?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.1. Introduction
Haemorrhage stroke is a major issue for public health care. The prevalence of intracranial aneurysms in the general population ranges between 3 and 6.6% (Wardlaw and White Citation2000). Fortunately, very few aneurysms rupture. The incidence of ruptured aneurysms is approximately 0.5% per year. However, patients are stressful and wants to be treated. But, endovascular treatment of unruptured aneurysms, which is the safest treatment, is not without risk and has about 1% mortality rate (Sluzewski et al. Citation2001). So, unruptured intracranial aneurysms represent a dilemma for the physicians.
In Citation2011 (Costalat et al.), we have proposed an indicator base on the relationship between the mechanical properties of wall aneurysm tissues and the rupture risk. First conclusions suggested that the softer tissue is, the higher rupture risk is.
The present study aims to confirm these results, initiates with only 16 samples, studying 26 more aneurysms.
2. Methods
2.1. Patients and clinical data
A total of sixty-three patients treated for ruptured or unruptured aneurysms by surgical clipping were recruited by four French neurosurgical teams. The research study protocol was approved by the local ethical committee in each center. A consent form was signed by patients with normal neurological status, or by the relatives in all other cases. For each patient, clinical, and radiological information was collected concerning age, gender, aneurysm status (ruptured/unruptured) … All documented risk factors were recorded in order to be related to the biomechanical behavior of each aneurysm.
2.2. Biomechanical testing methodology
Immediately after resection, each aneurysm wall was progressively frozen at −80 °C. One hour before mechanical testing, aneurysm samples were thawed at ambient temperature. Then a regular rectangular piece was dissected from the aneurysm preserving maximum length. This was a delicate point due to the small size of the samples. Many of them were lost during this cutting.
Once cut, uniaxial stretch tests were carried out on the sample within a warmed (37 °C) physiological liquid in order to simulate in vivo conditions. This testing device was composed of a Texture Analyzer (TA-XT2, Stable Microsystems, UK) with a 50 N load cell. Aneurysm strips were fixed by glue on each extremity to aluminium grips.
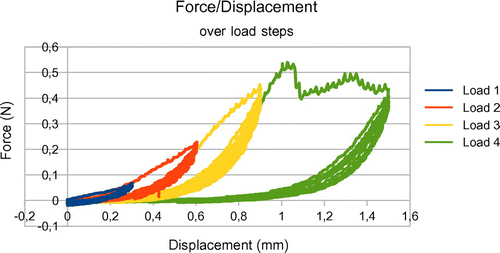
The uniaxial stretch tests consisted of a series of increasing load steps until rupture of the sample (see Figure ). For each load step the sample was stretched then unloaded and that was repeated five times. The extension rate was small enough to not consider viscous phenomena (0.01 mm/s). A baseline tension of 0 N was applied to the strip before starting each test.
2.3. Data post-processing: mechanical parameter identifications
Only the measurements from the last elongation of load step 1 were used to obtain more realistic mechanical characterization of the data, in the range of physiological solicitations. Using the assumption that the specimen was subjected to a uniform traction, the engineering stress, noted Π, was computed, and the engineering strain, noted ε, registered. Once the strain/stress graph was obtained, we proceeded to a curve fitting using a Sequential Least Squares Programming algorithm in order to determine the corresponding hyperelastic model and its coefficients. In our cases, the best match was obtained with an incompressible 2 parameters Yeoh model. Let F be the measured force, the initial section and λ = 1 + ε the elongation of the sample, the behavior law is given by the following equation:
where mechanical parameters and C2 have to be identified.
