1. Introduction
Injuries to digital collateral nerves after hand trauma are frequent and results from surgical treatment are often poor (Schmidhammer et al. Citation2007). Characterization of mechanical behaviour of healthy collateral nerves is necessary to produce numeric models (Chang et al. Citation2015) and enhance the comprehension of nerve repair and regeneration process. There is no standard in the literature to characterize healthy nerve mechanics. The mechanical properties of the repaired nerve should be as similar as the healthy nerves, following the principles of mechanobiology (Aubry et al. Citation2015). The aim of this study was to characterize the mechanical properties of human digital collateral nerves to look for a correlation with density as an intrinsic property of materials.
2. Methods
2.1. Harvesting and nerve conservation
After nerve dissection on 4 cadaveric hands, 18 digital collateral nerves of 5 cm average length, were harvested and preserved by immersion in a NaCl 0.9% solution (Nishida et al. Citation2015) to be tested. Each nerve was labelled to identify the corresponding finger during the tensile test.
2.2. Tensile test
Nerves were positioned in a frame of emery paper, sutured and glued with ethyl-2-cyanoacrylate (Loctite, Henkel, Germany). One drop of Cyanoacrylate was carefully applied on the zone in contact with emery paper. Once positioned and aligned (Figure ) in the machine (Instron® 3345 single column tensile test machine) the tensile test was performed at speed of 6 mm/min. During the test the nerve was constantly rehydrated with a NaCl 0.9% solution. Diameter was measured in the middle of the nerve with a calibrated camera and Image J software (Schindelin et al., 2007). The Young modulus was measured with Origin 8.5® as the slope corresponding from the elastic zone of each nerve, where a linear regression fitted the curve.
2.3. Density and failure pattern
Density was measured using a pycnometer and registered for each nerve. The test was recorded by a high-speed camera (Mako® G-131) to identify the failure pattern according to whether the failure occurs in distal, middle and proximal part of the nerve. Finally, the diameter was measured using ImageJ software (Schindelin et al. Citation2012) in a posterior image analysis.
3. Results and discussion
Results presented concern 3 thumbs, 2 index fingers, 6 middle fingers, 3 ring fingers and 4 pinky fingers (Table ). There is no statistical significant difference between the studied variables among fingers.
Table 1. Results of the mechanical characteristics of digital collateral nerves (mean values by finger type).
The failure pattern was distal in 72.22%, middle 11.11% and proximal in 16.67% of cases in contrast to the results presented by Goldberg et al. (Citation2007).
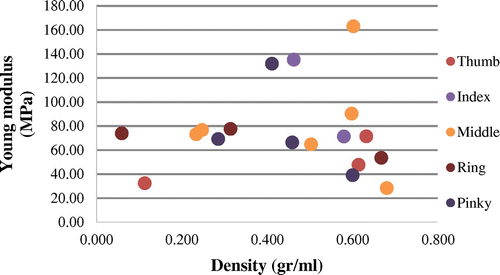
Our study presented a specific modulus characterization of human digital collateral nerves (Figure ), we do not found other studies to compare our results. A pattern for each finger was not found in contrast with dispersed results, even between finger types. We propose that tissue characterization should involve intensive properties to describe their mechanical behaviour. This article was limited to a scanty number of nerves; further studies would be addressed on this subject.
4. Conclusions
We presented the human digital collateral nerve specific modulus constituted of 18 nerves. The failure pattern was distal in 70% cases. Further studies in nerve composition must be conducted to enhance the knowledge of nerve biomechanical behaviour. There is a lack in standardization for testing biological tissues. There are no studies comparing intensive properties of nerves to describe their mechanical behaviour.
Acknowledgements
We acknowledge the European Wrist Arthroscopy Society EWAS for the donation of the cadaveric hands.
We acknowledge Illaria Illuminati, Mohamed Swaisi, Antonio Lopez Camacho, for their collaboration in this project.
References
- Aubry D, Gupta M, Ladoux B, Allena R. 2015. Mechanical link between durotaxis, cell polarity and anisotropy during cell migration. Phys Biol. 12:26–37.
- Chang CT, Chen YH, Lin CCK, Ju MS. 2015. Finite element modeling of hyper-viscoelasticity of peripheral nerve ultrastructures. J Biomech. 48:1982–1987.10.1016/j.jbiomech.2015.04.004
- Goldberg SH, Jobin CM, Hayes AG, Gardner T, Rosenwasser MP, Strauch RJ. 2007. Biomechanics and histology of intact and repaired digital nerves: an in vitro study. J Hand Surg Am. 32:474–482.10.1016/j.jhsa.2006.12.008
- Nishida N, Kanchiku T, Ohgi J, Ichihara K, Chen X, Taguchi T. 2015. Mechanical properties of nerve roots and rami radiculares isolated from fresh pig spinal cords. Neural Regen Res. 10:1869–1873.10.4103/1673-5374.170319
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods. 9:676–682.10.1038/nmeth.2019
- Schmidhammer R, Zandieh S, Hopf R, Schultz A, Gogolewski S, Hertz H, Redl H. 2007. How to improve the results of peripheral nerve surgery. In: Millesi H, Schmidhammer R, editors. Springer-Verlag/Wien.