?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Keywords:
1. Introduction
Bone remodeling characterizes the lifelong turnover and adaptation of bone tissue to its mechanical and biochemical environment. This phenomenon is of major importance, as it governs healing as well as osseointegration around implants (Haïat et al. Citation2014).
Several scales and physics are involved in bone remodeling. Indeed, changes of the mechanical environment of bone at the organ scale lead to apposition and resorption of bone material at the microstructural scale which, in turn, result in an evolution of the mechanical properties of bone tissue, at the tissue scale. Moreover, biochemical stimuli can also trigger bone remodeling.
In this work, we present a thermodynamically consistent framework for describing the evolution of bone elastic properties at the tissue scale. Such an approach allows to consider not only mechanics, but also phenomena related to biological activity. In particular, we focus on the stress-driven rotation of the principal axes of the elastic tensor of bone. Rotation of bone material axes describes, at the tissue scale, the reorientation of bone microstructure induced by the macroscopic loading.
2. Methods
In the present study, bone tissue is described as an orthotropic elastic medium whose elastic properties evolve in time according to the mechanical environment (DiCarlo et al. Citation2006). The remodeling process is represented as the rotation of the bone microstructure which is linked to the macroscopic loading.
The complete motion of a material point of such a medium requires a two-layer description: On the one side, its position in space (gross motion); On the other side, the rotation of its material axes. A generalized virtual power principle, encompassing the virtual power expended by velocities related to both the gross motion and the rotation of the material axes, leads to the continuum balance laws. A constitutive theory is then built on the definition of a strain energy density depending on the elastic strain and of the orientation of the material axes. Finally, the formulation of the dissipation principle leads to a remodeling evolution law giving an explicit relationship coupling the dissipation related to the remodeling (the fourth-order tensor D), the stress and strain tensors (the second-order tensors S and E, respectively), the rotation of the material axes (the second-order rotation tensor R) and its time derivative ():
where [S, E] = SE − ES. Remodeling equilibrium is achieved when material properties no longer evolve, corresponding to a stationary state of the rotation. This condition corresponds to an equilibrium at the macroscopic scale, which does not account for the modeling and remodeling cell activity at the lower scale.
It is worth noting that this model predicts the principal axes of the strain and stress tensors to be locally collinear at the remodeling equilibrium (Sansalone et al. Citation2011). Thus, a physically sound condition for remodeling equilibrium (Cowin Citation1986) is recovered without any ad hoc assumption.
3. Results and discussion
The model was tested on simple 2D toy models.
Firstly, the model was applied to a macroscopic unit of bone tissue to follow the evolution of the local orientation of the material as well as strain energy until equilibrium. When constant strain conditions are applied, the medium tends to minimize the strain energy to reach equilibrium. This result reads as a sound condition to the achievement of equilibrium.
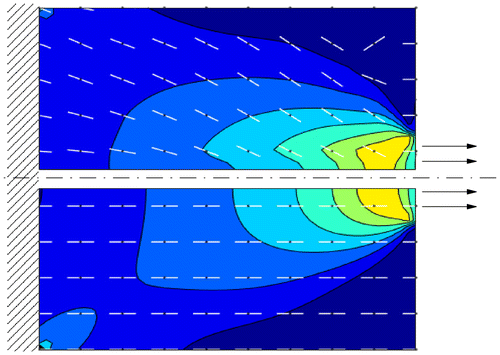
On the other hand, we monitored the evolution of a square piece of bone subjected to a boundary load with a finite element analysis. The prediction of the rotation of the principal axes of the material in simple loading configurations is consistent with the superimposed boundary conditions (see Figure ). This result shows the ability of the model to simulate the material response to non-uniform stress configurations.
4. Conclusions
A novel, thermodynamically sound model of bone remodeling was proposed. The preliminary results obtained in 2D show that the model can describe the response of the material to non-uniform stress configurations, which shows the potential of this approach. Model predictions for in vivo biomechanical loading configurations need to be further tested, including the specific case of tissue surrounding an implant. To this end, suitable experimental data will be identified. The present model can also integrate the mechanobiological phenomena regulating bone remodeling (Lerebours et al. Citation2016). However, this would require a reliable description of the biochemical stimuli of bone remodeling (Lemaire et al. Citation2011). This matter is out of the scope of this paper and will be addressed in future works. Eventually, it should be mentioned that our model is only able to describe the global remodeling taking place in a medium. However, bone remodeling is a lifelong process and remodeling equilibrium results from the balance of bone resorption and apposition. So far, our model can only describe the net result of these two biological processes and is not able to distinguish between such a ‘dynamical’ equilibrium state – where the two biological processes are balanced – and the complete absence of remodeling – where the two biological process are simply stopped. In order to distinguish between these two very different situations, a finer description of the individual biological processes leading to bone remodeling should be introduced, e.g. as proposed in (DiCarlo et al. Citation2009) in the scope of growing aneurysms.
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program [grant agreement number 682001], [project ERC Consolidator Grant 2015 BoneImplant].
References
- Cowin S. 1986. Wolf’s law of trabecular architecture at remodeling equilibrium. J Biomech Eng. 108:83–88.10.1115/1.3138584
- DiCarlo A, Naili S, Quiligotti S. 2006. Sur le remodelage des tissus osseux anisotropes. CR Mecanique. 334:651–661.10.1016/j.crme.2006.06.009
- DiCarlo A, Sansalone V, Tatone A, Varano V.. 2009. Growth and remodelling of intracranial saccular aneurysms. Proceeding of the COMSOL Conference 2009; Oct 14–16; Milan, Italy.
- Haïat G, Wang HL, Brunski J. 2014. Effects of biomechanical properties of the bone–implant interface on dental implant stability: from in silico approaches to the patient’s mouth. Ann Rev Biomed Eng. 16:187–213.10.1146/annurev-bioeng-071813-104854
- Lemaire T, Capiez-Lernout E, Kaiser J, Naili S, Sansalone V. 2011. What is the importance of multiphysical phenomena in bone remodelling signals expression? A multiscale perspective. J Mech Behav Biomed Mat. 4:909–920.10.1016/j.jmbbm.2011.03.007
- Lerebours C, Buenzli PR, Scheiner S, Pivonka P. 2016. A multiscale mechanobiological model of bone remodelling predicts site-specific bone loss in the femur during osteoporosis and mechanical disuse. Biomech Model Mechanobiol. 15:43–67.10.1007/s10237-015-0705-x
- Sansalone V, Naili S, Di Carlo A. 2011. On the rotary remodelling equilibrium of bone. Comput Methods Biomech Biomed Engin. 14(s1):203–204.10.1080/10255842.2011.595181