3. Results and discussion
For this type of behavior law, parameter is related to the Young’s modulus E, which is the slope at origin (λ = 1) of the function Π(λ). It could be easily demonstrated that
is equals to E/6. About parameter C2, it is representative of the curvature of the function Π(λ). The larger parameter C2 is, the higher rigidity of the material is. After the identification procedure, we gathered the values of parameters C2 in graphs of Figure . These values are sorted according to the aneurysm status before surgery and to the gender. On average, unruptured aneurysms are stiffer than ruptured ones. But, the results show also that some unruptured aneurysms have soft material properties. For both parameters, there seems to be an upper limit for ruptured aneurysms: 0.2 MPa for C1 and 10 MPa for C2. Moreover, we noticed that all ruptured aneurysms have a
lower than 3.7 MPa, except one which is equal to 7 MPa. Then we stratified aneurysms in 3 categories:
| • | Soft tissues are those having a | ||||
| • | Intermediates tissues are those having a | ||||
| • | Stiff tissues are those having a | ||||
Figure 2. Hyperelastic parameter C2 vs. status (Ruptured (red)/Unruptured (green)) and gender (Female (circle)/Male (square)) of the aneurysm.
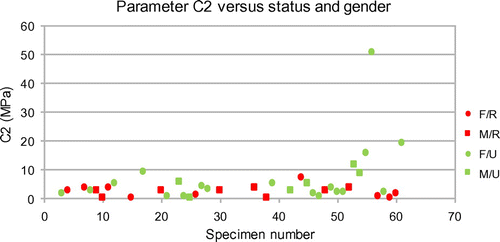
Almost certainly the physicians did the good choice in treating the unruptured aneurysms having a C2 lower than 3.7 MPa (soft tissues). In a general manner, both soft and intermediate tissues had a risk of rupture, because we found a ruptured aneurysm with a C2 equals to 7 MPa. At this time, only stiff tissues (C2 > 7 MPa) seem to be safe.
In terms of comparisons, we are able to compare parameter C1 with those obtained in the literature.
Valencia et al. (Citation2015) extracted from a Mooney-Rivlin hyperelastic model, an average value of parameters C01 + C10, equivalent to our C1, of about 0.17 MPa, while Toth et al. (Citation2007) found 0.12 MPa. They are of same order than our average value for C1: 0.11 MPa. Unfortunately, we cannot compare C2, which is however the most significant.
Because of the surgical resection, only aneurysms easily and safely accessible were selected, introducing a potential selection bias. Uniaxial strain/stress testing is not representative of the anisotropic behavior of the aneurysm wall in vivo, but the small size of the strips was a limitation to perform bi-axial tests. The taking of measures, such as thickness and the width, was difficult to carried out because samples were relatively irregular. So, we took average values, what can skew the estimation of C2. Because of the small sample sizes, in several cases, the hypothesis of pure traction, was not insured (the proportion of the length over the width was too low). This is an additional factor of error to be considered.
4. Conclusions
With a total of 42 analyzed aneurysms, our study confirms that ruptured aneurysms are mainly presenting a soft tissue and unruptured aneurysms, presenting a stiff material. But, contrary to what we observed in the previous study (Costalat et al. Citation2011), including only 16 samples, gender has no influence on material parameters. Aneurysmal tissues from males seem not to be stiffer than those of females and vice versa.
References
- Costalat V, et al. 2011. Biomechanical wall properties of human intracranial aneurysms resected following surgical clipping. J Biomechanics. 44(15):2685–2691.10.1016/j.jbiomech.2011.07.026
- Sluzewski M, et al. 2001. Rupture of intracranial aneurysms during treatment with Guglielmi detachable coils: incidence, outcome, and risk factors. J Neurosurg. 94:238–240.10.3171/jns.2001.94.2.0238
- Toth B, Nasztanovics F, Bojtar I. 2007. Laboratory tests for strength paramaters of brain aneurysms. Acta Bioeng Biomech. 9:3–7.
- Valencia A, et al. 2015. Mechanical test of human cerebral aneurysm specimens obtained from surgical clipping. J Mech Med Biol. 15:1550075.10.1142/S021951941550075X
- Wardlaw JM, White PM. 2000. The detection and management of unruptured intracranial aneurysms. Brain. 123(2):205–221.10.1093/brain/123.2.205