?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.1. Introduction
Bone is a living material in continual renewal (Frost Citation1987). Each year, 5% of trabecular bone and 20% of cortical bone is renewed. It undergoes continual adaptation under applied external mechanical constraints as initially phenomenologically modeled by (Wolff Citation1892). Many multi-scale and/or multi-physic theoretical, numerical models have followed since (see for example George Citation2017; Giorgio Citation2016; Madeo Citation2012; Scala Citation2016; Spingarn Citation2017) trying to predict the kinetics of bone remodeling. But, many difficulties remain in the precise understanding of the mechanotransduction processes (Lemaire Citation2015) driving this remodeling. For example, in orthodontic treatment, bone remodeling occurs due to the applied orthodontic forces ranging between 0.5 and 2.5 N (Wagner Citation2017). The periodontal bone will resorb in the compressed area while being reconstructed in the tensile area enabling teeth displacements (Cattaneo Citation2009). Under these low amplitude forces, the vascularization of the periodontal ligament is partially occluded in the compression zone while being dilated in the tension zone. Cell differentiation and activation is altered, due to nutriments variation, resulting in the bone remodeling. When compression is too important, the ligament is no longer vascularized and hyalinization occurs, resulting in a decrease in the amount of cells and death of the surrounding tissue (Liao Citation2016). While a weak force allows steady and continual tooth displacement, an important force induces abrupt movement and necrosis of the bone. In the current work, we study the variations in vascularization blood flow in the periodontal ligament and thus in the supply chain of nutrients and oxygen to predict cell recruitment, proliferation and migration assuming that bone resorption occurs by the osteoclasts proliferating in hypoxia (Arnett Citation2003) and bone reconstruction occurs by the osteoblasts proliferating with oxygen increase (Tuncay Citation1994).
2. Method
Bone remodeling being the result of numerous mechanobiological mechanisms, we propose to describe its evolution through the use of a biomechanical stimulus ΔS, describing a variation from the state of mechanobiological equilibrium, defined in the Lagrangian configuration (Madeo, Citation2012; Scala, Citation2016), and newly expressed as:(1)
(1)
where n is the total number of external sources (mechanical, biological, electrical, neurological…) involved in the process and αi are their weighting coefficients (), triggered by genetic and/or epigenetic factors, allowing to simultaneously control their impact on the overall response of the system as well as their interactions.
and
are the kinematical fields that associate to any material point its current (
) and reference (
) position respectively, and Di is a characteristic distance accounting for each independent effect. Among the potential external sources, we consider in this work: (i) the mechanical energy accounting for the compressive and tensile loads sustained by the bone cells and triggering bone growth and resorption respectively, (ii) the concentration of oxygen, glucose and other cell nutriments expressed as function of the hydrostatic pressure and of the vascular density in specifics regions of the system, and (iii) the osteoblasts and/or osteoclasts activity triggered by specific levels of oxygen and glucose concentration together with the intensity of the mechanical force applied. More specifically, the osteoblasts and osteoclasts recruiting and migration are described via two diffusion equations (Allena Citation2014; Schmitt, Citation2016) as follows
(2)
(2)
where cj is the cell density (with j being the osteoblasts or osteoclasts), t is the time, αj and βj are two coefficients of proliferation and differentiation respectively. The diffusion tensor D depends on the principal strains (∊i) and directions (θi) and reads(3)
(3)
with and ϕj two coefficients and I the identity matrix. The bone density variation in time ρb is given by
(4)
(4)
where rb and sb are the rates for bone resorption and synthesis respectively, depending on the positiveness of the defined mechanobiological stimulus, and is a fonction of the porosity φ* which reads
(5)
(5)
with k and b indicating the intensity and the maximum threshold of the bone remodeling process depending on the porosity. The parameters of equations (Equation1(1)
(1) ), (Equation4
(4)
(4) ) and (Equation5
(5)
(5) ) were identified using inverse methods from animal experimentations and modeling (Scala, Citation2016). For equations (Equation2
(2)
(2) ) and (Equation3
(3)
(3) ), the coefficients were quantified through histological analyses on sheep mandibles (Schmitt Citation2016).
