Abstract
Contact between a charged metal surface and an electrolyte implies a particular ion distribution near the charged surface, i.e. the electrical double layer. In this mini review, different mean-field models of relative (effective) permittivity are described within a simple lattice model, where the orientational ordering of water dipoles in the saturation regime is taken into account. The Langevin–Poisson–Boltzmann (LPB) model of spatial variation of the relative permittivity for point-like ions is described and compared to a more general Langevin–Bikerman (LB) model of spatial variation of permittivity for finite-sized ions. The Bikerman model and the Poisson–Boltzmann model are derived as limiting cases. It is shown that near the charged surface, the relative permittivity decreases due to depletion of water molecules (volume-excluded effect) and orientational ordering of water dipoles (saturation effect). At the end, the LPB and LB models are generalised by also taking into account the cavity field.
Introduction
The functional activity of cells in contact with an implant is determined by the physical properties of the cell membrane (CitationBoulbitch et al. 2001) and the material characteristics of the implant (CitationGongadze et al. 2011b). The most widely used implant material is titanium (CitationGongadze et al. 2011b), because it is not rejected by the body. The interactions between the charged metal implant surface and the surrounding bone tissue are essential for the successful integration of the bone implant. It was indicated recently that the strength of interaction between a charged titanium surface and osteoblast cells strongly depends on the properties of the intermediate electrolyte (CitationTeng et al. 2000; CitationOghaki et al. 2001; CitationSmith et al. 2004; CitationKabaso et al. 2011). Contact between a charged metal implant or electrode surface and an electrolyte implies a particular ion distribution near the charged surface, i.e. the electrical double layer (EDL) which is the subject of this work.
CitationHelmholtz (1879) treated the double layer mathematically as a capacitor, based on a physical model in which a layer of ions of opposite charge (counterions) with a single shell of hydration around each ion (the so-called Helmholtz layer) is adsorbed at the oppositely charged surface and neutralises its charge. CitationGouy (1910) and CitationChapman (1913) also considered the thermal motion of ions and pictured a diffuse double layer composed of counterions attracted to the surface and ions of the same charge (co-ions) repelled by it, embedded in a dielectric continuum of constant permittivity. Such a distribution of ions in the EDL can be described within the mean-field Poisson–Boltzmann (PB) theory (CitationGouy 1910; CitationChapman 1913; CitationStern 1924; CitationMcLaughlin 1989; CitationSafran 1994; CitationKralj-Iglič and Iglič 1996; CitationLamperski and Outhwaite 2002; CitationManciu and Ruckenstein 2002; CitationBivas 2006; CitationBivas and Ermakov 2007; CitationBazant et al. 2009), expressing the competition between electrostatic interactions and the configurational entropy of ions in the solution. The Gouy–Chapman diffuse double layer is more extended than the single molecular Helmholtz layer.
Within the standard PB theory (CitationCevc 1990), the finite size of ions is not taken into account (except by the Stern distance of closest approach); therefore, the number density of counterions at the charged surface may exceed the upper value corresponding to their close packing. Different attempts have been made to incorporate steric effects into a modified PB theory in order to prevent the prediction of an unrealistically high number densities of counterions close to the charged surface.
The first attempt to include the finite size of ions in PB theory was made by CitationStern (1924) who combined CitationHelmholtz (1879) and Gouy–Chapman models (CitationGouy 1910; CitationChapman 1913). In its simplest version, the Stern model considers only the finite size of the counterions whose centres can approach the charged surface only to a certain distance, the so-called outer Helmholtz plane (CitationButt et al. 2003).
Later CitationBikerman (1942) proposed a modified PB model (Bikerman model) to account for the finite size of ions and solvent molecules. Bikerman's modified PB equation and the corresponding Fermi–Dirac-like distribution of ions has been later derived and justified using other different methods (CitationGrimley and Mott 1947; CitationGrimley 1950; CitationFreise 1952; CitationDutta and Sengupta 1954; CitationEigen and Wicke 1954; CitationWiegel and Strating 1993; CitationKralj-Iglič and Iglič 1996; CitationLamperski and Outhwaite 2002). Among others, CitationFreise (1952) introduced the finite size of ions by a pressure-dependent potential, while CitationEigen and Wicke (1954) used a thermodynamic approach. More recently, Bikerman's predictions have been reformulated within the PB theory based on a lattice statistics model and density functional theory (CitationKralj-Iglič and Iglič 1996). The finite size of ions has also been described by other density functional approaches (CitationTrizac and Raimbault 1999; CitationBarbero et al. 2000) and by considering the ions and solvent molecules as hard spheres (CitationLamperski and Outhwaite 2002; CitationBiesheuvel and van Soestbergen 2007). Also Monte Carlo simulations are widely used in order to describe the finite-sized counterions (CitationBiesheuvel and van Soestbergen 2007; CitationTresset 2008; CitationIbarra-Armenta et al. 2009; CitationZelko et al. 2010).
