arm entry (). In addition, there were no significant differences between the control and EMS groups when exploratory behaviour (head dips, rearing and stretch attend postures) and number of grooming incidences were analysed ().
Figure 1 Behaviour on the elevated plus maze in control and EMS rats. (A) There was no significant difference in locomotion, as measured by the total number of arm entries between the two groups. (B) EMS rats showed a significant decrease in proportion of entries into the open arm as a percentage of total arm entries (*p < 0.05 t-test). (C) There was no significant difference in the percentage time spent in the open arms between control and EMS rats. (D) Although there was a trend for EMS rats to have a higher latency to the first open arm entry this was not statistically significant (p>0.05 Kruskal Wallis test). All graphs show the mean of the group ± SEM, n = 9 control, 11 EMS.
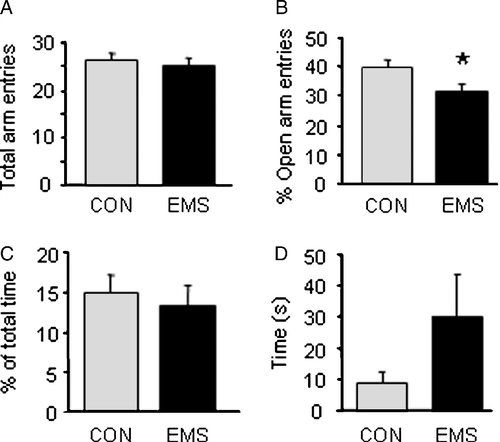
Figure 2 Exploratory behaviours measured on the EPM in control and EMS rats. (A) There was no significant difference between control and EMS rats in the number of head dips over the side of the open arms (as a proportion of time in arm). (B) There was no significant difference in the number of rearing events between the two groups (as a proportion of time in closed arm and hub). (C) There was also no significant difference in the number of stretch attend postures (C) or grooming events (D) exhibited between and EMS rats. All graphs show mean of group ± SEM, n = 9 control, 11 EMS.
c-fos mRNA expression
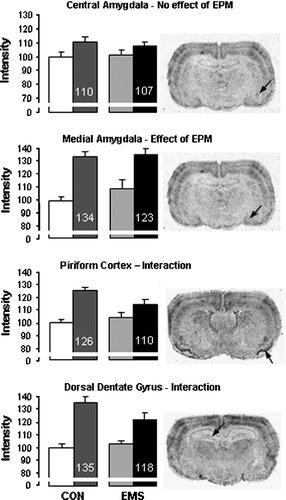
Control rats not exposed to the EPM showed a low level of c-fos mRNA expression throughout the brain regions examined (and in comparison to negative control sections). Exposure to the EPM caused a significant increase in c-fos mRNA expression (), with an overall effect of the maze seen when all regions were analysed by a multivariate (repeated measures) ANOVA (p < 0.001). There was no overall effect of EMS, however there was a trend for EMS rats (without exposure to the EPM) to have a slightly elevated level of c-fos mRNA expression ().
Table I. Relative expression of c-fos mRNA in different brain areas in control (CON) or EMS animals exposed to the EPM.
When brain areas were examined individually there was no effect of exposure to the EPM in two of the regions examined, the central amygdala (Figure 3A) and the pontine region (). However, a significant increase in c-fos mRNA expression was seen in a number of other regions after exposure to the EPM. Within the forebrain these included the cingulate, barrel and piriform cortices (), medial amygdala (Figure 3B), lateral septum, caudate putamen, the CA1, CA2 and CA3 subfields of the dorsal hippocampus, and all subfields of the ventral hippocampus. Within the hindbrain increased expression was seen in the dorsal raphe nucleus (DRN) and the cerebellum. Within the locus coeruleus c-fos mRNA expression was only detectable after the EPM, but there was no significant difference between EMS and Control rats (t-test). A significant interaction between plus maze and neonatal treatment group was seen in two regions, the piriform cortex (p < 0.05) and the dorsal dentate gyrus (p < 0.05) (, D).
Figure 3 c-fos mRNA expression in various brain regions in control (CON) and EMS rats either in the home cage or after exposure to the EPM (left and right hand bars of each pair, respectively). Data are from the central (A) and medial (B) amygdala, piriform cortex (C) and dorsal dentate gyrus (D) and values are shown as a percentage of the values in control rats not exposed to the EPM (note the break in the axis). The value indicated on the bars is the mean value expressed as percentage of its own control (no EPM). Statistical analysis indicated that these four areas respectively showed no effects (A), an effect of EPM alone (B), and a significant effect of EPM and interaction (C,D) -see for test details. Photomicrographs on the right hand side show representative regions from an EMS, EPM-exposed rat with the areas analysed indicated by the arrow. All graphs show mean of group ± SEM, n = 9 control, 11 EMS.