A Finite Element (FE) method is used to predict bone kinetic remodelling when applied to different mechanobiological stimuli such as for example orthodontic forces. The major challenge lays in the identification and importance of each external source, their mutual interactions as well as the kinetics of each process.
3. Results
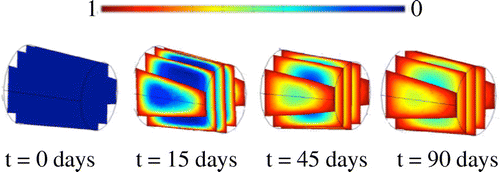
Some preliminary results have been obtained both in 2D (Schmitt, Citation2016) and 3D (Fig. ) on the modeling of cell colonization of a porous titanium scaffold under simple cyclic bending. The cells migrate from the exterior to the interior of the implant following the principal directions and after 90 days most of the space is fulfilled with mature osteoblasts cells (Fig. ).
4. Conclusion
A number of individual mechanical and biological phenomena are integrated within a single mechanobiological stimulus for bone remodeling. Each effect influences the bone density evolution and the remodeling process. The implications of these different effects could help the surgeons in the understanding and optimization of bone surgery parameters.
References
- Allena R and Maini PK. 2014. Reaction-diffusion finite element model of lateral line primordium migration to explore cell leadership. B. Math. Biol. 76(12):3028–3050.
- Arnett TR, et al. 2003. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol. 196(1):2–8.
- Cattaneo PM, et al. 2009. Strains in periodontal ligament and alveolar bone associated with orthodontic tooth movement analyzed by finite element. Orthod Craniofac Res. 12(2):120–128.
- Frost J. 1987. Bone “Mass” and the “Mechanostat”: A proposal. Anat. Rec. 219:1–9.
- George D, et al. 2017. Examples of multiscale and multiphysics numerical modeling of biological tissues. Bio-Med. Mat. Eng. 28(S1):S15–S27.
- Giorgio I, et al. 2016. A visco-poroelastic model of functional adaptation in bones reconstructed with bio-resorbable materials. Biomech Model Mechanobiol. 15:1325–1343.
- Lemaire T, et al. 2015. Three-scale multiphysics modeling of transport phenomena within cortical bone. Math. Prob. Eng. 398970.
- Liao Z, et al. 2016. Biomechanical investigation into the role of the periodontal ligament in optimizing orthodontic force: a finite element case study. Arch Oral Biol. 66:98–107.
- Madeo A, et al. 2012. A second gradient continuum model accounting for some effects of microstructure on reconstructed bone remodeling. C.R. Mécanique. 340(8):575–589.
- Scala I, et al. 2016. Mechanically-driven bone remodeling simulation: application to LIPUS treated rat calvarial defects. Math. Mech. Sol. in press, DOI:10.1177/1081286516651473.
- Schmitt M, et al. 2015. Diffusion model to describe osteogenesis within a porous titanium scaffold. Comp. Meth. Biomech. Biomed. Eng. 19(2):171–179.
- Spingarn C, et al. 2017. Multiphysics of bone remodeling: a 2D mesoscale activation simulation. Bio-Med. Mat. Eng. 28(S1):S153–S158.
- Tuncay OC, et al. 1994. Oxygen tension regulates osteoblast function. Am J Orthod Dent. Orthop. 105(5):457–463.
- Wagner D, et al. 2017. Mechanical equilibrium of forces and moments applied on orthodontic brackets of a dental arch: correlation with literature data on two and three adjacent teeth. Bio-Med. Mat. Eng. 28(S1):S169–S177.
- Wolff J. 1892. Das gesetz der transformation der knochen. 1re éd. Pro Business. 300 p.