?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.1. Introduction
Coronary heart disease is mainly related to cholesterol plaque build up which causes hardening and narrowing of the arteries, consequently reducing or blocking blood flow to the heart. Several design requirements should be taken into account when developing new coronary stent. Traditionally a new stent design is developed by experimental trial-and-error method: this process is very time consuming and does not often fully reveal potential failures. Hence, Finite Element Analysis (FEA) has been used extensively in recent years for research and development of stents as an integral part of the design process (Hsiao et al. Citation2012). These methodologies allow testing virtual prototypes, but the question about the optimal design can be raised, taking into account all the geometrical, structural and material point of view.
In that context, mathematical optimization algorithms are very efficient, and can lead to find the best design, and avoiding trial and error tryout procedures (Ning et al. Citation2009). Indeed, the combination of finite element analysis and optimization procedure (Sanjay et al. Citation2011), enables to precisely predict stent deployment and detect product defects as stress concentrations and fractures in a design stage, thereby reducing design and prototyping costs to a considerable extent. However, numerical simulations and optimization algorithms have to be sufficiently robust in order to avoid the high computational time and guarantee severe manufacturing precisions. In this work, in order to improve the mechanical properties of stents, the Sequential Quadratic Programming (SQP) algorithm is coupled with the FE simulation using Abaqus software and python script. This optimization process aims
| • | to decrease the stent surface to ensure a better flexibility | ||||
| • | to avoid the high strain locations which is directly correlated to the fatigue criteria. | ||||
| • | to avoid a critical value of Foreshortening in order to restore a large surface of the blood flow in the artery. | ||||
2. Methods
2.1. Numerical Modelling
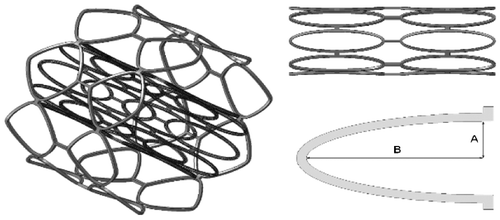
The stent is characterized by ring elements (Figure ) made of stainless steel material AISI316L, only a fraction of the ring will be modelled and optimized considering the strut parametrized by two design variables (A, B, Figure ).
The FEA is conducted using Abaqus finite element code using linear hexahedrons elements (C3D8I).
Two loading conditions are considered: radial expansion and bending combined with torsion of the stent. The radial strength of the stent is explored by expanding the stent in the radial direction using a cylinder. The expansion is simulated by constraining only the displacement of the strut in the axial direction and a radial displacement Ur = 2Rstent is imposed to the cylinder, Rstent being the initial radius of the stent.
2.2. Design optimization
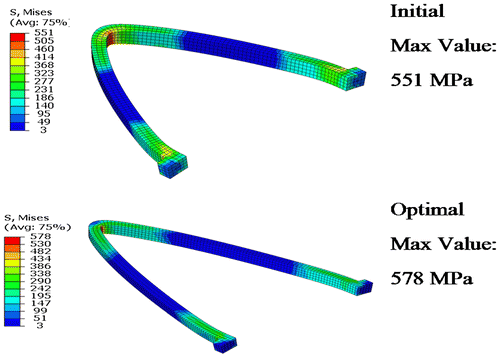
When the strut dimensions are very small, the stent flexibility is improved. Avoiding a decrease of the strut Foreshortening allows restoring the blood flow in the artery in the heart muscle tissue. The objective of the optimization algorithm is to find optimal strut dimensions to minimize the strut surface, with specific constraints like the Foreshortening stent, and the maximum equivalent plastic strain. This means that residual stresses after stent expansion should be below the ultimate tensile strength (σUTS = 580 MPa) and consequently the fatigue resistance of the stent will be ensured.
When the stent self-expands and reaches the artery diameter, it still generates radial load against the artery wall and positions itself against the artery wall with Foreshortening. In order to restore the blood flow, the first inequality constraint function is defined to avoid that the Foreshortening stent in the strut decreases more than a critical value Fc = 10 mm. Another constraint function
is defined in order to improve the long-term performance of the stent, particularly in decreasing the risk of fatigue fracture: strain amplitude (Equation 1) in the strut should be less than the critical value
as discussed by Wiersma et al. Citation2006.
(1)
(1)
The constraint functions or
are selected in a way that they are negative if respectively the Foreshortening or strain amplitude obtained in the optimal structure are higher than a critical value Fc, or
else they will be positive. Given these conditions, the nonlinear optimization problem may be expressed as follow:
(2)
(2)
Where J(x) is the normalized objective function, S(x) is the surface of the strut, S0 is the initial strut surface, x is the vector of the optimization parameters: x = {A,B}. xl and xu represent respectively the lower and upper limits: xl = [0.3 1] and xu = [0.5 2].
3. Results and discussion
In order to improve the stent design, Finite Element Method (FEM) simulations provide quantitative measures of the stent surface, the Foreshortening stent’s strain amplitude and mean strain which are generated by the cyclic pulsating load. These FE simulations were run after the geometry of the strut was updated according to the optimization loops. The summary of the optimization results is reported in Table . It can be observed that the optimization algorithm gives an improvement of the strut surface, which gives more flexibility of the stent. The objective function is then decreased by 12% compared to the initial solution.
Table 1. Summary of the optimization results.
Initial configuration of the stent shows that the Foreshortening does not respect the requirements of the optimization problem. During the optimization operations, the objective function (strut surface) decreases with optimal value equal to 21.88 mm2. At the same time, both constraints are respected. The residual stresses after stent expansion (Figure ) is below the ultimate tensile strength (σUTS = 580 MPa) and consequently the fatigue resistance of the stent is ensured.
4. Conclusions
This study accomplishes an optimization of the design of a coronary stent, based on a parametric CAD model. Several objective and constraint functions have been used in the optimization problem, such as life duration, or flexibility. The relationship between stent over-sizing, strut dimensions and fatigue life of stent were demonstrated: It has been shown that the Foreshortening decreased with decreasing stent over-sizing. The strains amplitude and mean strains follows the requirements of stent design for the criteria of fatigue life. This study illustrates the feasibility of optimization process of a biomechanical device, showing very interesting results for future strut development.
References
- Hsiao H, Chiu Y, Lee K, Lin C. 2012. Computational modeling of effects of intravascular stent design on key mechanical and hemodynamic behavior. Computer-Aided Design. 44:757–765.
- Ning L, Hongwu Z, Huajiang O. 2009. Shape optimization of coronary artery stent based on a parametric model. Finite Element in Analysis and Design. 45:468–475.
- Sanjay P, Georges L, Nick PC, Neil WB. 2011. Multiobjective design optimisation of coronary stents. Biomaterials. 32:7755–7773.
- Wiersma S, Dolan F, Taylor D. 2006. Fatigue and fracture in materials used for micro-scale biomedical components. Bio-Medical Materials and Engineering. 16:137–146.