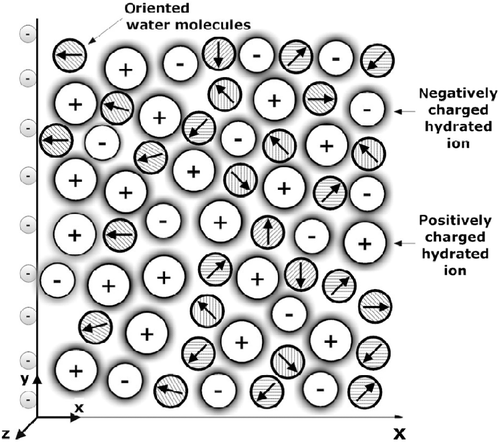
An oft-stated assumption in most PB models is that the relative permittivity in the electrolyte is constant (CitationMcLaughlin 1989; CitationCevc 1990; CitationHianik and Passechnik 1995; CitationLamperski and Outhwaite 2002; CitationButt et al. 2003). But actually, close to the charged surface the water dipoles cannot move as freely as further away from it. Besides, due to accumulation of counterions near the charged metal surface, the water molecules are partially depleted from this region (see e.g. CitationGruen and Marčelja 1983; CitationButt et al. 2003; CitationIglič et al. 2010; CitationGongadze et al. 2011a, Citation2011c). In addition, the dipole moment vectors of water molecules at the charged metal surface are, due to the strong electric field of the charged surface, partially oriented towards the surface, while all orientations of water dipoles further away from the charged surface are equally probable. The water orientation near the charged membrane surface is important for many biological processes such as binding of ligands to active sites of enzymes, transport of ions through channel proteins or adhesion of cells to an implant surface (CitationMcLaughlin 1989; CitationCevc 1990; CitationIsraelachvili and Wennerström 1996; CitationButt et al. 2003; CitationArsov et al. 2009; CitationGongadze et al. 2011a, Citation2011b; CitationKabaso et al. 2011).
As shown in the past, the properties of the EDL may be influenced by the ordering of water molecules in the region of the EDL (CitationGruen and Marčelja 1983; CitationOuthwaite 1983; CitationCevc 1990; CitationCoalson and Duncan 1996; CitationIsraelachvili and Wennerström 1996; CitationButt et al. 2003; CitationManciu and Ruckenstein 2004; CitationArsov et al. 2009) and the depletion of water molecules (CitationGongadze et al. 2011a). Close to the charged surface the orientation of water molecules may result in spatial variation of permittivity (CitationOuthwaite 1976; CitationGongadze et al. 2011a, Citation2011b; CitationButt et al. 2003). However, in the absence of an explicit consideration of the orientational ordering of water molecules, the assumption of constant permittivity is largely a consequence of the constant number of water molecules in PB theory. Considering this effect, Outhwaite developed a modified PB theory of the EDL composed of a mixture of hard spheres with point dipoles and finite size ions (CitationOuthwaite 1976, Citation1983). The problem was also considered within lattice statistics (CitationIglič et al. 2010; CitationGongadze et al. 2011a).
In this mini review, we describe the mean-field density functional theory of spatial variation of permittivity of an electrolyte solution in contact with a charged surface by taking into account the orientational ordering of water molecules within the lattice statistical mechanical approach, assuming that ions and solvent molecules occupy sites in a square lattice. In the model, the relative permittivity is consistently related to the spatial distribution of electric field strength and the distribution of ions. The finite volume of ions and water molecules (CitationLamperski and Outhwaite 2002; CitationIglič et al. 2010) in the electrolyte solution is taken into account. Accordingly, the number density of water is not constant in the whole electrolyte solution (CitationIglič et al. 2010; CitationGongadze et al. 2011a).
In the present article, we compare the predictions of the mean-field lattice EDL model considering the orientational ordering of water molecules () in the Langevin–Bikerman (LB) model for finite-sized ions and in the Langevin–Poisson–Boltzmann (LPB) model for point-like ions. The Bikerman model for finite-sized ions and zero dipole moments of water molecules (CitationBikerman 1942) is derived as a limiting case of the LB model. The interplay between water ordering and the volume-excluded effect in the decrease of permittivity near the charged surface is discussed. The dependence of the relative permittivity and the electric potential on the distance from the charged plane in the vicinity of charged surface is compared in different models. Comparison between the predictions of both Langevin models and the Stern model is discussed. In the LB and LPB models (CitationIglič et al. 2010; CitationGongadze et al. 2011a), the cavity and reaction field as well as the structural correlations between water dipoles (CitationOnsager 1936; CitationKirkwood 1939; CitationBooth 1951; CitationFröhlich 1964; CitationFranks 1972) are not taken into account. Therefore, at the end of the article, generalisation of the LPB and LB models by taking into account the cavity field in the saturation regime (CitationBooth 1951) (i.e. at high values of electric field strength) is presented within the Booth–Poisson–Boltzmann (BPB) model for point-like ions and within the modified Langevin–Bikerman (MLB) model for finite-sized ions (CitationGongadze and Iglič 2012).
LB model considering the finite size of ions and spatial variation of the relative permittivity
We consider a planar uniformly charged surface in contact with a solution of monovalent ions (counterions and co-ions) and water dipoles of finite size. The charged surface bears a charge with a surface charge density . The x-axis of the Cartesian coordinate system points in a direction from the charged plane to the bulk solution (). The water molecules are assumed to have non-zero dipole moments (
). A lattice with an adjustable lattice site is introduced in order to describe the system of water dipoles and salt ions. All lattice sites are occupied by ions or water dipoles. For the sake of simplicity, we assume that a single lattice site is occupied by only one ion or water. No empty lattice sites are allowed in the model. If the volume of one lattice site is closer to the volume of a single water molecule than to the volume of a single ion, this means that we allow partial overlapping of the ions at number densities which are close to the saturation densities of the ions. Oppositely, if the volume of the lattice site is closer to the volume of a single ion, this means the water dipole describes a cluster of water molecules.
