1. Introduction
The human inner ear hosts a fundamental component of the mechanisms regulating balance and maintaining gaze: the vestibular system. It can detect motion thanks to three semicircular canals (SCC), which act as sensors of angular acceleration of the head, using deflections of the cupular diaphragm to generate neural information. This system is often damaged after a head trauma, as a consequence of a traffic accident, a domestic fall or a violent sport activity as well as from blast injury, and this damage causes significant difficulty and reduced quality of life for the accident victim. As the vestibular system is very small, complex to access surgically and breakable when extracted, an accurate understanding of its behaviour has not yet been achieved. One way to improve our knowledge on it is to develop and use analytical models such as Finite Element (FE) analysis tools.
Consequently, this study aims to investigate the mechanical behaviour of that peripheral neural sensor to help surgeons in their diagnosis and treatment, and to develop better support for the victim after a head trauma.
2. Methods
To this end, a 3 D FE model of a lateral SCC was developed using the Altair™ Hyperworks™ Software.
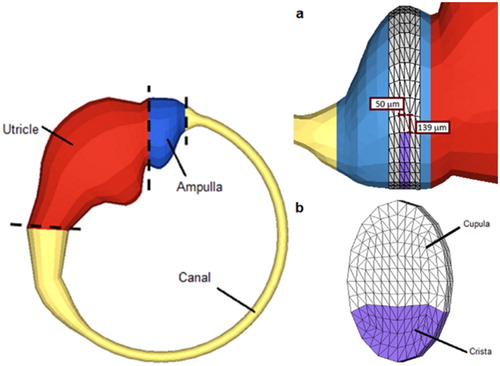
The geometry of the model was extracted from anatomical measurements (Curthoys and Oman Citation1987) of the human lateral SCC. The FE model () includes the following components: membranous structures on the outer surface of the model, divided between canal, ampulla and utricle; a cupular diaphragm, composed of a cupula and a crista, in the ampullar compartment; and an endolymphatic fluid filling the remainder of the enclosed volume. Membranous structures are represented by 4-nodes quadrilateral 2 D elements (with a given implicit thickness of 0.01 mm), while the other structures are made of 8-nodes hexahedral 3 D elements. The whole mesh consists of 17,112 nodes and 18,882 elements. The mesh is also perfectly continuous.
Figure 1. Membranous walls of the FE model (left), meshed cupula and crista (right b) inside the ampulla (right a).
The fluid in the endolymphatic space is considered to be a Newtonian homogenous quasi-incompressible viscous fluid. It has been attributed a density of 1,020 kg.m−3. For the kinematic viscosity, literature reports values in the order of magnitude of 10−4 m2.s−1 (Rajguru et al. Citation2004). The solid structures of the model (the membranes and the cupular diaphragm) was considered as being linear elastic materials. Literature values were taken into account for the choices of the Young moduli of cupula (1 Pa) and membranes (100 Pa, Kassemi et al. 2005). For the crista, Young modulus (1 MPa) was based on other known tissues of similar composition. The rigidity, i.e., the Young’s modulus, of the cupula, which is the most deformable part of the model, has to be lower than that of the membranes, while the crista rigidity has to be much higher. Also, the density of the cupula (1,020 kg.m−3) must be equal to that of the surrounding endolymphatic fluid to avoid mass and inertia effects, while the density of the other materials is that of water (1,000 kg.m−3), of which they are mostly made. The fluid/structural coupling followed an Arbitrary Lagrangian Eulerian approach.
In order to represent the perilymphatic space influence, it was decided to include its confining role on the membranes: the pressure difference between the endolymph and the perilymph was implemented in the mechanical modeling. A difference of 0.2 mPa (De Paolis et al. Citation2017) was therefore used. It can be noted that the perilymphatic space was not entirely meshed in the model. Actually meshing this whole space with an exact geometry would have drastically augmented the duration of calculations, while using a simplified perilymphatic space (for example, meshing two layers of elements around the free membranes) would provide incorrect results according to tests that were achieved.