Discussion
Effect of exposure to the elevated plus maze
Exposure to the EPM resulted in an increase in c-fos mRNA expression in specific areas of the brain of male Hooded–Lister rats. A significant increase in expression was seen in many areas associated with anxiety, such as the frontal cortex, paraventricular nucleus, hippocampus, medial amygdala and DRN, and is consistent with areas previously shown to display increased c-fos mRNA or Fos protein expression following exposure to the EPM (Silveira et al. Citation1993; Duncan et al. Citation1996; Hinks et al. Citation1996; Salome et al. Citation2004). EPM-induced immediate-early gene expression in the barrel cortex or caudate putamen has not been previously reported, and may represent the sensory and motor components of the behavioural response, respectively. Within the hippocampal formation our data showed an increase in c-fos mRNA in both the dorsal and ventral sub-fields. Previous reports suggest that the dorsal hippocampus plays a preferential role in learning and memory (particularly spatial memory), whereas the ventral hippocampus is preferentially involved in anxiety processing (Bannerman et al. Citation2004). Interestingly the EPM-induced increase was greater in the dorsal compared to ventral regions, suggesting that the stimulus may have a greater spatial memory component than anxiety component.
c-fos mRNA expression was detected in the pontine region and central amygdala, but did not differ between control and EPM rats, suggesting that these areas are not involved in the processing of this particular stimulus. Interestingly, the differential response seen between sub-regions of the amygdala (increase in medial, no effect in central) supports evidence from other studies that the medial amygdala responds to predominantly psychological stress whereas the central amygdala responds to more physical stressors. For example, very few Fos positive cells are seen in the amygdala of control rats, large numbers are seen in the medial amygdala after noise, restraint or forced swim stress, whereas central amygdala activation is greatest in rats exposed to haemorrhage or immune challenge (Dayas et al. Citation2001). Indeed it may be that the central amygdala is actually inhibited during psychological stress (Day et al. Citation2005).
Effect of early maternal separation
The long-term consequences of maternal separation (also referred to as maternal deprivation) were first studied by Hofer and Levine in the 1970s but the association between early adverse experience and poor mental health has led to a large number of studies since the early 1990s. A wide number of different methodologies have been used, from prolonged single episodes of maternal separation to repeated shorter episodes, the latter being suggested as a better model of poor maternal care and with less effects due to nutritional deficit. Variations in the literature include strain of rat used, timing and duration of separation and the treatment of the control group (Lehmann and Feldon Citation2000). In this study we chose to follow a previously published protocol that had been shown to have long-lasting effects on behaviour and biochemistry in Hooded Lister rats (Matthews et al. Citation1996, Citation2001).
Although the EMS paradigm resulted in a significant reduction in number of open arm entries on the EPM, indicating a lasting change in behavioural reactivity, in the unstimulated (control) state there was no overall significant effect of EMS on the level of c-fos mRNA expression in the brain regions studied. Following exposure to the EPM the pattern of c-fos mRNA expression did not differ between control and EMS rats, but there was an interaction between EPM exposure and EMS in the piriform cortex and the dentate gyrus: in these areas there was a smaller increase in gene expression after exposure to the EPM compared to the control rats, with the reduced increase reaching statistical significance within the dorsal dentate gyrus.
The piriform cortex is the main cortical region involved in olfactory processing and the synaptic plasticity within this region is considered to allow learning of odour experience (Wilson Citation2003). Exposure to a novel environment, such as the EPM, evokes strong exploratory reactions in the rat and, hence, also olfactory stimulation. Previous studies have also demonstrated an increase in c-fos mRNA expression in the piriform cortex after the EPM (Silveira et al. Citation1993; Hinks et al. Citation1996; Salome et al. Citation2004). The reduced c-fos mRNA expression after the EPM in EMS rats, compared to controls, in this study suggests the stress-induced activity of the piriform cortex is inhibited. This may indicate that the increased anxiety shown by the EMS rats is reducing olfactory stimulation. Interestingly, patients with post-traumatic stress disorder are less proficient in odour detection (Vasterling et al. Citation2000). In addition, the piriform cortex makes extensive connections to other cortical structures, including gustatory centres through which it is able to influence feeding behaviour (Fu et al. Citation2004). In this respect, it is of interest that the EMS rats examined in this study showed decreased body weight at adulthood compared to controls.