The free energy of the system (functional) F can be written as
In most EDL models, the relative permittivity is taken into account a priori rather than deriving it as in the present work. For this purpose, the average microscopic volume charge density should be considered by including the contribution of the local net ion charges and the dipole moments, presented by the polarisation P (see e.g. CitationEvans and Wennerström 1994; CitationJackson 1999):
In thermal equilibrium F adopts a minimum with respect to the functions ,
and
. The results of the variational procedure are (CitationIglič et al. 2010):
The above expression for (Equation (38)) is consistent with the usual definition of relative permittivity (CitationGongadze et al. 2010, Citation2011a):
In this paper, the LB Equation (37) was solved numerically for planar geometry using the finite element method (FEM) within the Comsol Multiphysics 3.5a Software program package (COMSOL AB, Stockholm, Sweden). The space dependence of (Equation (38)) in Equation (36) was taken into account in an iterative procedure, where the initial value of
is constant equal to the permittivity of the bulk solution. The boundary conditions (44) and (45) are taken into account.
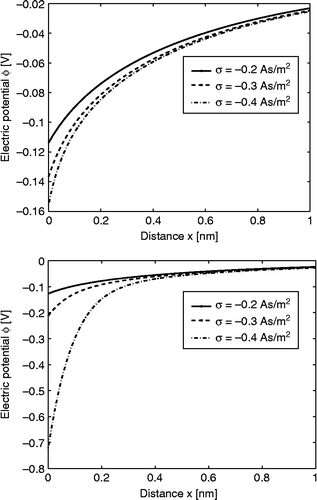
shows the calculated spatial dependence of for three values of the surface charge density
. The decrease in
towards the charged surface for larger values of
is a consequence of the increased orientational ordering of water dipoles (saturation effect) and the increased depletion of water molecules () near the charged surface due to accumulation of counterions ().
Figure 2 Relative permittivity (Equation (38)) as a function of the distance from the charged surface x within the LB model for finite-sized ions. Three values of surface charge density were considered:
,
and
. Equation (36) was solved numerically as described in the text. The dipole moment of water
, bulk concentration of salt
, bulk concentration of water
, where
is the Avogadro number.
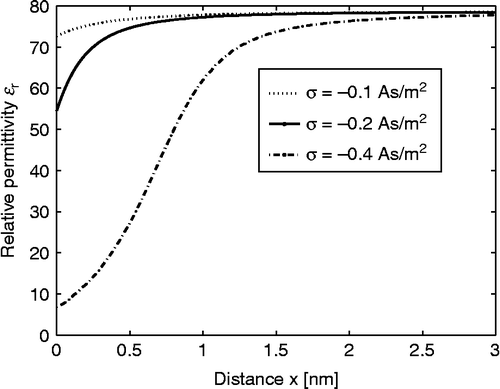
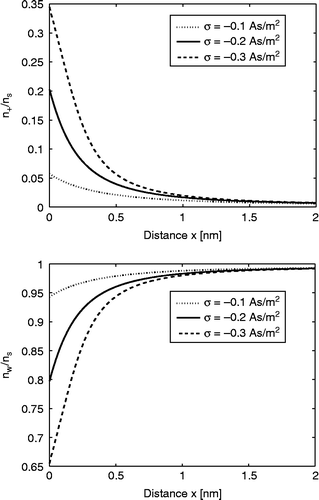
Figure 3 The relative number density of counter ions and water Langevin dipoles
as a function of the distance from the charged surface x (calculated using Equations (21) and (23), respectively) within the LB model for finite-sized ions. Three values of surface charge density were considered:
,
and
. Equation (36) was solved numerically as described in the text. The other values of the model parameters are the same as in Figure 2.
The distribution functions (2122(23) can also be derived without minimisation of the system free energy by using only Boltzmann factors within lattice statistics (CitationGongadze et al. 2011c). Here again the finite size of molecules is considered by assuming that ions and water dipoles are distributed in a lattice, where each lattice site is occupied by only one of the three molecular species (cations, co-ions and water molecules).
Since in the bulk solution, i.e. far away from the charged surface, the number densities of water molecules (), counterions (
) and co-ions (
) are constant, their number densities can be expressed in a simple way by calculating the corresponding probabilities that a single lattice site is occupied by one of the three particle types in the electrolyte solution (counterions, co-ions and water molecules):
Bikerman and PB models
In the limit of , the particle distribution functions (2122(23) transform into Fermi–Dirac-like distributions in the form (CitationBikerman 1942; CitationGrimley and Mott 1947; CitationGrimley 1950; CitationFreise 1952; CitationDutta and Sengupta 1954; CitationEigen and Wicke 1954; CitationWiegel and Strating 1993; CitationIglič and Kralj-Iglič 1994; CitationKralj-Iglič and Iglič 1996):
LPB model considering spatial variation of the relative permittivity for point-like ions
In this section, we describe the LPB mean-field model of the EDL for point-like ions, where the spatial variation of permittivity (i.e. orientational ordering of water dipoles) is taken into account. Again we consider a planar-charged surface with surface charge density in contact with a water solution of monovalent ions (counterions and co-ions). Unlike in Section 2, the finite volume of ions and water in the electrolyte solution is not taken into account. Accordingly, the volume density of water is constant in the whole electrolyte solution (Equation (57)) (CitationKralj-Iglič and Iglič 1996), while the configurational entropy of the ions can be expressed by Equation (5). Therefore, the free energy of the system F can be written as (see also Equation (1))
The LPB Equation (37) was solved numerically for planar geometry using the FEM within the Comsol Multiphysics 3.5a Software program package as already described above. The space dependence of (Equation (69)) in Equation (67) was taken into account in an iterative procedure, where the initial value of
is constant and equal to the permittivity of the bulk solution. The boundary conditions (70) and (71) are taken into account.
