1. Introduction
The main cause (90%) of cancer-related deaths is due to metastasis, spreading of cancer to distant sites in the body. Metastasis requires cells to dynamically adapt to the changing environments that they traverse, inducing changes in cell morphology, intracellular structure and dynamics, cell-cell interactions, as well as cell interactions with a changing microenvironment. Accordingly, we and others have shown that metastatic cells exhibit faster cytoskeletal dynamics and are internally (Gal and Weihs Citation2012) and externally softer than non-invasive cells. Concurrently, metastatic cancer cells apply stronger adhesive, traction forces as compared to benign (Massalha and Weihs Citation2017).
We have recently discovered the existence of a mechanically invasive subpopulation of metastatic cancer cells that can rapidly (<2 hours) and forcefully indent an elastic, physiological stiffness, synthetic, impenetrable gel. In breast cancer cell lines, we have shown that the mechanically invasive cells indent the gels to depths of 1-10μm, whereas benign breast cells do not indent (Kristal-Muscal et al. Citation2013; Dvir et al. Citation2015; Merkher and Weihs Citation2017); the cell diameter is 20 μm. We have observed that indenting cells exhibit a coordinated reorganization of their mechanostructure to apply force (Dvir et al. Citation2015), utilizing mechanisms that rely on the cytoskeleton dynamics. The mechanically invasive subpopulation of cells is also highly migratory and invasive as determined by Boyden chamber assay that takes 48-96 hrs (AlvarezElizondo and Weihs 2017).
2. Methods
2.1. Cell culture
Cell lines (from ATCC) were cultured in their appropriate media and were used up to passage 30. Tumor samples were obtained from local hospitals. Cells were extracted from freshly resected tissue samples by mincing, enzymatic degradation, debris filtration (100 µm), and blood-cell lysis.
2.2. Gel preparation
Polyacrylamide gels were prepared according to our published protocols (Merkher and Weihs Citation2017). In short, gels were prepared at stiffness of 2.4 kPa with 200-nm fluorescent beads embedded in their surface; those serve as fiducial markers for the gel surface.
2.3. Mechanical invasiveness, cell indentation assay
Cells are seeded on gels and changes in focal plane of particles at the gel surface is used to reveal indentations and their depths (Merkher and Weihs Citation2017).
3. Results and discussion
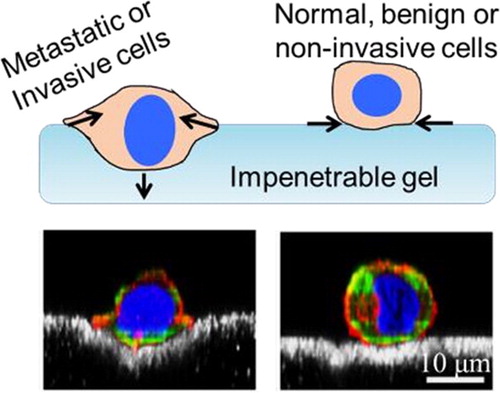
Interactions of cells on a soft gel indicate existence of a mechanically invasive subpopulation of cells (). When cancer cells are densely seeded, closely situated yet still in a single layer, we note that a subset of metastatic cells (>40%) is able indent gels to depths on the scale of cell radius (Merkher and Weihs Citation2017). In contrast, benign and non-invasive cancer cells indent at lower amounts (<20%) and to lower depths. These measurements supplement and strengthen our observations in single cells, providing a rapid tool for evaluation of the metastatic potential of cells through their mechanical invasiveness.
Figure 1. Mechanically invasive subpopulations of cancer cells actively indent cells, while benign or non-invasive cancer cells only apply adhesive, lateral forces. (Top) Schematic of indenting and nonindenting cells; (Bottom) Confocal images of indenting metastatic cells and non-indenting benign cells (Adapted from (Dvir et al. Citation2015)); beads at gel surface are in white.
We observe measurable differences in the mechanobiological interactions of invasive and noninvasive subpopulations of commercial cancer cell lines. Using cell lines from breast and pancreatic cancers we establish the mechanical invasiveness measure. We demonstrate that the mechanical invasiveness measure is applicable to various solid cancers (e.g., breast, pancreatic, and skin cancers). Furthermore, we show that the mechanical invasiveness agrees with the clinical histopathology in patients and also with the long term outcome in patients with rapidly invasive cancers.
The interactions of cells with their surroundings is of course also affected by the microenvironment mechanostructure, e.g., the topology and biomechanics. Thus, we show that metastatic cancer cells differ from benign cells when seeded on varying surface topographies. Specifically, we show that metastatic and benign cells differ in their morphology, alignment, adherence, and proliferation, when seeded on flat, porous or micro-patterned surfaces. The short term and long term interactions indicate that e.g., benign cells are geared towards generating continuous cell layers and eventual tissue, while cancer cells independently seek optimal regions for adherence. Changes in tissue mechanostructure can thus facilitate invasion of cancer cells. Aptly, we show that the biomechanics of in vivo tissue can change (in terms of stiffness and elasticity) following long-term exposure to microparticles shed from breast cancer cells.
4. Conclusions
We show that the mechanobiological interactions of cells with their surroundings can provide rapid (2 hr) cancer diagnosis and a prognosis for the likelihood of metastasis. The biomechanics of the tumor and metastatic site microenvironment, which we also show is modified by the presence of cancer in the body. Our innovative mechanical invasiveness measure is independent of tumor genetics, and governed by the strength and invasive propensity of the cells, and is that widely applicable to different solid tumors.
Additional information
Funding
References
- Alvarez-Elizondo MB, Weihs D. 2017. Cell-gel mechanical interactions as an approach to rapidly and quantitatively reveal invasive subpopulations of metastatic cancer cells. Tissue Eng Part C Methods. 23(3):180–187.
- Dvir L, Nissim R, Alvarez-Elizondo MB, Weihs D. 2015. Quantitative measures to reveal coordinated cytoskeleton-nucleus reorganization during in vitro invasion of cancer cells. New J Phys. 17(4):043010.
- Gal N, Weihs D. 2012. Intracellular mechanics and activity of breast cancer cells correlate with metastatic potential. Cell Biochem Biophys. 63(3):199–209.
- Kristal-Muscal R, Dvir L, Weihs D. 2013. Metastatic cancer cells tenaciously indent impenetrable, soft substrates. New J Phys. 15(3):035022.
- Massalha S, Weihs D. 2017. Metastatic breast cancer cells adhere strongly on varying stiffness substrates, initially without adjusting their morphology. Biomech Model Mechanobiol. 16(3):961–970.
- Merkher Y, Weihs D. 2017. Proximity of metastatic cells enhances their mechanobiological invasiveness. Ann Biomed Eng. 45(6):1399–1406.