3. Results and discussion
The FE model of the lateral SCC is validated against measurements from clinical practice. The test used is a case of sudden stop of the head, in 100 ms, following rotation, namely the Head Impulse Test of Halmagyi and Curthoys (1988).
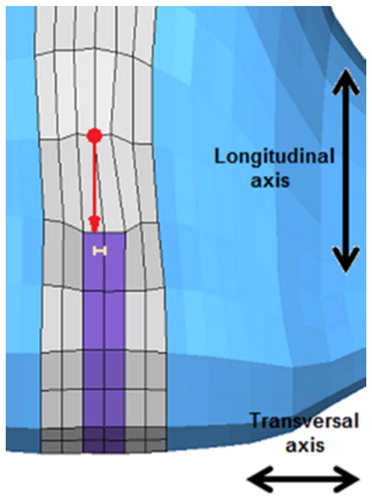
Results () of the reference simulation, concerning the cupula displacements and relaxation durations, were in agreement with known data from literature (Njeugna et al. Citation1992). In fact, a 17 μm transversal deflection of the cupula is calculated in the simulation, and is comparable to the 21 μm transversal deflection measured in the experiments (Halmagyi and Curthoys 1988). Moreover, a 1.23 s relaxation duration of the cupula is obtained in the simulation, and is comparable to the 1.8 to 5 s relaxation duration that is measured in the experimental tests (Njeugna et al. Citation1992).
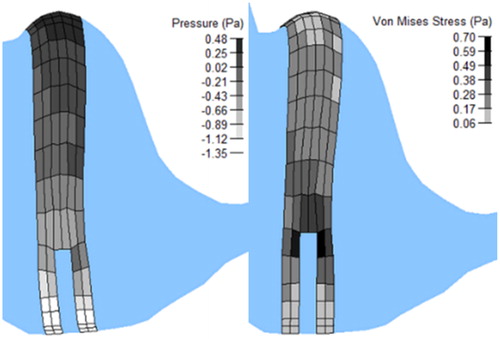
Pressures ( left) and Von Mises stresses ( right) in the cupula were also calculated in order to estimate the levels such variables may reach in that kind of anatomical structure. Nevertheless, these maximal values, i.e., a pressure of 0.48 Pa and a Von Mises of 0.70 Pa, could not be compared to experimentally measured values because of lack of such data in literature. Nevertheless, the pressure in particular (P = (σ11+σ22+σ33)/3) experiences values that are in the range of magnitude that are expected for such biological soft tissues.
4. Conclusions
This study describes the development and the validation against experimental data and clinical observations of a FE model of a human lateral SCC of the inner ear. This model is of particular interest for the understanding of vestibular physiology, in order to help ENT practitioners in their positional diagnostic and therapeutic manoeuvres after a head trauma. In addition, this model could also prove useful for clinical purpose, in cases of atypical pathologies of the inner ear. In fact, there is no other way to obtain such knowledge of this very small and fragile system.
References
- Curthoys IS, Oman CM. 1987. Dimensions of the horizontal semicircular duct, ampulla and utricle in the human. Soto. 103(3–4):254–261.
- De Paolis A, Watanabe H, Nelson JT, Bikson M, Packer M, Cardoso L. 2017. Human cochlear hydrodynamics: a high resolution μCT-based finite element study. J Biomech. 50:209–216.
- Halmagyi GM, Curthoys IS. 1988. A clinical sign of canal paresis. Arch Neurol. 45(7):737–739.
- Kassemi M, Deserranno D, Oas JG. 2005. Fluid-structural interactions in the inner ear. Comput Struct. 83(2–3):181–189.
- Njeugna E, Eichhorn JL, Kopp C, Harlicot P. 1992. Mechanics of the cupula: effects of its thickness. J Vestib Res. 2(3):227–234.
- Rajguru SM, Ifediba MA, Rabbitt RD. 2004. Three-dimensional biomechanical model of benign paroxysmal positional vertigo. Ann Biomed Eng. 32(6):831–846.