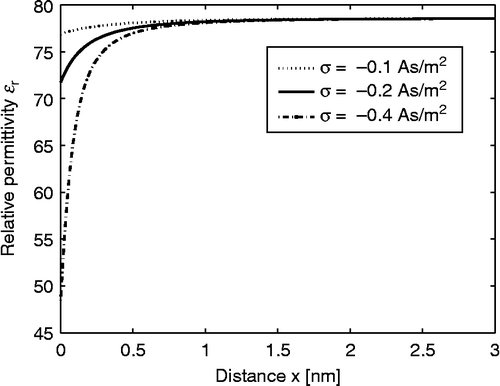
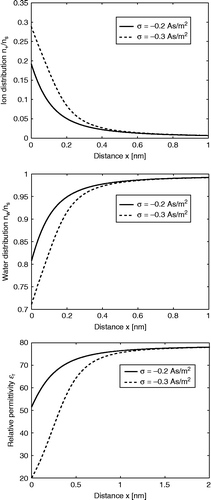
shows the spatial dependence of calculated within the LPB model for point-like ions (Equation (69)) using two values of the surface charge density
. The decrease in
towards the charged surface is now a consequence of the increased orientational ordering of water dipoles near the charged surface only. Therefore, it is less pronounced than in the case of the LB model for finite-sized ions (), where the depletion of water molecules near the charged surface additionally decreases
.
Figure 4 Relative dielectric permittivity (Equation (69)) as a function of the distance from the charged surface x within the LPB model for point-like ions. Three values of surface charge density were considered:
, σ = − 0.2 As/m2 and
. The LPB equation (67) was solved numerically as described in the text. The dipole moment of water
, bulk concentration of salt
, bulk concentration of water
, where
is the Avogadro number.
For , we can expand the Langevin function in Equation (72) into a Taylor series up to the cubic term:
to get:
In the approximation of a small electrostatic energy and small energy of dipoles in the electric field compared to thermal energy, i.e. small and small
, also the relative permittivity within the LB model for finite-sized ions (Equation (38)) can also be expanded into a Taylor series to get:
Comparison of LPB and LB models
Comparison of the approximative expression for the relative permittivity , calculated within the LB model for finite-sized ions (Equation (75)) and within LPB theory for point-like ions (Equation (73)), we can see that the first three terms in the expansions are equal in both models. The third term represents the effect of orientation of water molecules in the electric field near the charged membrane surface. The fourth term in Equation (75) describes the decrease in
near the charged membrane surface due to depletion of water dipoles, because of the accumulation of counterions. Based on Equations (75) and (73), it can be concluded that the relative permittivity of the electrolyte near the charged membrane surface is reduced relative to its bulk value due to preferential orientation of water molecules and due to depletion of water molecules in the close vicinity of the charged surface.
shows the electric potential as a function of the distance from the charged planar surface (x) calculated within the LPB model and the LB model. It can be seen that the potential drop near the charged surface is largest in the LB model which takes into account the finite size of ions, while in the LPB model for point-like ions the potential drop is smaller; this can be explained by the larger value of near the charged surface for point-like ions than for finite-sized ions, as shown in and .
Figure 5 Electric potential as a function of the distance from the charged planar surface x within the LPB model for point-like ions (upper figure) and within the LB model for finite-sized ions (lower figure) for three values of the surface charge density;
,
and
. The dipole moment of water
, bulk concentration of salt
and bulk concentration of water
.
