1. Introduction
The preferential development of atherosclerotic lesions near arterial branches and bifurcations suggests that localizing factors such as fluid mechanical disturbances are involved in the etiology of the disease. Numerous experimental and computational studies of arterial flow fields have demonstrated the highly dynamic nature of flow disturbance at arterial branches (Karino et al. Citation1990; Cheer et al. Citation1998). In the aorta in vivo, major branches are often in close anatomic proximity and thus interact fluid mechanically with one another. A prominent example where atherosclerotic lesions are prevalent is in the abdominal aorta where two major branches, the celiac and superior mesenteric arteries, emerge from the aorta along the ventral wall to form T-junctions. Interestingly, the spacing between these two branches varies significantly among individuals and across species, and this spacing is expected to have a profound impact on the fluid mechanical interactions between the two branches and thus on the overall flow field in the abdominal aorta. How fluid mechanical interactions between two aortic branches modulate flow disturbance in the vicinity of each branch remains incompletely understood. The goal of the present study is to computationally investigate the effect of fluid mechanical interactions between the celiac and superior mesenteric arteries on the local flow field and wall shear stress distribution.
2. Methods
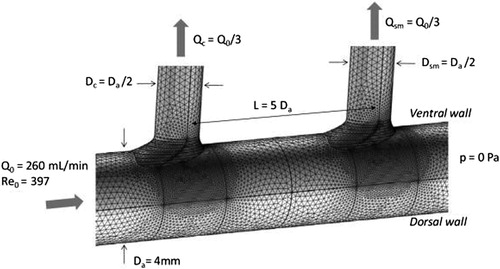
We performed computational fluid dynamic simulations of the flow field in a rigid wall model of the abdominal aorta with two successive T-junctions emanating from the same aortic aspect, as would be the case for the celiac and superior mesenteric arteries. The spacing between the two branches was varied over a wide range, and aortic Reynolds numbers in the range 10 to 1000 were studied. The fluid was assumed to be Newtonian with the density and viscosity of blood. Both steady and sinusoidal pulsatile flow were simulated. The simulations were conducted using the commercial multi-physics code COMSOL. depicts the geometry and flow conditions for the base case simulations.
3. Results and discussion
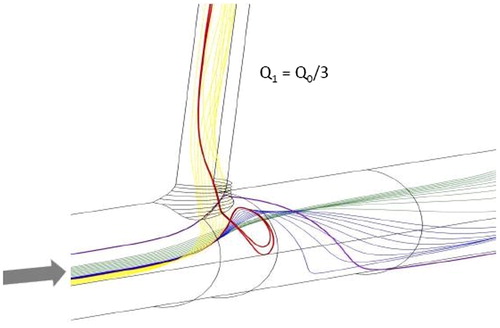
Previous experimental and computational studies of the flow field at the aorto-celiac junction have demonstrated the presence of thin zones of separation and flow reversal along the lateral walls of the aorta, leading to low wall shear stress levels in those regions (Barakat et al. Citation1997; Cheer et al. Citation1998). Our present simulations show that for steady flow, these zones are always present in the case of a single aortic junction as long as a critical Reynolds number of approximately 100 is exceeded. An example is illustrated in . The flow in these zones is derived from streamlines coming from the middle lateral region of the aortic lumen that are deflected towards the branch but insufficiently to enter it directly. These streamlines impact the flow divider at the distal wall of the branch before looping backwards along the lateral walls to form the separation regions, and they then follow complex trajectories to enter the branch.
Figure 2. Illustration of the flow separation and streamline reversal zone along the lateral wall of a single aortic branch.
In the case of two aortic junctions, the simulations show that whether or not the aortic lateral looping patterns occur depends in a complex manner on both the Reynolds number and the spacing between the two branches. More specifically, for both very small and very large values of inter-branch spacing, the looping patterns are present at both branches over practically the entire range of Reynolds numbers studied (100–900). On the other hand, at intermediate inter-branch spacing, lateral loops are present at the upstream but not the downstream branch, suggesting that the two branches experience fundamentally different forms of flow disturbance.
All the above was for the case of steady flow. In pulsatile flow, the lateral looping patterns periodically grow and shrink. Therefore, for intermediate values of inter-branch spacing, the two branches experience markedly different patterns of directional oscillations in wall shear stress. A particularly interesting observation is that along the lateral walls of the proximal branch, the wall shear stress reverses direction once during the cardiac cycle, whereas two episodes of shear stress directional reversal during each cycle are observed for the distal branch. This finding indicates that the temporal gradients of wall shear stress are considerably different for the two branches.
4. Conclusions
The present results highlight the importance of inter-branch spacing in determining the nature of flow disturbance in the aorta. For a particular type of aortic flow disturbance, namely recirculating flow loops along the lateral aortic wall, a surprising finding is that both very small and very large values of inter-branch spacing lead to similar patterns in this disturbance whereas intermediate values of inter-branch spacing are associated with a fundamentally different pattern. These findings have important implications for efforts aimed at determining the hemodynamic parameters that best correlate with the localization of atherosclerosis.
Additional information
Funding
References
- Barakat AI, Karino T, Colton CK. 1997. Microcinematographic studies of flow patterns in the excised rabbit aorta and its major branches. BIR. 34(3):195–221.
- Cheer AY, Dwyer HA, Barakat AI, Sy E, Bice M. 1998. Computational study of the effect of geometric and flow parameters on the steady flow field at the rabbit aorto-celiac bifurcation. Biorheology. 35(6):415–435.
- Karino T, Motomiya M, Goldsmith HL. 1990. Flow patterns at the major T-junctions of the dog descending aorta. J Biomech. 23(6):537–548.