1. Introduction
The mitral valve is the cardiac valve separating the left atrium from the left ventricle. It is composed of four elements: the anterior and posterior leaflets; the annulus which serves as insertion site in the heart muscle for the leaflets; the chordae tendineae which are attached to pillars in the ventricular wall and ensure that the very flexible leaflets remain within the ventricle; the papillary muscles which are located at the tip of the chordae tendineae and actively modify the tension acting on the leaflets. In the physiological case, the valve is open during diastole letting blood flow into the ventricle, and close during systole: the leaflets then create a hermetic seal between the two chambers preventing blood from regurgitating into the left atrium when ejected into the aorta. Mitral insufficiency is a valvular heart disease caused by a leaky mitral valve. It is the most frequent valvular pathology in Western countries after aortic narrowing upon calcification (Singh et al. Citation1999).
Mitral valve scan is the gold standard to obtain geometrical information. Anatomical studies have shown that human mitral valve measures may differ among patients (Gunnal et al. Citation2012; Khalighi et al. Citation2017), but generic patterns exist (Lau et al. Citation2010; Drach et al. Citation2015), which opens up the possibility to define parametric models of typical mitral valves. Several valve models have been developed in physiological conditions (Kunzelman et al. Citation2007; Lau et al. Citation2010; Shen et al. Citation2017), but pathological conditions have been little studied. The aim of the present study is to develop a geometrical model of mitral valves in both healthy and pathological states.
2. Methods
2.1. Mitral valve definition
A mitral valve parametric model was created using FreeCAD software and Fortran subroutines, assuming a left-to-right symmetry and average geometrical parameter values (). It is constructed by defining points along the edges of the leaflets, which are then interpolated using b-splines.
Table 1. Geometrical parameters used for the model.
The annulus of the mitral valve is defined using a planar D-shape, similarly to the Carpentier-Edwards Physio annuloplasty ring (Edwards Lifesciences). The papillary muscles tips are placed at fixed positions obtained from medical data (Sakai et al. Citation1999). The chordae tendineae are created as uniformly distributed chords going from the edges of the leaflets to the papillary muscle tips.
2.2 Model of the mitral function
The closure of the mitral valve is simulated using a structure-only finite element method with the software ADINA v9.5. The impact of the blood flow dynamics on the valve deformation is indeed negligible when the valve is closed (Toma et al. Citation2017).
We solve for the displacement of the leaflets when subjected to a pressure profile composed of an initial linear ramp up to 0.1 s, followed by a constant plateau. The annulus and papillary muscle tips are fixed during the computation. The leaflet and chordae tendineae are assumed to be isotropic linear elastic, with Young modulus E = 3 MPa, Poisson ratio v = 0.45 and density ρ = 103 kg/m3. The contact is accounted for implicitly with a constraint function parameter set at 10−9. We use a second-order implicit time integration scheme called Bathe scheme (Noh et al. Citation2013) and assume large displacements.
The leaflets are discretized using 4-node 3D-shells of maximum length 8 × 10−1 mm. The chordae tendineae are discretized using 2-node truss elements deforming in traction-only. The pathological case shown was obtained by rupturing some of the chordae tendineae attached to the anterior leaflet. It corresponds to a case where an asymmetry appears.
The linear part of the problem is solved directly using
LU decomposition and the non-linear part using the Newton-Raphson algorithm with a maximum of 15 iterations. A time step of 10−3 s is used from 0 s to 0.1 s and of 10−2 s hereafter. In addition, an adaptive time step method is used if convergence difficulties arise, the time step being then reduced to 10−7 s and 10−5 s, respectively.
3. Results and discussion
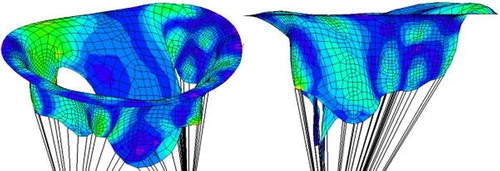
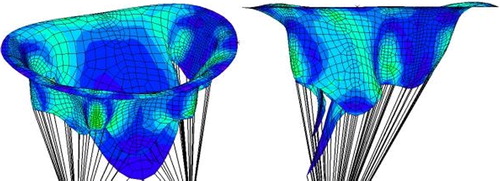
The stress distribution is plotted in the pathological () and healthy () cases. In the pathological case, a zone of higher stress than in the healthy case forms along the annulus. The valve is also not fully closed, which is a consequence of the lack of chordae tendineae attached to the anterior leaflet. The area of passage between the two leaflets can be quantified at systole to estimate valvular regurgitation. The anterior leaflet can be seen to protrude above the annulus plane in the pathological case, while it remains under it in the healthy case. The rupture of the chordae tendineae thus leads to the appearance of prolapse, which can be more easily detected thanks to imaging techniques than valve leakage itself, and can be of use to diagnose mitral regurgitation.
4. Conclusion
A geometrical model of the mitral valve capable of modeling healthy and pathological mitral valves has been built. The model allows to test several mitral valve geometries, and to assess in each case the mitral valve function using a finite element method. The model might be of use to numerically test medical procedures on mitral valves.
Additional information
Funding
References
- Drach A, Khalighi AH, ter Huurne FM, Lee CH, Bloodworth C, Pierce EL, Jensen MO, Yoganathan AP, Sacks MS. 2015. Population-averaged geometric model of mitral valve from patient-specific imaging data. J Med Devices. 9:030952.
- Gunnal SA, Farooqui MS, Wabale RN. 2012. Study of mitral valve in human cadaveric hearts. Heart Views. 13(4):132.
- Khalighi AH, Drach A, Bloodworth CH, Pierce EL, Yoganathan AP, Gorman RC, Gorman JH, Sacks MS. 2017. Mitral valve chordae tendineae: topological and geometrical characterization. Ann Biomed Eng. 45(2):378–393.
- Kunzelman KS, Einstein DR, Cochran RP. 2007. Fluid-structure interaction models of the mitral valve: function in normal and pathological states. Phil Trans R Soc B. 362(1484):1393–1406.
- Lau KD, Diaz V, Scambler P, Burriesci G. 2010. Mitral valve dynamics in structural and fluid-structure interaction models. Med Eng Phys. 32:1057–1064.
- Noh G, Ham S, Bathe KJ. 2013. Performance of an implicit time integration scheme in the analysis of wave propagations. Comp Struct. 123:93–105.
- Sakai T, Okita Y, Ueda Y, Tahata T, Ogino H, Matsuyama K, Miki S. 1999. Distance between mitral anulus and papillary muscles: anatomic study in normal human hearts. J Thorac Cardiov Sur. 118:636–641.
- Shen X, Wang T, Cao X, Cai L. 2017. The geometric model of the human mitral valve. Plos One. 12(8):e0183362.
- Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, Lehman B, Benjamin EJ. 1999. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol. 83(6):897–902.
- Toma M, Einstein DR, Bloodworth CH, Cochran RP, Yoganathan AP, Kunzelman KS. 2017. Fluid-structure interaction and structural analyses using a comprehensive mitral valve model with 3D chordal structure. Int J Numer Meth Biomed Eng. 33(4):e2815.