Stern model and the distance of closest approach
Stern model
Within the Stern model (CitationStern 1924; CitationButt et al. 2003), the concentration of charged ions obeys the Boltzmann distribution law (Equations (55) and (56)), while the electrostatic potential is determined by the PB equation (Equation (58)). What makes the Stern model different from the usual PB (Gouy–Chapman) theory for point-like ions (CitationGouy 1910; CitationChapman 1913; CitationMcLaughlin 1989; CitationSafran 1994) is the distance of closest approach of ions (b) to the charged surface, so the PB Equation (58) in the simplest version of the Stern model is replaced by
Stern–Langevin–Poisson–Boltzmann and Stern–Langevin–Bikerman models
Generalisation of the above Stern model within LPB theory for point-like ions includes the orientational ordering of water dipoles, while the ions are still considered as point-like particles as described in Section 4:
A further generalisation of the Stern model is the Stern–Langevin–Bikerman (SLB) model for finite-sized ions (Section 2):
SLB model with a step function
In order to better capture the discrete character of the thin layer of ordered water molecules at the charged surface, the continuous dependence of relative permittivity (Equation (82)) in the region
, where
is the width of a single lattice site, may be described by a step function. Hence
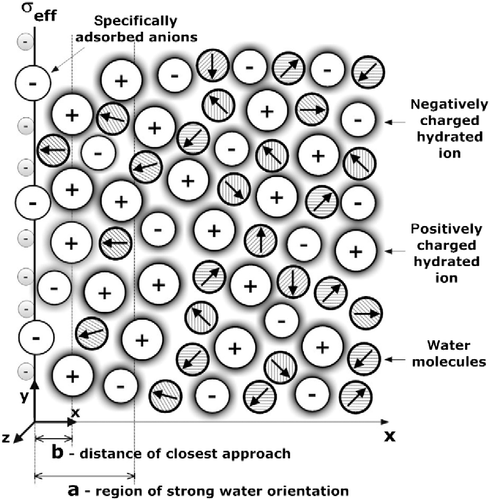
Figure 6 Charge distribution SLB model (CitationGongadze et al. 2011c), where in the interval is the region of strong water orientation and b is the distance of closest approach. The surface charge density
incorporates the negatively charged metallic surface, as well as the specifically bound negatively charged ions (CitationButt et al. 2003).
Boundary conditions
As already shown above, the boundary conditions at the charged surface are consistent with the condition of electro-neutrality of the whole system:
Due to the screening effect of the negatively charged surface caused by the accumulated cations, far away from the charged metal surface the electric field strength tends to zero, which means that the electric potential is constant. As already taken into account in the above derivations (see e.g. Equation (31)), we assume:
Spatial variation of electric potential in different models
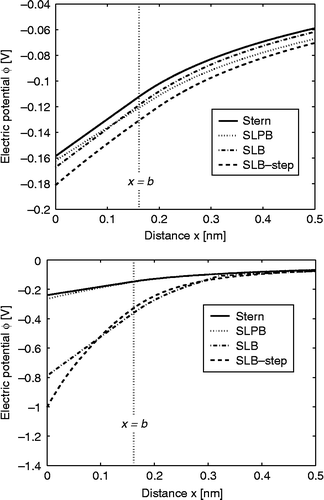
shows the electric potential as a function of the distance from the charged planar surface (x) calculated within the classical Stern model, the Stern–Langevin–PB (SLPB) model, the SLB model and the SLB model with the relative permittivity represented as a step function. The potential drop near the charged surface is the smallest in the Stern model where is constant everywhere in solution and equal to its bulk value. As expected, the electric potential changes linearly in the region
, but then for x>b the slope (i.e. the electric field strength) changes substantially (see ). The main reason for such behaviour is that the electric field strength close to the charged surface in the region
(where the free ions are depleted) is determined by the boundary condition at the charged metal surface (at x = 0). Therefore, in this region the electric field strength is
.
Figure 7 Electric potential as a function of the distance from the charged planar surface x (
) within the Stern model (Equation (77)), the SLPB model for point-like ions (Equation (79)), the SLB model for finite-sized ions (Equation (81)) and the SLB model with a step function for finite-sized ions (Equation (83)) for c = 0, where in all four cases the distance of closest approach
was taken into account. The value of the surface charge density was considered to be:
(upper figure) and
(lower figure). The remaining parameters used are dipole moment of water,
; bulk concentration of salt,
and bulk concentration of water,
, where
is Avogadro number.
Cavity and reaction field
The effective dipole moment of the water molecule should be known before a satisfactory statistical mechanical study of water and aqueous solutions is possible (CitationAdams 1981). The dipole moment of a water molecule in liquid water differs from that of the isolated water molecule because each water molecule is further polarised (i.e. the dipole moment is further increased) and orientationally perturbed by the electric field of the surrounding water molecules (CitationAdams 1981). Accordingly, in the above-described treatment of water ordering close to the saturation limit at high electric field within the LPB and LB models, the effective dipole moment of water is larger than the dipole moment of an isolated water molecule
. However, it is also larger than the dipole moment of a water molecule in clusters
and the dipole moment of an average water molecule in the bulk
(CitationDill and Bromberg 2003) since the cavity and reaction fields as well as structural correlations between water dipoles (CitationFröhlich 1964; CitationFranks 1972) were not explicitly taken into account in the LPB and LB models.
In the past treatment of the cavity and reaction fields and the correlations between water dipoles in the CitationOnsager (1936), CitationKirkwood (1939) and CitationFröhlich (1964) models were limited to the case of small electric field strengths, i.e. far away from saturation limit considered in the LB model and also in the LPB model. Generalisation of the Kirkwood–Onsager–Fröhlich theory in the saturation regime was performed by CitationBooth (1951). However, Booth's model does not consider the excluded volume effect in an electrolyte solution near a charged surface as described in the LB model and is therefore appropriate only for the LPB model. Therefore in this section, first the LPB model (CitationGongadze et al. 2011a) is generalised to take into account the cavity field, as well as the structural correlations between the water dipoles close to the saturation regime by utilising the Booth expression for relative permittivity. At the end, generalisation of LB model is also given by taking into account the cavity field (but not the structural correlations between water dipoles) in the saturation regime important in consideration of an electrolyte solution in contact with highly charged surface (CitationGongadze and Iglič 2012).
