1. Introduction
In prosthetic dentistry, elements of implant-supported fixed partial dental prosthesis (such as superstructures, bars and trans-gingival abutments) are conventionally manufactured in Ti6Al4V (Wataha, Citation2002). This titanium alloy is traditionally manufactured using subtractive processes (numerically controlled milling of a casted Ti6Al4V block). Additive manufacturing such as selective laser melting (SLM) appears to be a promising alternative because it creates the opportunity to produce objects with complex geometries, strong individualization and less material waste (Van Noort Citation2012).
We previously showed that SLM manufactured Ti6Al4V fulfilled the mechanical requirements of the ISO 22674 standard related to the manufacturing of prosthetic parts in dentistry (Crenn et al. Citation2018). However, this process modifies surface properties and topography (Benedetti et al. Citation2017) which are essential parameters affecting gingiva reaction (Nikkhah et al. Citation2012) and plaque retention (Subramani et al. Citation2009). Since abutments, overdenture and implants bars are in direct contact with the gingiva, their surface are highly polished in order to limit bacteria accumulation responsible for gingiva inflammation (Bollen et al. Citation1997). This final polishing step is usually performed by a dental technician before clinical application.
Here, we explore the influence of the manufacturing process and of the surface finishing of Ti6Al4V on the response of human gingival fibroblasts (HGFs) in order to conclude on the potential of SLM manufacturing in prosthetic dentistry on a biological point of view.
2. Methods
2.1. Specimens preparation
Four groups of Ti6Al4V specimens were designed and prepared using different manufacturing processes and surface finishing ().
Table 1. Definition of the study groups, SEM images and roughness measurements.
2.2. Surface roughness
Surface roughness was measured using Alicona Infinite focus microscope on 3 locations per samples (n = 3 per group). Filtering parameters were determined according to the specifications of the ISO 4288 standard. Results were analyzed using Student t-test (α = 0.05).
2.3. E HGFs behaviour
2.3.1. Cytotoxicity study
HGFs obtained from primary cultures (1st passage) were seeded on Ti6Al4V samples at 40.000 cells/ml (n = 5 per group). Direct cytotoxicity was assessed at day 1 and 3 according to the ISO 10993-5 standard. Percentage of cell proliferation and viability were analyzed using Live/Dead™ Cell viability. Metabolic activity was measured using rezaruzine test (alamar Blue®). Polyolefin polymer discs were used as control. Significance was assessed using Prism 7 software (GraphPad v7.0a) with Kruskal-wallis statistical test (p value < 0.05).
2.3.2. Cell morphology characterization
For the evaluation of cell morphology, samples were seeded with HGFs (2nd passage) at 5.000 cells/ml and observed using SEM after fixation at day 14 (n = 3 per group).
3. Results and discussion
3.1. Surface roughness
No statistically significant difference was observed between SLM manufactured and numerically controlled milled samples after mechanical polishing: 68 nm ± 26 nm vs. 94 ± 63 nm. Thus, surface finishing erases the difference in surface topography introduced by the chosen process ().
3.2. HGFs behaviour
HGFs were viable and proliferated on all disc types at day 3 (). For each specimen type, metabolic activity was significantly increased at day 3 compared to day 1, displaying cell proliferation. No significant difference was observed between all groups at day 1. However, metabolic activity was decreased for as-built samples compared to control at day 3.
Figure 1. LIVE/DEAD staining images of HGFs cells on the surface of Ti6Al4V discs after 3 days at incubation.
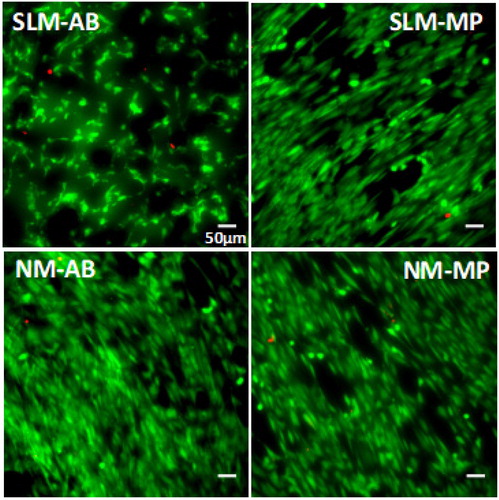
Cell viability was higher to 97% for all groups. Numerically milled specimens with mechanical polishing (NM-MP) showed a slightly higher %viability (p = 0.016) than SLM as-built (SLM-AB) at day 1 and 3. No significant difference was shown compared to other groups.
At day 14, HGFs presented different morphologies according to sample surfaces. Cells were elongated in shape, slender and stretched out on MP surfaces. More lamellipodia were observed on the SLM-AB, suggesting a better adhesion.
4. Conclusion
Taken together, our data show that the mechanically polished titanium surface obtained by SLM is compatible for the realization of dental prosthesis in direct contact with gingiva. However, cell shapes on polished samples suggest a moderate adhesion compared to unpolished samples. It remains to investigate the influence of surface roughness and treatments to improve soft tissue integration.
Acknowledgements
The authors would like to thank Prof. B. Salmon and Dr G. Jouanny, S. Le Goff from URB2i and the Centre des Matériaux (Mines ParisTech) for SEM images; and Edentech and Circles4labs for providing specimens.
References
- Benedetti M, Torresani E, Leoni M, Fontanari V, Bandini M, Pederzolli C, Potrich C. 2017. The effect of post-sintering treatments on the fatigue and biological behavior of Ti-6Al-4V ELI parts made by selective laser melting. J Mech Behav Biomed Mater. 71:295–306.
- Bollen CM, Lambrechts P, Quirynen M. 1997. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: a review of the literature. Dent Mater. 13(4):258–269.
- Crenn MJ, Benoit A, Attal JP, Fromentin O. 2018. Comparison of mechanical properties of CAD/CAM-milled and selective laser-melted Ti-6Al-4V for dental superstructures. Int J Prosthodont. 31(6):591–593.
- Nikkhah M, Edalat F, Manoucheri S, Khademhosseini A. 2012. Engineering microscale topographies to control the cell-substrate interface. Biomaterials. 33(21):5230–5246.
- Van Noort R. 2012. The future of dental devices is digital. Dent Mater. 28(1):3–12.
- Subramani K, Jung RE, Molenberg A, Hammerle C. 2009. Biofilm on dental implants: a review of the literature. Int J Oral Maxillofac. 24(4):616–626.
- Wataha JC. 2002. Alloys for prosthodontic restorations. J Prosthet Dent. 87(4):351–363.
