1. Introduction
Because many cardiovascular diseases are related to blood flow features, being able to predict how blood flows would open a window towards better diagnostic and treatment capabilities. To this respect, Computational Fluid Dynamics (CFD) is a natural complement to advanced medical imaging techniques which suffer from a lack of spatial resolution and cannot measure the stresses induced by the flow (pressure, wall shear stress). Still, several aspects make computational hemodynamics very challenging. Many (medical) questions related to blood flows are relevant to the bigger arteries/veins of the cardiovascular system. The typical length scale ranges from 1 mm to a few centimetres and the typical Reynolds number can be as high as a few thousands. At these scales, blood is practically considered as a homogeneous fluid with either constant viscosity (typically μ = 3 × 10−3 Pa.s) or a shear-thinning behaviour. Typical issues that must be properly handled are then related to the highly complex and deformable geometry of the domain where blood flows (Chnafa et al. Citation2014), the transitional nature of the wall-bounded blood flow (Nicoud et al. Citation2011), the interaction with thin and highly deformable membranes like valve leaflets (Sigüenza et al. Citation2018), thrombus formation due to the presence of biomedical materials (Méndez Rojano et al. Citation2018) and of course validation (Sigüenza et al. Citation2016; Zmijanovic et al. Citation2017).
2. Methods
The flow solver YALES2BIO (imag.umontpellier.fr/∼yales2bio/) developed at IMAG for macro and micro hemodynamics was used to compute the flow within the Food and Drug Administration’s (FDA) medical nozzle benchmark model (Hariharan et al. Citation2011). In this solver, the turbulence activity, if any, is represented by the large-eddy-simulation technique where the largest structures are resolved explicitly and the effect of the sub-grid scales is modeled. In YALES2BIO, the Sigma model (Nicoud et al. Citation2011) is used since it is well-suited to represent weak turbulence at moderate Reynolds numbers. The geometry of the FDA case consists of an inlet section, a 10° half-conical convergent nozzle, a throat section connected to a larger downstream pipe through a sudden expansion section. When the Reynolds number is large enough, the experimental data shows that the jet issued from the throat becomes turbulent and breaks down at some position within the downstream pipe.
3. Results and discussion
As detailed previously (Zmijanovic et al. Citation2017), several simulations were performed to establish the capability of the flow solver to predict the position of the breakdown to turbulence.
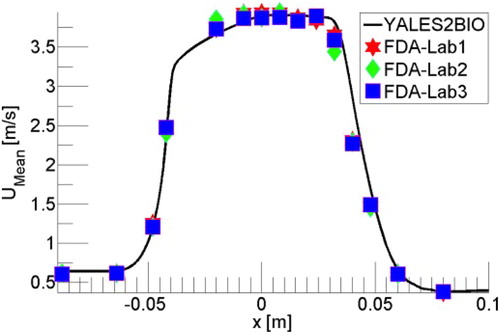
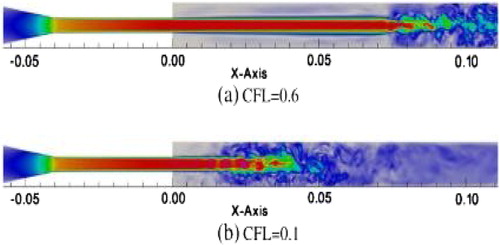
When a rather fine mesh is used (50 M cells), shows that the agreement with the experimental data can be excellent. Unfortunately, this result is not robust to changes of numerical parameters, as illustrated in which shows how the jet breakdown changes when the CFL number (scaled time step) is decreased from 0.6 to 0.1
In the present case, because the transition of a symmetric jet is very sensitive to all the details, the jet breakdown was observed (Zmijanovic et al. Citation2017) to be also impacted by the numerical scheme and the mesh refinement. Irrespective of the good comparison shown in , the lack of robustness of the numerical results makes them unacceptable, although this aspect is virtually not discussed in the literature.
It turns out that the numerical results can be made robust to all the numerical parameters cited above (time step, numerical scheme, mesh resolution) by recognizing that the inlet velocity profile is not a perfect Poiseuille parabolic profile but rather contains a small amount of random fluctuations (Zmijanovic et al. Citation2017). Some amount of fluctuations was necessarily present in the experiment, although not documented. Interestingly, the stabilization of the results is achieved independently of the amplitude of the fluctuations (the range 0.5% to 5% was tested successfully). Note also that the whole range of flow regimes considered experimentally (from laminar to fully turbulent) can be computed accurately and in a robust and predictive way thanks to the procedure proposed. As a matter of fact, similar observations regarding the importance of inlet perturbations were made recently by another group using a rather different, finite-element-based, numerical strategy (Bergersen et al. Citation2019).
4. Conclusions
In many studies, validating a numerical strategy simply amounts to reaching a good agreement with experimental data. This is in fact not always sufficient as the robustness of the numerical results to physical/numerical parameters may be so large that a good agreement may be reached by chance. In view of the results presented, it is important that robustness is established on top of accuracy to validate a numerical methodology.
References
- Bergersen AW, Mortensen M, Valen-Sendstad K. 2019. The FDA nozzle benchmark: “In theory there is no difference between theory and practice, but in practice there is. Int J Numer Meth Biomed Eng. 35(1):e3150.
- Chnafa C, Mendez S, Nicoud F. 2014. Imagebased large-eddy-simulation in a realistic left heart. Computer Fluid. 94:173–187.
- Hariharan P, Giarra M, Reddy V, Day SW, Manning KB, Deutsch S, Stewart SFC, Myers MR, Berman MR, Burgreen GW, et al. 2011. Multilaboratory particle image velocimetry analysis of the FDA benchmark nozzle model to support validation of computational fluid dynamics simulations. J Biomech Eng. 133(4):041002.
- Méndez Rojano R, Mendez S, Nicoud F. 2018. Introducing the pro-coagulant contact system in the numerical assessment of device-related thrombosis. Biomech Model Mechanobiol. 17(3):815–826.
- Nicoud F, Toda HB, Cabrit O, Bose S, Lee J. 2011. Using singular values to build a subgrid-scale model for large eddy simulations. Phys Fluid. 23(8):085106.
- Sigüenza J, Mendez S, Ambard D, Dubois F, Jourdan F, Mozul R, Nicoud F. 2016. Validation of an immersed thick boundary method for simulating fluid-structure interactions of deformable membranes. Comput Phys. 322:723–746.
- Sigüenza J, Pott D, Mendez S, Sonntag S J, Kaufmann TAS, Steinseifer U, Nicoud F. 2018. Fluid-structure interaction of a pulsatile flow with an aortic valve model: a combined experimental and numerical study. Int J Numer Meth Biomed Eng. 34(4):e2945–19.
- Zmijanovic V, Mendez S, Moureau V, Nicoud F. 2017. About the numerical robustness of biomedical benchmark cases: interlaboratory FDA’s idealized medical device. Int J Numer Meth Biomed Eng. 33(1):e02789.