BPB model
To take into account the cavity field, as well as structural correlations between water dipoles within the LPB model, the LPB equation for point-like ions (see Equations (67) and (68)):
In the approximation of small energy of dipoles in the electric field compared to the thermal energy, the relative permittivity within the BPB model for point-like sized ions (Equation (93)) can be expanded in a Taylor series to get
Figure 8 Relative permittivity as a function of the magnitude of electric field strength (E) within the LPB model (Equation (69)) and BLP model (Equation (93)) for point-like ions and
, where
is the Avogadro number. In the case of the LPB model, the effective dipole moment of water
, while in the BLP model the dipole moment of water
and
.
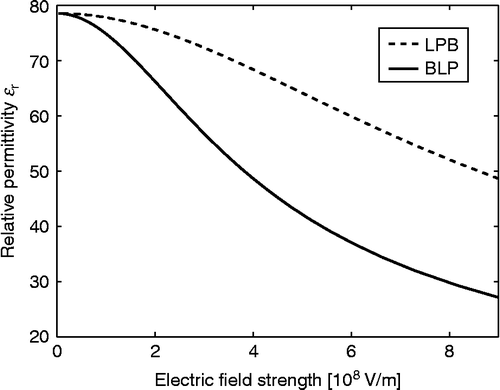
shows the relative permittivity as a function of the magnitude of the electric field strength (E) within the LPB and Booth–Langevin–Poisson (BLP) models, both for point-like ions. It can be seen that in the two models
decreases with increasing E; however in the BLP, it drops already at around
to a half of its bulk value. Obviously, including the cavity field and structural correlations between water dipoles leads to a stronger saturation of the relative permittivity than by only considering the orientational ordering of water molecules.
MLB model
In the model, electronic polarisation is taken into account by assuming that the point-like rigid (permanent) dipole embedded in the centre of the sphere with a volume equal to the average volume of a water molecule in the electrolyte solution. The permittivity of the sphere is taken to be , where
is the optical refractive index of water. The relative (effective) permittivity of the electrolyte solution
can be then expressed as
In the approximation of small electrostatic energy and small energy of dipoles in the electric field compared to thermal energy, i.e. small and small
, the relative permittivity within the MLB model for finite-sized ions (Equation (117)) can be expanded into a Taylor series (assuming
) to get (CitationGongadze and Iglič 2012):
The value is also close to the experimental values of the effective dipole moment of water molecules in clusters
and in bulk solution
(CitationDill and Bromberg 2003). The MLB model does not, however, neglect the main (qualitative) predictions of the LB model where all the equations (including the expression for the relative permittivity) have a similar structure as in the MLB, only the effective value of the water dipole moment
is larger. Moreover, for
and
, the equations of the above described MLB model transform into equations of LB model.
shows the calculated spatial dependence of relative number density of counter ions , water dipoles
and
within MLB model in planar geometry for two values of the surface charge density
. The decrease in
towards the charged surface is pronounced with increasing
and is a consequence of the increased depletion of water molecules near the charged surface (due to excluded volume effect as a consequence of counterions accumulation near the charged surface) and increased orientational ordering of water dipoles (saturation effect). Comparison between the predictions of the LB model and the presented MLB model shows the stronger decrease in relative permittivity of the electrolyte solution near the highly charged surface stronger in MLB model.
Figure 9 The relative number density of counter ions , water dipoles
(calculated using Equations (111) and Equation (113) and relative permittivity
(Equation (117)) as a function of distance from a planar-charged surface x (adapted from CitationGongadze and Iglič 2012). Two values of surface charge density were considered:
and
. Equation (118) was solved numerically taking into account the boundary conditions (120) and (121) as described in the text. Values of parameters assumed are dipole moment of water,
; bulk concentration of salt,
; optical refractive index,
; bulk concentration of water,
, where
is Avogadro number.
Conclusions
To conclude, in this work we described different modifications of the PB model of the EDL by introducing the orientational ordering of water molecules (also close to the saturation regime) and the finite size of molecules. The corresponding LPB model for point-like ions and the LB model and generalised Stern model for finite-sized ions were derived. The Bikerman model is derived as the limiting case of the LB model for finite-sized ions. It is shown that due to the increased magnitude of the electric field in the vicinity of the charged surface in contact with the electrolyte solution, the relative permittivity of the electrolyte solution in this region is decreased. The predicted decrease in the relative permittivity relative to its bulk value is the consequence of the orientational ordering of water dipoles in the vicinity of the charged surface (saturation effect). Due to accumulation of counterions near the charged surface, the number density of water molecules near the charged surface is decreased and as a result the relative permittivity is additionally decreased (excluded volume effect).
The electric field may influence the dipole moment of the water in two ways. First, it perturbs the average orientation of the water dipole, and second, it induces an increase in the magnitude of the water dipole moment, mainly by elastic displacement of the atomic electrons relative to their respective nuclei (CitationFröhlich 1964). The magnitude of the induced water dipole moment is determined by the polarisability of the molecule, i.e. the proportionality coefficient between the induced dipole moment and , where
is the local electric field strength as defined above (Equation (101)).
In order to (partially) capture these two effects in our theoretical description of the permittivity of water, we applied the concept of the cavity field (CitationOnsager 1936; CitationFröhlich 1964) in the MLB (Gongadze–Iglič) model (valid also in the saturation limit) by simultaneously taking into account the volume-excluded effect. To our knowledge this was done for the first time. The corresponding analytical expression for the spatial dependence of the relative (effective) permittivity of the electrolyte solution near the charged surface was derived (CitationGongadze and Iglič 2012).
