1. Introduction
Critical to the detection of vulnerable plaque (VPs) rupture is quantification of their anisotropic mechanical properties (CitationFinet et al. 2004, CitationNayak et al. 2017). A number of biomechanical studies have identified peak cap stress amplitude as a major key predictor of vulnerability to rupture. Quantifying intraplaque stress distribution, in order to predict plaque rupture, has been a challenge. To overcome this obstacle, the local strain has been measured based on intravascular ultrasound (IVUS) sequences. If we know the anisotropic local mechanical properties of the atherosclerotic lesion, then the intraplaque stress can be directly quantified. Therefore, on the basis of IVUS strain images, CitationCéspedes et al. (2000) proposed an isotropic elasticity-palpography technique (E-PT). His approach was later improved by our group (CitationDeleaval et al. 2013) to account for the non-concentric anatomic shape of the VPs. Even though these studies highlighted original, potential and promising ‘homogenized’ approaches for improving the evaluation of VP rupture, they did not overcome a main limitation related to the anisotropic mechanical behavior of the arterial wall and the atherosclerotic lesion media.
2. Material and Methods
2.1 Anisotropic elasticity index
The present biomechanical study was designed to extend the theoretical framework of the improved isotropic E-PT (CitationDeleaval et al. 2013) by considering the anisotropic mechanical properties of the arterial wall and lesion constituents. Based on the continuum mechanics theory prescribing the strain field, an anisotropic index (AI) was defined.
This extended anisotropic E-PT was successfully applied to several coronary lesions of patients imaged in vivo with IVUS at the Cardiologic Hospital Louis Pradel of Lyon. The robustness and performance of the new anisotropic elasticity-palpography index were also investigated with respect to noise, which may affect the prediction of plaque vulnerability.
3. Results
To test the numerical performance of the proposed anisotropic E-PT algorithm, a finite element (FE) method was used to generate our input set of intraplaque displacement and radial strain fields.
We used VP geometries of patients imaged in vivo with an IVUS system operating at 40 MHz, since realistic human VP geometries were needed to perform the FE simulations. The resulting FE displacement and radial strain fields computed in the cross-sectional plane of the pathological artery and the associated internal and external contours of the coronary were the only inputs of our inverse model.
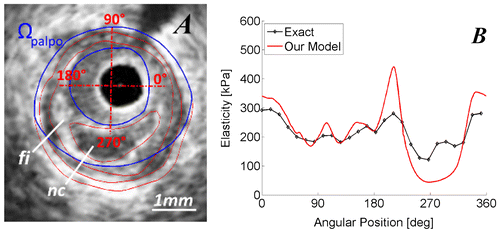
The performance of the anisotropic E-PT to detect and characterize a vulnerable plaque is showed on Figure .
4. Conclusion and clinical implication
The new anisotropic E-PT was successfully applied to several patients’ coronary lesions. Our preliminary results showed that this original technique is sufficient to detect and identify VPs.
Stabilization of vulnerable plaque remains a significant clinical problem (CitationAbela et al. 2011). Studies conducted to analyze the structural variation in the fibrous cap and necrotic core with specific drug treatments (e.g., all statins, angiotensin converting enzyme inhibitors, etc...) revealed an enhancement in plaque stability (CitationNozue et al. 2012). Our group showed (CitationFinet et al. 2004) that a very slight increase in the mechanical properties of plaque constituents, namely the hardening of the lipidic necrotic core, can tilt a VP from instability to stability. Therefore, the proposed improved anisotropic elasticity-palpography imaging technique is promising since it provides a non-invasive approach to analyze in vivo the evolution of the anisotropic mechanical properties of atherosclerotic plaques during drug therapies.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
Ricardo D. Coppel Vizcarra and Armida Gomez would like to thank the Mexican National Council for Science and Technology (CONACYT) and the Nuevo Leon Institute of Innovation and Technology Transfer (I2T2).
References
- Abela GS, Vedre A, Janoudi A, Huang R, Durga S, Tamhane U. 2011. Effect of Statins on Cholesterol Crystallization and Atherosclerotic Plaque Stabilization. Am J Cardiol. 107:1710–7.10.1016/j.amjcard.2011.02.336
- Céspedes EI, de Korte CL, van der Steen AF. 2000. Intraluminal ultrasonic palpation: assessment of local and cross-sectional tissue stiffness. Ultrasound Med Bio. 26(3):385–396.10.1016/S0301-5629(99)00169-6
- Deleaval F, Bouvier A, Finet G, Cloutier G, Yazdani SK, Le Floc’h S, Clarysse P, Pettigrew RI, Ohayon J. 2013. The intravascular ultrasound elasticity-palpography technique revisited: A reliable tool for the in vivo detection of vulnerable coronary atherosclerotic plaques. Ultrasound in Med Bio, 39(8):1469–81.10.1016/j.ultrasmedbio.2013.03.001
- Finet G, Ohayon J, Rioufol G. 2004. Biomechanical interaction between cap thickness, lipid core composition and blood pressure in vulnerable coronary plaque: impact on stability or instability. Coron Artery Dis;15:13–20.10.1097/00019501-200402000-00003
- Nayak R, Huntzicker S, Ohayon J, Carson N, Dogra V, Schifitto G, Doyley MM. 2017. Principal Strain Vascular Elastography: Simulation and Preliminary Clinical Evaluation. Ultrasound Med Bio; 43(3):682–699.10.1016/j.ultrasmedbio.2016.11.010
- Nozue T, Yamamoto S, Tohyama S, Umezawa S, Kunishima T, Sato A, Miyake S, Takeyama Y, Morino Y, Yamauchi T, et al. 2012. Statin treatment for coronary artery plaque composition based on intravascular ultrasound radiofrequency data analysis. Am Heart J; 163:191–9 e1.10.1016/j.ahj.2011.11.004