A significant interaction between exposure to the maze and EMS was also seen in the dorsal dentate gyrus, with lower levels of activation in the EMS group. The dentate gyrus provides an afferent pathway to the CA3 region of the hippocampus and is involved in inhibition of hippocampal activity. It is also one of the few areas where neurogenesis can occur in the adult brain (Cameron and Gould Citation1994). The hippocampus has been strongly implicated in anxiety processing, although this is predominantly controlled by the ventral hippocampus (Bannerman et al. Citation2004). The dentate gyrus has been reported to show increased c-fos mRNA expression after the plus maze in several previous studies (Hinks et al. Citation1996; Salome et al. Citation2004). Furthermore, in the dentate gyrus there is some evidence that cells expressing c-fos mRNA after stress (acute swim and restraint stress) are actually inhibitory interneurones (Cullinan et al. Citation1995). Therefore, a reduction in activation might suggest less inhibition of the hippocampus, leading to increased anxiety-related activity. The EMS rats examined in this study may, therefore, be showing a decreased inhibition of stress-induced activation of the hippocampus.
In a previous study examining c-fos mRNA after exposure to the open arm of the EPM in two breeding lines of rat, one line showing high anxiety behaviour and the other low anxiety behaviour, significant differences in expression levels were found (Salome et al. Citation2004). Differential expression was seen in the hippocampal regions, including the dentate gyrus, with high anxiety rats showing a higher expression of c-fos mRNA. This is contrary to the present data where the more anxious EMS rats displayed lower expression. The reasons for this are unclear, but may suggest that different underlying anxiety states may affect neural reactivity in different ways. A number of mechanisms have been suggested to contribute to the neuronal plasticity seen after EMS, including alterations in 5-HT, corticotrophin releasing factor and BDNF levels (Fumagalli et al. Citation2007; Fenoglio et al. Citation2006; Pryce et al. Citation2005).
Conclusions and clinical significance
The present data show that early life stress can have a lasting effect both on the behavioural response to an anxiogenic stimulus and on the underlying neuronal activation. Functional imaging studies in humans have demonstrated that similar regions to those activated in rats exposed to the EPM are activated after presentation of stressful stimuli in healthy controls and in patients with anxiety disorders (Malizia Citation1999; Cannistraro and Rauch Citation2003). An increased understanding of the neurocircuitry involved in the response to anxiety provoking stimuli and the programming of this circuitry by early life stress may help understand the pathophysiology of anxiety disorders.
Acknowledgements
The authors are grateful to Mr. Mel Leitch and Dr. Richard McQuade for assistance with developing the in situ hybridization histochemistry measures and to Dr Sasha Gartside for assistance with the EMS technique.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abraham IM, Kovacs KJ. Postnatal handling alters the activation of stress-related neuronal circuitries. Eur J Neurosci 2000; 12: 3003–3014
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev 2004; 28: 273–283
- Briski K, Gillen E. Differential distribution of Fos expression within the male rat preoptic area and hypothalamus in response to physical vs. psychological stress. Brain Res Bull 2001; 55: 401–408
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 1994; 61: 203–209
- Cannistraro PA, Rauch SL. Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharmacol Bull 2003; 37: 8–25
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 1995; 64: 477–505
- Day HE, Nebel S, Sasse S, Campeau S. Inhibition of the central extended amygdala by loud noise and restraint stress. Eur J Neurosci 2005; 21: 441–454
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci 2001; 14: 1143–1152
- Duncan GE, Knapp DJ, Breese GR. Neuroanatomical characterization of Fos induction in rat behavioral models of anxiety. Brain Res 1996; 713: 79–91
- Emmert MH, Herman JP. Differential forebrain c-fos mRNA induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Res 1999; 845: 60–67
- Fenoglio KA, Brunson KL, Baram TZ. Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects. Frontiers Neuroendocrinol 2006; 27: 180–192