Acknowledgements
This work was supported by the Slovenian Research Agency grants J3-2120, J1-4109, J1-4136, J3-4108, P2-0232-1538 and DFG for project A3 in the Research Training Group 1505/1 “welisa”.
REFERENCES
- AdamsDJ. Theory of the dielectric constant of ice. Nature. 293(5832):447–449.
- ArsovZ, RappoltM, GrdodolnikJ. 2009. Weakened hydrogen bonds in water confined between lipid bilayers: the existence of a long-range attractive hydration force. Chem Phys Chem. 10(9–10):1438–1441.
- BarberoG, EvangelistaLR, OliveroD. 2000. Asymmetric ionic adsorption and cell polarization in liquid crystals. J Appl Phys. 87(5):2646–2648.
- BazantMZ, KilicMS, StoreyB, AjdariA. 2009. Towards an understanding of induced-charge electrokinetics at large applied voltages in concentrated solutions. Adv Colloid Interface Sci. 152(1–2):48–88.
- BiesheuvelPM, van SoestbergenM. 2007. Counterion volume effects in mixed electrical double layers. J Coll Int Sci. 316(2):490–499.
- BikermanJJ. 1942. Structure and capacity of the electrical double layer. Phil Mag. 33:384–397.
- BivasI. 2006. Electrostatic and mechanical properties of a flat lipid bilayer containing ionic lipids. Coll Surf A. 282–283(1):423–434.
- BivasI, ErmakovYA. 2007. Elasticity and electrostatics of amphiphilic layers. In: Leitmannova LiuA, editor. Advances in planar lipid bilayers and liposomes. Vol. 5. Amsterdam: Elsevier. p. 313–343.
- BoothF. 1951. The dielectric constant of water and the saturation effect. J Chem Phys. 19(4):391–394.
- BoulbitchA, GuttenbergZ, SackmannE. 2001. Kinetics of membrane adhesion mediated by ligand receptor interaction studied with a biomimetic system. Biophys J. 81(5):2743–2751.
- ButtHJ, GrafK, KapplM. 2003. Physics and chemistry of interfaces. 1st ed.Weinheim: Wiley-VCH Verlag.
- CevcG. 1990. Membrane electrostatics. Biochim Biophys Acta. 1031(3):311–382.
- ChapmanDL. 1913. A contribution to the theory of electrocapillarity. Philos Mag. 25(148):475–481.
- CoalsonRD, DuncanA. 1996. Statistical mechanics of a multipolar gas: a lattice field theory approach. J Phys Chem. 100(7):2612–2620.
- DillKA, BrombergS. 2003. Molecular driving forces. New York and London: Garland Science.
- DuttaM, SenguptaM. 1954. A theory of strong electrolytes in solution based on new statistics. Proc Natl Inst Scif India. 20:1–11.
- EigenM, WickeE. 1954. The thermodynamics of electrolytes at higher concentrations. J Phys Chem. 58(9):702–714.
- EvansDF, WennerströmH. 1994. The colloidal domain: where physics, chemistry, biology, and technology meet, Advances in artificial engineering series. New York: Wiley-VHC.
- FranksF. 1972. Water. A comprehensive treatise. In: The physics and physical chemistry of water. Vol. 1. New York: Plenum Press.
- FreiseV. 1952. Zur Theorie der Diffusendoppelschicht. Z Elektrochem. 56:822–827.
- FröhlichH. 1964. Theory of dielectrics. Oxford: Clarendon Press.
- GongadzeE, IgličA. 2012. Decrease of permittivity of an electrolyte solution near a charged surface due to saturation and excluded volume effects. Bioelectrochemistry, 10.1016/j.bioelechem.2011.12.001.
- GongadzeE, BohincK, van RienenU, Kralj-IgličV, IgličA. 2010. Spatial variation of permittivity near a charged membrane in contact with electrolyte solution. In: IgličA, editor. APLBL. Vol. 11. Amsterdam: Elsevier. p. 101–126.
- GongadzeE, van RienenU, Kralj-IgličV, IgličA. 2011a. Langevin Poisson–Boltzmann equation: point-like ions and water dipoles near charged membrane surface. Gen Physiol Biophys. 30(2):130–137.
- GongadzeE, KabasoD, BauerS, SlivnikT, SchmukiP, van RienenU, IgličA. 2011b. Adhesion of osteoblasts to a nanorough titanium implant surface. Int J Nanomed. 6:1801–1816.
- GongadzeE, van RienenU, Kralj-IgličV, IgličA. 2011c. Generalized Stern models of an electric double layer considering the spatial variation of permittivity and finite size of ion in saturation regime. Cell Mol Biol Lett. 16(4):576–594.
- GouyMG. 1910. Sur la constitution de la charge electrique a la surface d'un electrolyte. J Physique (France). 9(1):457–468.
- GrimleyTB. 1950. The contact between a solid and an electrolyte. Proc R Soc Lond A. 201(1064):40–61.
- GrimleyTB, MottNF. 1947. The contact between a solid and a liquid electrolyte. Discuss Faraday Soc. 1:3–11.
