1. Introduction
Knee joint kinematics involves two convex bone condyles on the distal end of the femur that slides against two concave bone condyles at the proximal end of the tibia. Each condyle is covered by a thin layer of soft tissue called articular cartilage that helps to decrease friction during sliding. Below each articular cartilage layer, there is subchondral mineralized zone (SMZ) which is a mineralized joint layer that attenuates axial impact forces encountered during dynamic joint loading. SMZ lies between trabecular bone and articular cartilage. It is composed of subchondral bone (SB) and calcified cartilage (CC). During pathological process that impacts knee joint kinematics such as osteoarthritis (OA), SMZ tissue is hardened and subject to sclerosis and early stages of SMZ sclerosis show a decrease in SMZ porosity. However, there is a lack of automatic method to detect decrease in SMZ porosity in mice, the most common animal model of OA. This lack of method prevents scientist to quantify efficiency and/or develop new therapy dedicated to OA. The current study presents a new algorithm to compute automatically 3D porosity and thickness of SMZ, SB and CC in both femur condyles from HR-μCT slides of mouse wild type.
2. Methods
2.1. Bone scanning
μCT scans (SCANCO Medical AG) have been performed on a CD1 mice, 8-week-old mice femur with 1.2 μm voxel size and 4.080 mm field of view. The following code of segmentation has been customized in Matlab (Mathworks, Inc., Natick, Massachusetts, United States). To assess the validity of our method of segmentation, 3D visualization of each mineralized layers has been performed using Image J (Schneider et al. Citation2012). Image J has been solely used for 3D visualization purposes.
2.2. Segmentation of each mineralized layers of the SMZ
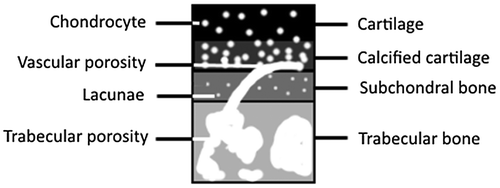
To segment CC, we used the difference in porosity between CC and bony layers because CC is mainly composed of chondrocytes porosity (5.80 μm) that are not present in any bony layers. Layers layout is presented on Figure . Presence of chondrocytes which appear in dark can increase contrast between CC and bony layers by blurring images. A multi-threshold has been applied to obtain a mask of CC based on Otsu’s method (Otsu Citation1979). Image grey values have been separated into several classes until pixels representing CC were completely separated from others. To segment TB, a boundary method similar to Edelsbrunner and Mücke (Citation1994) based on granulometry has been performed by contouring trabecular porosity. To isolate SB, the only bony zone directly between CC and TB has been segmented.
2.3. Porosity segmentation
SMZ porosity is composed of chondrocytes which lie within CC, lacunae which lie within SB, and vascular porosity which lies in both layers. Porosities layout is presented Figure . To segment vascular porosity, we tracked the common porosity within both TB and SMZ. Then, remaining porosity within SB is lacunae and remaining porosity within CC is chondrocytes.
3. Results and discussion
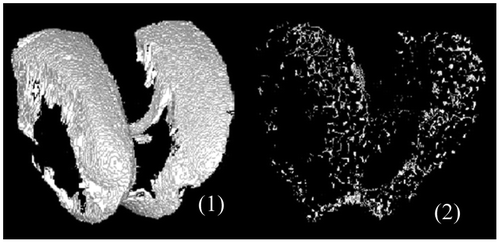
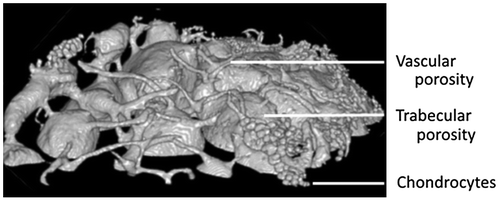
A realistic reconstruction of vascular porosity network, osteocytes lacunae within SB, and chondrocytes within Calcified Cartilage, has been obtained from a segmentation of the CC based on multi thresholding and percentage of chondrocytes porosity. Regarding vascular porosity geometrical properties and percentage, values obtained with our new algorithm spread in the range of the literature. Figure presents 3D reconstruction of SMZ and vascular porosity within SMZ.
Within SB, lacunae percentage of porosity is lower than vascular porosity (respectively 1.17% (±0.18) and 2.40% (±0.7)). Conversely, in calcified cartilage, vascular porosity is lower than chondrocytes porosity (respectively 12.83% (±2.60) and 0.94% (±0.95)) (Malinin and Ouellette Citation2000).
Regarding chondrocytes and vascular porosity orientation, our results do not provide any specific organization, but 3D visualization of vascular network showed a general tendency of vessels to be perpendicular to CC until contact; and then they turn to be tangential (Figure ). This can be explained by the main role of vascular porosity to bring nutrients to cartilage. Thus, tangential orientations promote exchanges with cartilage by increasing contact surface.
Our technique of segmentation segments chondrocytes within CC and provides for the first time a mean value for chondrocytes diameter of 5.80 μm (±0.57) in mice. Mean diameter of vascular porosity was 9.75 μm (±0.96) and mean of osteocytes lacunae diameter was 3.86 μm (±0.18).
Interestingly and for the first time, our 3D reconstructions show some vascular porosity infiltrations within numerous chondrocytes forming chambers (Figure ). These infiltrations are due to SMZ damages. Damages provoke a biological response by SMZ that potentiates progression of cartilage damage in OA (Burr and Radin Citation2003).
CC thickness was 40.69 μm (±16.31), SB thickness was 64.54 μm (±34.09) and SMZ thickness was 85.38 μm (±41.51). Our results regarding CC, SMZ and SB thickness shows that it doesn’t present homogeneous values as it has been previosuly found in human (Berteau et al. Citation2015).
4. Conclusions
Our study presents for the first time a 3D reconstruction of SMZ in mice knee joint with a realistic reconstruction of vascular porosity network, osteocytes lacunae and chondrocytes. Furthermore, we have been able to quantify porosity diameters, percentage of porosity, and pores direction in CC and SB in a rodent model. Our algorithm is of major value in future quantification of alterations of porosity caused by SB sclerosis during early OA.
Acknowledgements
We gratefully acknowledge Scanco Medical AG for having gratefully performed the high resolution μCT scans.
References
- Berteau JP, Mielke G, Morlock MM, Huber G. 2015. Morphological and biomechanical analyses of the subchondral mineralized zone in human sacral facet joints: Application to improved diagnosis of osteoarthritis. Clin Anat. 28(4):538–544.
- Burr DB, Radin EL. 2003. Microfractures and microcracks in subchondral bone: are they relevant to osteoarthrosis? Rheumatic Dis Clin North Am. 29(4):675–85.
- Edelsbrunner H, Mücke EP. 1994. Three-dimensional Alpha Shapes. ACM Trans Graph. 13(1):43–72.
- Malinin T, Ouellette EA. 2000. Articular cartilage nutrition is mediated by subchondral bone: a long-term autograft study in baboons. Osteoarthritis Cartilage. 8(6):483–91. DOI:10.1053/joca.1999.0324.
- Otsu Nobuyuki. 1979. A threshold selection method from gray-level histograms. N/A. 9(1):62–66. DOI:10.1109/tsmc.1979.4310076.
- Schneider Caroline A, Rasband Wayne S, Eliceiri Kevin W. 2012. NIH Image to ImageJ: 25 Years of Image Analysis. Nat Methods. 9(7):671–75. DOI:10.1038/nmeth.2089.