- Fu W, Sugai T, Yoshimura H, Onoda N. Convergence of olfactory and gustatory connections onto the endopiriform nucleus in the rat. Neuroscience 2004; 126(1033)1041
- Fumagalli F, Molteni R, Racagni G, Riva MA. Stress during development: impact on neuroplasticity and relevance to psychopathology. Prog Neurobiol 2007; 81: 197–217
- Hinks GL, Brown P, Field M, Poat JA, Hughes J. The anxiolytics CI-988 and chlordiazepoxide fail to reduce immediate early gene mRNA stimulation following exposure to the rat elevated X-maze. Eur J Pharmacol 1996; 312: 153–161
- Ingram CD. Pathways and transmitter interactions mediating an integrated stress response. Handbook of stress and the brain, part 1: The neurobiology of stress, T Steckler, NH Kalin, JMHM Reul. Techniques in the Behavioral and Neural Sciences, Elsevier, Amsterdam 2005
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing?. Rev Neurosci 2000; 11: 383–408
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science 1997; 277: 1659–1662
- Malizia AL. What do brain imaging studies tell us about anxiety disorders?. J Psychopharmacol 1999; 13: 372–378
- Matthews K, Dalley JW, Matthews C, Tsai TH, Robbins TW. Periodic maternal separation of neonatal rats produces region- and gender-specific effects in biogenic amine content in post-mortem adult brain. Synapse 2001; 40: 1–10
- Matthews K, Wilkinson LS, Robbins TW. Repeated maternal separation of preweanling rats attenuates behavioral responses to primary and conditioned incentives in adulthood. Physiol Behav 1996; 59: 99–107
- McQuade R, Leitch MM, Gartside SE, Young AH. Effect of chronic lithium treatment on glucocorticoid and 5-HT1A receptor messenger RNA in hippocampal and dorsal raphe nucleus regions of the rat brain. J Psychopharmacol 2004; 18: 496–501
- Menard JL, Champagne DL, Meaney MJ. Variations of maternal care differentially influence ‘fear’ reactivity and regional patterns of cFos immunoreactivity in response to the shock-probe burying test. Neuroscience 2004; 129: 297–308
- Ottoni EB. EthoLog 2.2: A tool for the transcription and timing of behavior observation sessions. Behav Res Methods Instrum Comput 2000; 32: 446–449
- Pacak K, Baffi JS, Kvetnansky R, Goldstein DS, Palkovits M. Stressor-specific activation of catecholaminergic systems: Implications for stress-related hypothalamic–pituitary–adrenocortical responses. Adv Pharmacol 1998; 42: 561–564
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 1985; 14: 149–167
- Pezzone MA, Lee WS, Hoffman GE, Rabin BS. Induction of c-Fos immunoreactivity in the rat forebrain by conditioned and unconditioned aversive stimuli. Brain Res 1992; 597: 41–50
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res 1993; 18: 195–200
- Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev 2005; 29: 649–674
- Salome N, Salchner P, Viltart O, Sequeira H, Wigger A, Landgraf R, Singewald N. Neurobiological correlates of high (HAB) versus low anxiety-related behavior (LAB): Differential Fos expression in HAB and LAB rats. Biol Psychiat 2004; 55: 715–723
- Schreiber SS, Tocco G, Shors TJ, Thompson RF. Activation of immediate early genes after acute stress. Neuroreport 1991; 2: 17–20
- Senba E, Matsunaga K, Tohyama M, Noguchi K. Stress-induced c-fos expression in the rat brain: Activation mechanism of sympathetic pathway. Brain Res Bull 1993; 31: 329–344
- Sharp FR, Sagar SM, Hicks K, Lowenstein D, Hisanaga K. c-fos mRNA Fos, and Fos-related antigen induction by hypertonic saline and stress. J Neurosci 1991; 11: 2321–2331
- Silveira MC, Sandner G, Graeff FG. Induction of Fos immunoreactivity in the brain by exposure to the elevated plus-maze. Behav Brain Res 1993; 56: 115–118
- Smith MA, Banerjee S, Gold PW, Glowa J. Induction of c-fos mRNA in rat brain by conditioned and unconditioned stressors. Brain Res 1992; 578: 135–141
- Vasterling JJ, Brailey K, Sutker PB. Olfactory identification in combat-related posttraumatic stress disorder. J Trauma Stress 2000; 13: 241–253
- Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav 1999; 66: 293–302
- Wilson DA. Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons. J Neurophysiol 2003; 90: 65–72
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary–adrenal activity. J Neurosci 2004; 24: 2974–2982
- Windle RJ, Gamble LE, Kershaw YM, Wood SA, Lightman SL, Ingram CD. Gonadal steroid modulation of stress-induced hypothalamo-pituitary–adrenal activity and anxiety behavior: role of central oxytocin. Endocrinology 2006; 147: 2423–2431