- GruenDWR, MarčeljaS. 1983. Spatially varying polarization in water. J Chem Soc Faraday Trans. II. 79(2):225–242.
- HelmholtzH. 1879. Studien über elektrische Grenzschichten. Ann Phys. 7:337–382.
- HianikT, PassechnikVI. 1995. Bilayer lipid membranes: structure and mechanical properties. Dordrecht: Kluwer Academic Publishers. p. 138–157.
- HillTL. 1986. An introduction to statistical thermodynamics. New York: Dover Publications.
- Ibarra-ArmentaJG, Martin-MolinaA, Quesada-PerezM. 2009. Testing a modified model of the Poisson–Boltzmann theory that includes ion size effects through Monte Carlo simulations. Phys Chem Chem Phys. 11(2):309–316.
- IgličA, Kralj-IgličV. 1994. Influence of finite size of ions on electrostatic properties of electric double layer. Electrotecnical Rev. 61(3):127–133.
- IgličA, GongadzeE, BohincK. 2010. Excluded volume effect and orientational ordering near charged surface in solution of ions and Langevin dipoles. Bioelectrochemistry. 79(2):223–227.
- IsraelachviliJN, WennerströmH. 1996. Role of hydration and water structure in biological and colloidal interactions. Nature. 379(6562):219–225.
- JacksonJD. 1999. Classical electrodynamics. New York: Wiley and Sons, Inc.
- KabasoD, GongadzeE, PerutkováŠ, Kralj-IgličV, MatschegewskiC, BeckU, van RienenU, IgličA. 2011. Mechanics and electrostatics of the interactions between osteoblasts and titanium surface. Comput Meth Biomech Biomed Eng. 14(5):469–482.
- KirkwoodJG. 1939. The dielectric polarization of polar liquids. J Chem Phys. 7(10):911–919.
- Kralj-IgličV, IgličA. 1996. A simple statistical mechanical approach to the free energy of the electric double layer including the excluded volume effect. J Phys II France. 6(4):477–491.
- LamperskiS, OuthwaiteCW. 2002. Volume term in the inhomogeneous Poisson–Boltzmann theory for high surface charge. Langmuir. 18(9):3423–3424.
- ManciuM, RuckensteinE. 2002. Lattice site exclusion effect on the double layer interaction. Langmuir. 18(13):5178–5185.
- ManciuM, RuckensteinE. 2004. The polarization model for hydration/double layer interactions: the role of the electrolyte ions. Adv Coll Int Sci. 112(1–3):109–128.
- McLaughlinS. 1989. The electrostatic properties of membranes. Ann Rev Biophys Chem. 18:113–136.
- OghakiM, KizukiT, KatsuraM, YamashitaK. 2001. Manipulation of selective cell adhesion and growth by surface charges of electrically polarized hydroxyapatite. J Biomed Mater Res. 57(3):366–373.
- OnsagerL. 1936. Electric moments of molecules in liquids. J Am Chem Soc. 58(8):1486–1493.
- OuthwaiteCW. 1976. A treatment of solvent effect in the potential theory of electrolyte solution. Mol Phys. 31(5):1345–1357.
- OuthwaiteCW. 1983. Towards a mean electrostatic potential treatment of an ion-dipole mixture or a dipolar system next to a plane wall. Mol Phys. 48(3):599–614.
- SafranS. 1994. Statistical thermodynamics of surfaces, interfaces, and membranes. Reading (MA): Addison-Wesley Publishing Company.
- SmithIO, BaumannMJ, McCabeLR. 2004. Electrostatic interactions as a predictor for osteoblast attachment to biomaterials. J Biomed Mater Res A. 70(3):436–441.
- SternO. 1924. Zur Theorie der elektrolytischen Doppelschicht. Z Elektrochemie. 30:508–516.
- TengNC, NakamuraS, TakagiY, YamashitaY, OhgakiM, YamashitaK. 2000. A new approach to enhancement of bone formation by electrically polarized hydroxyapatite. J Dent Res. 80(10):1925–1929.
- TressetG. 2008. Generalized Poisson–Fermi formalism for investigating size correlation effects with multiple ions. Phys Rev E. 78(6):061506.
- TrizacE, RaimbaultJL. 1999. Long-range electrostatic interactions between like-charged colloids: steric and confinement effects. Phys Rev E. 60(6):6530–6533.
- WiegelFW, StratingP. 1993. Distribution of electrolytes with excluded volume around a charged DNA molecule. Mod Phys Lett B. 7(7):483–490.
- ZelkoJ, IgličA, Kralj-IgličV, KumarPBS. 2010. Effects of counterion size on the attraction between similarly charged surfaces. J Chem Phys. 133(20):204901.
Appendix: Configurational entropy of electrolyte solution
We consider the configurational entropy of the solution composed of counterions and co-ions. Following the classical well-known approach (CitationHill 1986), the finite sizes of ions (macroions) are considered within the lattice model (CitationKralj-Iglič and Iglič 1996). The system is divided into cells of equal volume . In the particular cell chosen, there are
counterions and
co-ions. The number of spatial arrangements of non-interacting counterions and co-ions in a small cell with M lattice sites is (CitationHill 1986):
If we assume that ,
, everywhere in the solution, as well as
, we can expand the third term in Equation (136) up to quadratic terms to get (see for example CitationKralj-Iglič and Iglič 1996):