1. Introduction
In Europe 350 thousand people die every year of lung cancer, which is considered as the leading cause of all cancer deaths. Radiation therapy is one of the most common treatments for cancer, aims at delivering a lethal dose of ionizing radiation to tumour tissues while sparing the surrounding tissues from the adverse effects of radiation. Breathing is an active and complex process where the respiratory motion is non-reproducible, and the breathing periodicity, amplitude and motion path of patients’ organs, Intracycle and inter-cycle variations of respiratory patterns are observed during the respiration (Ehrhardt et al. 2013).
In literature, various finite element (FE) models have been developed and used to predict the respiratory motion. Unfortunately, most of the time, the authors have used a single organ (lung) with nonrealistic boundary conditions, or the lung motion is simulated using simple displacement boundary conditions which are not realistic and do not take into account the real physiological respiratory motion. The authors in (Eom et al. Citation2010) have proposed a FE modeling of the lung motion using a generic P-V curve, which is not patient specific. Recently, patient specific biomechanical model of the lung motion from 4 D CT images for half respiratory cycle have been proposed (Fuerst et al. Citation2015), where the motion is not constrained by any fixed boundary condition. The authors have respectively used 4 and 16 pressure zones on the sub-diaphragm and thoracic cavity, which is not physiologically realistic. In our last work
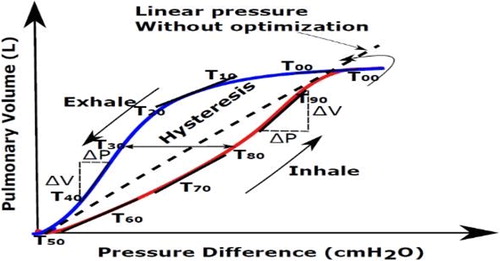
(Giroux et al. Citation2017), we have proposed and developed patient specific biomechanical model coupled and controlled by personalized physiological pressure/volume curve (compliance). In this paper, we have applied and compared two scenarios (): 1) The linear pressure (ramp amplitude) for the whole cycle without any optimization of the lung pressure at different respiratory states (named without optimization) and 2) Optimized lung pressure and diaphragm force using an automatic tuning algorithm based on inverse FE analysis methodology during a whole respiratory cycle and at different states (with optimization).
2. Materials and methods
Lungs and the external skin are segmented automatic- ally from 4 D CT Scan Images, using graylevel threshold algorithms available within ITK-SNAP library. However, the automatic segmentation of the diaphragm and lung tumors remains quite challenging and suffer from a lack of reproducibility. Thus, the correct segmentation of lung tumors can only be achieved by medical experts. To extract the mediastinum structure, we have used the different segmentation masks of the lungs, thorax, the inner thoracic region and the diaphragm. Finally, using marching cubes, we have extracted triangular surface meshes corresponding to each organ. These surfaces meshes are simplified and smoothed and then converted into tetrahedral meshes suited for finite element analysis. The volumetric meshes obtained from our developed approach were inserted into finite element ABAQUS solver in order to perform finite element analysis. The deformation of lungs is controlled and driven by simulated ribcage (mimic the external intercostal muscles) and diaphragm actions. Pleural sliding on the inside of the ribcage has also been considered. For the diaphragm, we have applied the radial direction of muscle forces, and simple homogeneous Dirichlet boundary condition is applied to the lower part of the diaphragm, which is attached to the rib cage. For each rib a rigid transformation is calculated automatically by finite helical axis method (Ladjal et al. Citation2015). The chest pressure-volume relationships (Compliance) is calculated and determined at different respiratory states by an optimization framework based on inverse FE analysis methodology, by minimizing the volume lungs errors, between the respiratory volume (calculated from CT scan images at each state) and the simulated volume (calculated by biomechanical simulation). In our simulations, the diaphragm and lungs tissues are considered as compressible, isotropic and hyperplastic Saint-Venant Kirchhoff (SVK) model. The mediastinum, the thorax and skin tissues are considered isotropic with linear elastic behavior. The mechanical properties of the different organs used in our simulations are given in (Ladjal et al. Citation2015, Giroux et al. Citation2017).
3. Results and discussion
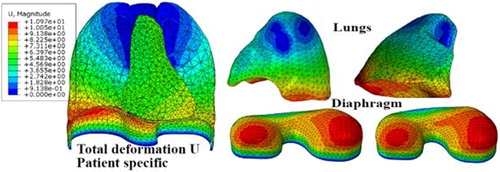
In this paper, we have evaluated the model accuracy on two selected patients, 4DCT scan data from DIRLab Dataset (Castillo et al. Citation2009) where the entire thorax was visible. We have compared the FE simulation results on 300 landmarks, at end inspiration (EI), end expiration (EE) states, and 75 landmarks at each intermediate respiratory state. shows the deformation of the lungs and diaphragm during breathing. We can observe the maximum displacement of the diaphragm on the right-posterior (RP) and left-posterior (LP) sides. For the lungs deformation, the maximum displacement occurring in the posterior region along the superior-inferior (SI) direction.
shows the comparison study between our FE simulation results and the ground-truth displacement vectors for two patients, without and within the optimization algorithm. These results show that the developed patient specific biomechanical model coupled within the lung-pressure/diaphragm-force optimization algorithm of the respiratory system is in a good agreement with the experimental data.
Table 1. Average landmark lung error during exhalation at different respiratory states.
We have also evaluated the tumour motion and trajectory identified in 4 D CT scan images. These trajectories are compared and evaluated with the trajectory obtained by FE simulation, during one complete breathing cycle. The results demonstrate that our patient specific biomechanical model coupled within the optimization algorithm for tumor lung tracking is very accurate, with the average mean error during the whole cycle less than 3 mm. This could be a potential tool to provide valuable location-specific tumor motion information for medical physician to reduce the margins between clinical target volume (CTV) and planning target volume (PTV).
4. Conclusions
We have developed an accurate and automatic tuning algorithm of lung pressure parameter based on patient specific biomechanical model of respiratory system during the whole respiratory cycle. We can observe that the proposed physically based FE model able to predict correctly and with the high precision the lung tumour displacements including the real physiological lung-pressure/diaphragm-force. In order to track and to compute the lung tumour position in clinical situation, this model will be correlated and driven by a set of external parameters (thorax outer-surface motion, air flow…) measured directly during each treatment session.
Additional information
Funding
References
- Castillo E, Castillo R, Martinez J, Shenoy M, Guerrero T. 2009. Four-dimensional deformable image registration using trajectory modeling. Phys Med Biol. 55(1):305–327.
- Eom J, Xu XG, De S, Shi C. 2010. Predictive modelling of lung motion over the entire respiratory cycle using measured pressure-volume data, 4DCT images, and finite-element analysis. Med Phys. 37(8):4389–4400.
- Ladjal H, et al. 2015. Biomechanical modeling of the respiratory system: human diaphragm and thorax, computational biomechanics for medicine new approaches and new applications. Comput Biomech Med. 101–115.
- Fuerst B, Mansi T, Carnis F, Salzle M, Zhang J, Declerck J, Boettger T, Bayouth J, Navab N, Kamen A, et al. 2015. Patient-specific biomechanical model for the prediction of lung motion from 4D CT images. IEEE Trans Med Imaging. 34(2):599–607.
- Giroux M, et al. 2017. Biomechanical Patient Specific Model of the Respiratory System Based on 4D CT Scans and Controlled by Personalized Physiological. 20th International Conference Medical Image Computing and Computer Assisted Intervention, MICCAI, 13 September 2017, Quebec, Canada. p. 216–223.